Abstract
Context
Powerful demographic trends toward reproductive aging of human populations, older age at first childbirth, and lower birth rates will profoundly influence the health, vitality, and economies of human societies and deserve greater attention in health policy and research.
Evidence Acquisition
Information on birth rates, fertility rates, and outcomes of assisted reproductive technologies were obtained from databases of government agencies (census data, Centers for Disease Control and Prevention).
Evidence Synthesis
Fecundity declines with advancing age, especially in women >35 years and men >50 years. Advanced parental age adversely affects pregnancy outcomes for the mother and the offspring and increases the offspring’s risk of chromosomal disorders, neurodegenerative diseases, and birth defects. Because of increased life expectancy, today people will spend a major portion of life in a period of reproductive senescence; diseases associated with reproductive senescence will influence the health and well-being of middle-aged and older adults. Inversion of the population age pyramid will affect health care costs, retirement age, generational distribution of wealth, and the vitality of societies. Actions can be taken to mitigate the societal consequences of these trends. An educational campaign to inform young people about the trade-offs associated with postponement of childbirth will enable them to make informed choices. Some repositioning of research agenda and health care policies is needed to address the public health threat posed by reproductive aging.
Conclusion
The consequences of low fertility rates and delayed parenthood on our nation’s health, vitality, and economic growth should be considered when crafting research, health, and economic policies.
Reproductive aging will profoundly influence health, vitality, and economies of human societies; redirection of health and economic policies is needed to mitigate its adverse societal consequences.
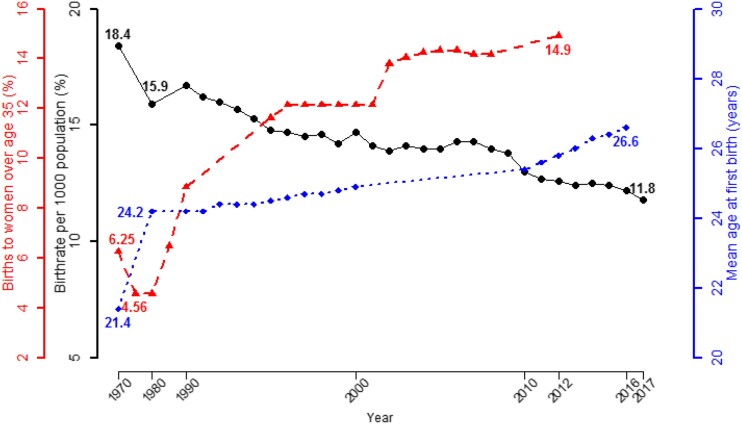
Birth rates in the United States, which had declined for nearly 200 years—except during a short baby boom period after World War II—have trended below replacement levels since 1971 (1‒3) (Fig. 1). A growing proportion of couples are having their first child after age 30 years, and an increasing proportion are postponing pregnancy beyond age 35 years (1‒4) (Fig. 1). First birth rates for women aged 35 to 39 years increased sixfold from 1.7 per 1000 women in 1973 to 11.0 per 1000 women in 2012; similarly, first birth rates for women aged 40 to 44 years increased fourfold from 0.5 to 2.3 per 1000 women from 1985 to 2012 (4). A confluence of socioeconomic factors, including the availability of contraceptives that enabled couples to separate their sexual and procreative lives, increased workforce participation and changing career expectations of women, and a higher age at reproductive union, have motivated men and women to postpone childbearing to an older age (3–5). Today, the extended time required to complete university education, be gainfully employed, and feel financially secure enough to raise a family provides strong motivation for young people to delay first childbirth (3, 5). Furthermore, men and women can expect to spend a substantial fraction of their life span in a period of reproductive senescence, which may affect their health and quality of life. Thus, the historical transition toward reproductive aging of the population, older age at first childbirth, and lower birth rates will profoundly influence the health, vitality, and economy of the United States and deserves greater attention in health policy development and research.
Figure 1.
Birth rates per 1000 population, mean age of mother at first childbirth, and proportion of infants born in the United States to women older than 35 y of age since 1970. The birth rate per 1000 population declined from 18.4 in 1970 to 11.8 in 2017 (black circles). The mean age of mothers at first childbirth increased from 21.4 y in 1970 to 26.6 y in 2016 (blue diamonds). The proportion of all infants born in the United States to mothers older than 35 y increased from 4.6% in 1970 to 14.9% in 2012 (red triangles). For 1990 through 2017, rates are estimated as of July 1; for 1970 and 1980, rates are estimated as of April 1 and are redrawn from Centers for Disease Control and Prevention data (1).
Although we have used US statistics to exemplify these demographic trends, similar trends toward lower total fertility rates have been observed in most developed countries (5). Countries such as Japan, Singapore, Germany, Italy, Spain, and Ukraine already are experiencing negative population growth. In most European countries, the majority of births now take place in women younger than 30 years (6). The average age of women at childbirth in Organization for Economic Cooperation and Development countries now stands at 30 years (7).
Declining birth rates are not unique to developed countries. Total fertility rates also have decreased markedly in rapidly developing countries such as China, India, and South Korea. If current birth rates persist, China’s population will begin to decline in 2025 (8). India’s 2016 census data indicate that fertility rates in nine of 29 states had reached the replacement level of 2.1 children per woman, and in an additional 12 states, fertility rates had fallen below replacement level to 2.0 children per woman (9). The average age of women at first childbirth in Chile, Turkey, and Mexico ranges from 28 to 32 years (7)!
Fecundity declines with advancing maternal age, particularly after age 35 years (6, 10), owing to a marked reduction in the number of oocytes (from a peak of 6 to 7 million during fetal life to ∼1 million at birth; 300,000 at puberty; 25,000 at age 37 years, and 1000 at age 51 years) and an increase in the proportion of poor-quality oocytes. Success rates of in vitro fertilization (IVF) decline steeply in women older than 35 years compared with younger women (11–13). Thus, the probability of a clinical pregnancy or a live birth in any month starts declining in women in their late twenties and declines steeply after age 35 years. Although men continue to father children well into old age, increasing paternal age also is an independent risk factor for reduced fertility (14–16). Compared with men 21 to 25 years old, men older than 50 years have lower sperm motility and sperm morphology, a higher frequency of sperm tail defects, lower fecundity, and increased time to pregnancy (15).
The postponement of childbearing until older age increases the risk of involuntary childlessness because of the adverse effects of advanced maternal and paternal age per se on fecundity; increased risk of comorbidities such as obesity, cancer, and cardiometabolic disorders associated with advancing age that may indirectly affect fecundity; and age-related changes in reproductive behaviors (4, 6). The expectations for family size also have decreased over the past 100 years. As couples get older, difficulties in conceiving due to reduced fecundity, the high cost of assisted reproductive technologies, and the challenges young people face in managing the demands of career and family may lead to indecision, ambivalence, and eventually a reluctant acceptance of childlessness.
Risks to the Health of the Mother and the Offspring Posed by Advanced Parental Age
Advanced parental age adversely affects pregnancy outcomes for the mother and the offspring (6, 14–16). Maternal age >35 years is associated with poor pregnancy outcomes for the mother: increased risks of ectopic pregnancy, preeclampsia, gestational diabetes, placental disorders, and maternal mortality (6). The offspring of older mothers (older than 35 years) are at increased risk of spontaneous abortion, stillbirth, preterm birth, low birth weight, and chromosomal errors (6, 16). Postponement of childbearing increases the likelihood of using ovulation induction or IVF treatment, both of which raise the risk of multiple pregnancies, morbidity, and mortality for the mother and the offspring (11–13). Advanced maternal age and increasing use of assisted reproductive technologies are important contributors to the increasing rates of multiple births over the past 30 years (11, 12).
Increased maternal and paternal age at conception may increase the offspring’s risk of certain chromosomal disorders, neurodegenerative diseases, and other birth defects (14–16). The risk of trisomy 21 (Down syndrome) is strongly associated with advanced maternal age; its incidence increases from 0.8 case per 1000 live births for mothers 25 to 29 years of age to 13.2 cases per 1000 live births for mothers 40 to 44 years of age (16). Advanced parental age also is associated with an increased risk of trisomy 13 and 18, Klinefelter syndrome, and possibly trisomy 21, although for the latter, the association is much stronger with maternal age than with paternal age (14, 15). Advanced paternal age is associated with increased risk of germ-line mutations in FGFR2, FGFR3, and RET genes and associated autosomal dominant diseases, such as achondroplasia, Pfeiffer syndrome, Crouzon syndrome, Apert syndrome, MEN2A, and MEN2B (14, 15). The incidence of achondroplasia increases nearly eightfold, from 1 in 15,000 in the general population to 1 in 1875 for children of men older than 50 years (17). Advanced paternal age also is associated with increased risk of neurodevelopmental disorders such as schizophrenia, autism, and bipolar disorders and cardiac malformations such as ventricular septal defects, atrial septal defects, and patent ductus arteriosus (14, 15).
Effect of Reproductive Aging on the Overall Health and Quality of Life of Middle-Aged and Older Men and Women
With few exceptions (e.g., short-finned pilot whales, killer whales, and some fish), most animal species in the wild do not live beyond their reproductive years (18). The remarkable increase in life expectancy during the past century—mostly as a result of improved sanitation and the availability of clean water, food, immunization, and treatments for infectious diseases—is an extraordinary human accomplishment. Today, women can expect to spend a greater proportion of their life span in menopause, which increases the risk of osteoporosis, cardiovascular disease, autoimmune diseases, sleep disorders, and possibly dementia (19). Likewise, age-related decline in testosterone levels in older men is associated with low libido, erectile dysfunction, reduced muscle mass and strength, physical dysfunction, mobility disability, whole body and visceral adiposity, and increased risk of fracture and low-grade late-life progressive depressive disorder (20). Therefore, preservation of steroidogenic functions of the gonad and optimizing hormone therapies to maximize their benefit/risk ratio are important research priorities.
Conditions related to reproductive aging—sexual dysfunction, testosterone deficiency, lower urinary tract symptoms, prostate cancer, and pelvic floor disorders—have emerged as major motivators for middle-aged and older adults to seek medical care. As a result of this growing unmet need, the number of specialized men’s health clinics that focus on these sexual and genitourinary problems have mushroomed around the United States (21). Similarly, the overall health and quality of life of middle-aged and older women is affected greatly by menopausal symptoms, cancers of the reproductive and accessory organs, urinary incontinence, pelvic floor disorders, and fertility preservation.
Reproductive Aging and the Health and Welfare of Future Generations
Reproductive aging poses a potential threat to the reproductive capacity, health, and welfare of current and future generations. Untreated infertility prevents the transmission of genetic disorders that may underlie infertility or comorbidities associated with infertility; successful fertility treatment may facilitate the transmission of these genetic disorders to offspring. The growing proportion of older adults conceiving through assisted reproduction may increase the likelihood of transmission of these genetic disorders to the offspring of older parents, thereby increasing rates of infertility and genetic disorders in future generations and posing complex ethical issues.
Shrinking populations could negatively affect labor force composition, economic growth, consumption, and the ability of current generations to pay for the support of older generations (3). The inversion of the population age pyramid, in which older adults outnumber the young, will affect health care costs, retirement age, generational distribution of wealth, economic growth rates, and the vitality of human societies.
Mitigating the Adverse Consequences of Reproductive Aging on Human Societies
Trends toward declining birth rates and increasing parental age at first childbirth are unlikely to reverse in the near future. However, several actions can be taken now to mitigate these demographic trends and their societal consequences. First, an informational campaign to educate the public and health care providers on the increased reproductive and health risks of delayed parenthood will enable individuals to make more informed decisions about their reproductive choices. Today, many young people in their mid to late 30s, who are just beginning to feel settled at the end of an extended period of higher education and their first job, are often surprised to learn about difficulties with conception and risks to the health of the mother and offspring due to advanced parental age. Therefore, this educational campaign to inform young people about the tradeoffs associated with postponing childbirth should be aimed at empowering them to make informed choices sufficiently early in their reproductive life.
Second, some redirection of research agendas and health care policy is needed to address public health concerns posed by reproductive aging. The field of reproductive aging is still nascent, and our knowledge of the mechanisms of reproductive aging is limited. A broad-based approach using preclinical as well as human models can enhance our fundamental understanding of the molecular mechanisms of germ cell and somatic cell aging in men and women. Therapeutic strategies to preserve gonadal germ cells, their niche, and gamete quality are much needed. These strategies require a better understanding of the molecular contributors to poor gamete quality in old age, whether they are intrinsic or extrinsic to germ cells. Because federally funded research of older gametes and their derived embryos is limited by federal laws, the literature in this field is increasingly founded on clinical experience in infertility and IVF centers rather than on rigorous fundamental research. The ability to preserve and mature germ cells ex vivo from tissue harvested before chemotherapy could expand fertility options for the increasing population of young people undergoing cancer treatment. Remarkable improvements in medical treatment of cancers and survival have focused attention on infertility, sex steroid deficiencies, sexual dysfunction, metabolic disorders, frailty/sarcopenia, osteoporosis, and depression among cancer survivors. Thus, mechanism-based strategies to prevent gonadal damage in response to cancer chemotherapeutic agents are needed.
The mechanisms of impaired sex steroid production in the aging male and female gonads remain poorly understood. Further research to determine the indications, timing, and optimization of sex-hormone treatment in menopausal women and older men would advance clinical practice and the health and well-being of a growing segment of the aging human population. An understanding of the mechanisms of tissue selectivity of sex hormones and their long-term safety can help optimize sex-hormone replacement in aging men and women. The effects of nutritional, environmental, and lifestyle factors on ovarian reserve, semen quality, and the trajectory of reproductive aging need further investigation. The nation’s research agenda should prioritize a long-range plan to understand the mechanisms of reproductive aging and minimize its potential adverse consequences on the health and well-being of middle-aged and older men and women.
Finally, although medical advances can increase fertility options for older couples and improve the health and well-being of older adults during postreproductive years, the causes of postponement of childbearing to an older age are largely socioeconomic; therefore, medical solutions—a necessary component of any multipronged strategy—alone are unlikely to reverse these powerful demographic trends (3). The long-term consequences of low fertility rates and delayed parenthood on a nation’s health, vitality, and economic growth should be considered in crafting realistic estimates of health care needs, economic growth, manpower projections, and entitlement outlays to guide health and economic policy and research priorities.
Acknowledgments
Disclosure Summary: S.B. has received research grants from the National Institute on Aging, the National Institute of Nursing Research, the National Center for Medical Rehabilitation Research of the National Institute of Child Health and Human Development, the Foundation of National Institutes of Health, the Patient-Centered Outcomes Research Institute, AbbVie, AliveGen, and Metro International Biotechnology and consulting fees from OPKO Pharmaceuticals and AbbVie and holds equity interest in Function Promoting Therapies, LLC. These research grants and contracts are managed by the Brigham and Women’s Hospital and overseen by the Office of Industry Interaction, Partners Healthcare System, in accordance with institutional policies. The remaining authors have nothing to disclose.
Glossary
Abbreviation:
- IVF
in vitro fertilization
Contributor Information
Shalender Bhasin, Research Program in Men’s Health: Aging and Metabolism, Boston Claude D. Pepper Older Americans Independence Center, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts.
Candace Kerr, Aging Physiology Branch, Division of Aging Biology, National Institute on Aging, Bethesda, Maryland.
Kutluk Oktay, Laboratory of Molecular Reproduction and Fertility Preservation, Department of Obstetrics and Gynecology, Yale University, New Haven, Connecticut.
Catherine Racowsky, Department of Obstetrics, Gynecology and Reproductive Biology, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts.
References and Notes
- 1. Centers for Disease Control and Prevention. National Vital Statistics System: birth data. Available at: www.cdc.gov/nchs/nvss/births.htm. Accessed 2 December 2018.
- 2. Infoplease. Live births and birth rates, by year. Available at: www.infoplease.com/us/births/live-births-and-birth-rates-year. Accessed 2 December 2018.
- 3. Stone L. Declining fertility in America. American Enterprise Institute, 2018. Available at: www.aei.org/wp-content/uploads/2018/12/Declining-Fertility-in-America.pdf. Accessed 2 December 2018.
- 4. Mathews TJ, Hamilton BE. Mean Age of Mothers Is on the Rise: United States, 2000–2014. NCHS data brief no. 232. Hyattsville, MD: National Center for Health Statistics; 2016. [PubMed] [Google Scholar]
- 5. Gustafsson S, Kalwij A. Education and Postponement of Maternity: Economic Analyses of Industrialized Countries: European Studies of Population. Vol 15. Dordrecht, Netherlands: Kluwer Academic Publishers, Springer; 2006. [Google Scholar]
- 6. Schmidt L, Sobotka T, Bentzen JG, Nyboe Andersen A; ESHRE Reproduction and Society Task Force. Demographic and medical consequences of the postponement of parenthood. Hum Reprod Update. 2012;18(1):29–43. [DOI] [PubMed] [Google Scholar]
- 7. OECD Family Database. Sf2.3: age of mothers at childbirth and age-specific fertility. Available at: www.oecd.org/els/soc/SF_2_3_Age_mothers_childbirth.pdf. Accessed 2 December 2018.
- 8. Statistics about the population growth in China, 2001–2011, World Bank, July 2012. Available at: https://data.worldbank.org/country/china. Accessed 2 December 2018.
- 9. Office of the Registrar General & Census Commissioner, India. SRS statistical report 2016. Available at: www.censusindia.gov.in/vital_statistics/SRS_Reports__2016.html. Accessed 2 December 2018.
- 10. Menken J, Trussell J, Larsen U. Age and infertility. Science. 1986;233(4771):1389–1394. [DOI] [PubMed] [Google Scholar]
- 11. Sunderam S, Kissin DM, Crawford SB, Folger SG, Boulet SL, Warner L, Barfield WD. Assisted reproductive technology surveillance – United States, 2015. MMWR Surveill Summ. 2018;67(3):1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Calhaz-Jorge C, De Geyter C, Kupka MS, de Mouzon J, Erb K, Mocanu E, Motrenko T, Scaravelli G, Wyns C, Goossens V; European IVF-Monitoring Consortium (EIM) European Society of Human Reproduction and Embryology (ESHRE). Assisted reproductive technology in Europe, 2013: results generated from European registers by ESHRE. Hum Reprod. 2017;32(10):1957–1973. [DOI] [PubMed] [Google Scholar]
- 13. Centers for Disease Control and Prevention. Assisted reproductive technology: national summary report. Available at: www.cdc.gov/art/ART2011/PDFs/ART_2011_National_Summary_Report.pdf. Accessed 2 December 2018.
- 14. Brandt JS, Cruz Ithier MA, Rosen T, Ashkinadze E. Advanced paternal age, infertility, and reproductive risks: a review of the literature. Prenat Diagn. 2018;39(2):81–87. [DOI] [PubMed] [Google Scholar]
- 15. American Society for Reproductive Medicine. Guidelines for sperm donation. Fertil Steril. 2004;82(Suppl 1):9–12. [DOI] [PubMed] [Google Scholar]
- 16. Goetzinger KR, Shanks AL, Odibo AO, Macones GA, Cahill AG. Advanced maternal age and the risk of major congenital anomalies. Am J Perinatol. 2017;34(3):217–222. [DOI] [PubMed] [Google Scholar]
- 17. Toriello HV, Meck JM; Professional Practice and Guidelines Committee. Statement on guidance for genetic counseling in advanced paternal age. Genet Med. 2008;10(6):457–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Croft DP, Brent LJN, Franks DW, Cant MA. The evolution of prolonged life after reproduction. Trends Ecol Evol. 2015;30(7):407–416. [DOI] [PubMed] [Google Scholar]
- 19. The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728–753. [DOI] [PubMed] [Google Scholar]
- 20. Bhasin S, Brito JP, Cunningham GR, Hayes FJ, Hodis HN, Matsumoto AM, Snyder PJ, Swerdloff RS, Wu FC, Yialamas MA. Testosterone therapy in men with hypogonadism: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2018;103(5):1715–1744. [DOI] [PubMed] [Google Scholar]
- 21. Bhasin S. A perspective on the evolving landscape in male reproductive medicine. J Clin Endocrinol Metab. 2016;101(3):827–836. [DOI] [PubMed] [Google Scholar]