Abstract
Context:
Deficient anterior pituitary with variable immune deficiency (DAVID) syndrome is a rare disorder in which children present with symptomatic adrenocorticotropic hormone (ACTH) deficiency preceded by hypogammaglobulinemia from B-cell dysfunction with recurrent infections, called common variable immunodeficiency (CVID). Subsequent whole exome sequencing studies have revealed germline heterozygous C-terminal mutations of NFKB2 as a cause of DAVID syndrome or of CVID without clinical hypopituitarism. However, to the best of our knowledge there have been no cases in which the endocrinopathy has presented in the absence of a prior clinical history of CVID.
Case Description:
A previously healthy 7-year-old boy with no history of clinical immunodeficiency presented with profound hypoglycemia and seizures. He was found to have secondary adrenal insufficiency and was started on glucocorticoid replacement. An evaluation for autoimmune disease, including for antipituitary antibodies, was negative. Evaluation unexpectedly revealed hypogammaglobulinemia [decreased immunoglobulin G (IgG), IgM, and IgA]. He had moderately reduced serotype-specific IgG responses after pneumococcal polysaccharide vaccine. Subsequently, he was found to have growth hormone deficiency. Six years after initial presentation, whole exome sequencing revealed a de novo heterozygous NFKB2 missense mutation c.2596A>C (p.Ser866Arg) in the C-terminal region predicted to abrogate the processing of the p100 NFKB2 protein to its active p52 form.
Conclusions:
Isolated early-onset ACTH deficiency is rare, and C-terminal region NFKB2 mutations should be considered as an etiology even in the absence of a clinical history of CVID. Early immunologic evaluation is indicated in the diagnosis and management of isolated ACTH deficiency.
A 7-year-old boy presenting with hypoglycemic seizure found to have secondary adrenal insufficiency, hypogammaglobulinemia, and growth hormone deficiency was found to have an NFKB2 mutation.
Deficient anterior pituitary with variable immune deficiency (DAVID) syndrome was first reported in 2011 in four children from three unrelated families with common variable immunodeficiency (CVID) and recurrent or severe sinopulmonary infections followed 1 to 7 years later by symptomatic adrenocorticotropic hormone (ACTH) deficiency (1). Subsequently, germline heterozygous mutations of the C-terminal region of NFKB2 were identified as a cause of DAVID syndrome (2). Fourteen individuals from 10 families with these mutations have been reported (2–7), and of these, 11 have had laboratory-confirmed secondary adrenal insufficiency occurring 1 year to decades after the onset of CVID (2–5). Here, we report the case of a 7-year-old boy with a de novo heterozygous C-terminal NFKB2 missense mutation who presented with clinical ACTH deficiency before any history suggestive of CVID. Written consent for publication of this case report was obtained from the patient and his parents.
Case Report
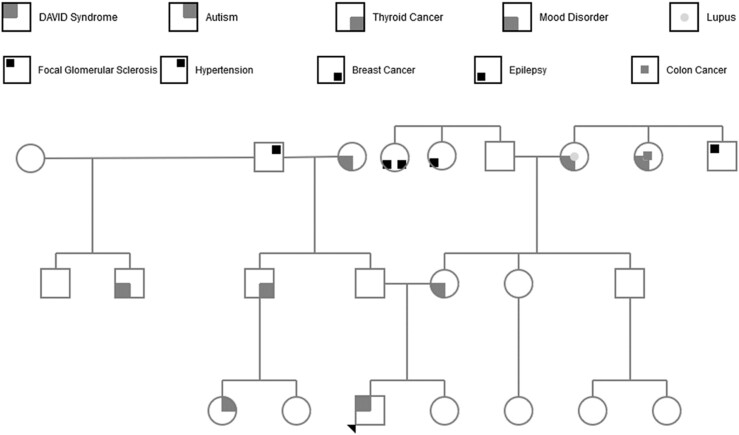
The patient was a previously healthy 7-year-old boy of northern European descent with normal growth and development. He had several episodes of acute otitis media during infancy but no frequent or severe sinopulmonary infections. There was no family history of endocrine or immunologic diseases (Fig. 1). One morning, he was found unresponsive in bed with dilated pupils, extremity twitching, and fecal incontinence. His parents called paramedics, who documented a fingerstick blood glucose of <25 mg/dL. Intravenous dextrose was administered, with an increase in blood glucose to 51 mg/dL. The patient’s mental status improved, and he took dextrose by mouth. On arrival at the emergency department, he had regained his baseline mental status, with a blood glucose of 95 mg/dL. Initial physical examination was unremarkable, with weight in 82nd percentile and height in the 85th percentile. Laboratory testing revealed hyponatremia (131 mmol/L), hyperkalemia (5.0 mmol/L), 4+ ketonuria, and mild elevation of creatine phosphokinase and liver transaminases. An extensive endocrine and metabolic workup revealed isolated acute ACTH deficiency. Computed tomography of the head revealed no hemorrhage, masses, or edema. Pituitary magnetic resonance imaging was concerning for partial empty sella that was ultimately described as a normal variant. Circulating antibodies to the anterior pituitary were tested on a research basis by Dr. Patrizio Caturegli at Johns Hopkins University School of Medicine; none were detected by immunofluorescence on human cadaveric pituitary sections (8). DNA sequencing for TBX19 mutations was negative.
Figure 1.
Extended pedigree for proband.
The patient was started on physiologic glucocorticoids, with gradual resolution of transaminase elevation, muscle weakness, and fatigue. At 10 years of age he was found to have diminished growth velocity with a low insulinlike growth factor-1, insulinlike growth factor-binding protein 3, and an inadequate growth hormone response to stimulation with clonidine and arginine. A bone age done at 10.5 years old was consistent with skeletal maturation of an 8-year-old. Growth hormone replacement therapy was initiated shortly thereafter. At age 11 he had spontaneous testicular enlargement and development of pubic and axillary hair. The patient has been monitored for central hypothyroidism and continues to have normal thyroid-stimulating hormone and free T4.
The patient was referred to immunology for consideration of atypical autoimmune etiologies, and he was initially evaluated for hypergammaglobulinemia, a feature of certain autoimmune diseases. This workup unexpectedly revealed significantly decreased serum immunoglobulin A (IgA), IgM, and IgG levels. T-cell and B-cell phenotyping and T-cell proliferative response to tetanus toxoid were normal. Antibody titers to previously administered tetanus, diphtheria, and Haemophilus influenzae type b vaccines were protective. A protective but nonrobust response to 7 of 12 tested serotypes was obtained after immunization with 23-valent unconjugated pneumococcal vaccine. The patient received a diagnosis of subclinical to mild CVID. No mutations were found in a number of CVID-associated genes (TNFRSF13B, SH2D1A, BTK, and FAS). Sequencing for mutations of AIRE, which are associated with autosomal recessive autoimmune polyendocrinopathy, revealed a monoallelic p.Lys114Asn amino acid substitution variant. However, this variant was also found in the patient’s asymptomatic mother and was therefore not considered pathogenic. His hypogammaglobulinemia remained stable >5 years after presentation. However, he experienced several mild sinopulmonary infections not necessitating intravenous antibiotics or hospitalization.
Whole exome sequencing performed 6 years after his hypoglycemia episode identified a germline heterozygous missense mutation (c.2596A>C) that resulted in a serine to arginine substitution at amino acid residue 866 (p.S866R) in the C-terminal region of NFKB2. This mutation was confirmed by Sanger sequencing and was absent in the patient’s parents and younger sister. The serine 866 residue is moderately evolutionarily conserved but is located in a mutation hotspot. According to the Grantham scale, this amino acid substitution was “moderately radical,” and mutation prediction software (NextGene and Genome Analysis Toolkit) predicted that this was a “deleterious” and “probably damaging” substitution.
Discussion
Symptomatic isolated ACTH deficiency presenting in childhood is extremely rare (9). Although children with biallelic mutations of the TBX19 gene often present with isolated ACTH deficiency, they usually do so as neonates or young infants (10). Other genetic causes of ACTH deficiency typically present later in childhood with additional clinical features, such as early-onset obesity and hypopigmentation of POMC deficiency (11) or recurrent infections as part of CVID in DAVID syndrome (1–5). Our patient’s clinical course is to the best of our knowledge unique in that symptomatic ACTH deficiency preceded any clinical signs of CVID. Although his hypogammaglobulinemia persisted, he continued to have a mild form of CVID, and he did not develop any of the characteristic B-cell phenotype abnormalities observed in other patients with NFKB2 mutations (2–7).
Family studies have shown that C-terminal region NFKB2 mutations act in an autosomal dominant manner to both perturb immune function and cause ACTH deficiency (2–7). The immunodeficiency invariably impairs B-cell function, with hypogammaglobulinemia and poor antigen-specific antibody responses, which usually present with sinopulmonary infections beginning in infancy or early childhood (1–7). The expression of clinically overt ACTH deficiency is more variable and involves only about two-thirds of the cases and is usually an isolated form of hypopituitarism. Growth hormone deficiency has been observed in only one other case of DAVID syndrome (1, 3).
Transcriptional regulation by nuclear factor κB proteins involves the nuclear translocation of members of the canonical pathway (p50/RelA or p50/c-Rel dimers) or noncanonical pathway (p52/RelB dimers). In the noncanonical pathway, the NFKB2 transcripts are first translated into a p100 precursor protein that undergoes activation-dependent cytoplasmic proteolytic processing into the smaller active p52 form after the phosphorylation of its serine 866 and serine 870 by inhibitor of nuclear factor kappa-B kinase 1 subunit (IKK1) (12). A p100 processing blockade appears to be important in the pathogenesis of DAVID syndrome, because such a blockade is either predicted or has been shown ex vivo in cellular studies for all the reported mutations (p.Ala867Val, p.Asp865Gly, pArg853X, p.Lys855X, p.Lys855SerFsX7, p.Asp865ValFsX17, p.Arg853AlaFsX29, and p.Ala867CysFsX19) (2–5). Our patient follows this pattern in having a de novo heterozygous C-terminal NFKB2 missense mutation, resulting in an amino acid substitution of serine 866 with arginine, preventing the phosphorylation of this residue by IKK1 and the p100 processing.
This identification of natural mutation of an IKK1 phosphorylation site in humans is particularly important in that it directly implicates this IKK1 phosphorylation event in the endocrine phenotype of DAVID syndrome. We speculate that the delayed and milder presentation of the immunodeficiency may be the result of a partial block of IKK1 phosphorylation, because it does not involve the other serine 870 residue that is phosphorylated. Most of the other reported upstream nonsense and frameshift mutations would abolish such phosphorylation.
How germline C-terminal NFKB2 processing mutations result in postnatal ACTH deficiency remains an enigma. There is no compelling evidence for a direct role of the NFKB2 pathway in pituitary cell development, homeostasis, or function or for NFKB2 deficiency resulting in autoimmune pituitary disease. It is also interesting that ACTH deficiency has been reported only with C-terminal NFKB2 mutations and not with other germline mutations that impair the activity of either the canonical pathway (NFKB1 and IKKG) or the noncanonical (NIK) pathway (3, 13).
Conclusions
Heterozygous C-terminal NFKB2 processing mutations are an important genetic cause of isolated autosomal dominant ACTH deficiency. As reported here, the endocrinopathy can present before any clinical evidence of CVID, whereas other patients may have clinical CVID for decades and remain asymptomatic for pituitary disease. Because of the rarity of acquired ACTH deficiency, pediatric patients who present with this disorder without history of medication exposure or trauma and with a normal or hypoplastic pituitary magnetic resonance imaging warrant quantitative immunoglobulin testing for DAVID syndrome. Given the clinical variability, consequences of undiagnosed disease, and ease of treatment, routine presymptomatic genetic testing of siblings and parents is recommended for these mutations.
Acknowledgments
This work was supported in part by funds from the Jeffrey Modell Foundation for Primary Immunodeficiency (to D.B.L.).
Disclosure Summary: The authors have nothing to disclose.
Abbreviations:
- ACTH
adrenocorticotropic hormone
- CVID
common variable immunodeficiency
- DAVID
deficient anterior pituitary with variable immune deficiency
- Ig
immunoglobulin
- IKK1
inhibitor of nuclear factor-κB kinase 1 subunit.
Contributor Information
Rayhan A. Lal, Division of Endocrinology, Department of Pediatrics, Stanford University School of Medicine, Stanford, California 94305 Division of Endocrinology, Department of Medicine, Stanford University School of Medicine, Stanford, California 94305.
Laura K. Bachrach, Division of Endocrinology, Department of Pediatrics, Stanford University School of Medicine, Stanford, California 94305
Andrew R. Hoffman, Division of Endocrinology, Department of Medicine, Stanford University School of Medicine, Stanford, California 94305
Jingga Inlora, Department of Genetics, Stanford University School of Medicine, Stanford, California 94305.
Shannon Rego, Department of Genetics, Stanford University School of Medicine, Stanford, California 94305.
Michael P. Snyder, Department of Genetics, Stanford University School of Medicine, Stanford, California 94305
David B. Lewis, Division of Allergy, Immunology & Rheumatology, Department of Pediatrics, Stanford University School of Medicine, Stanford, California 94305
References
- 1. Quentien MH, Delemer B, Papadimitriou DT, Souchon PF, Jaussaud R, Pagnier A, Munzer M, Jullien N, Reynaud R, Galon-Faure N, Enjalbert A, Barlier A, Brue T. Deficit in anterior pituitary function and variable immune deficiency (DAVID) in children presenting with adrenocorticotropin deficiency and severe infections. J Clin Endocrinol Metab. 2012;97(1):E121–E128. [DOI] [PubMed] [Google Scholar]
- 2. Chen K, Coonrod EM, Kumánovics A, Franks ZF, Durtschi JD, Margraf RL, Wu W, Heikal NM, Augustine NH, Ridge PG, Hill HR, Jorde LB, Weyrich AS, Zimmerman GA, Gundlapalli AV, Bohnsack JF, Voelkerding KV. Germline mutations in NFKB2 implicate the noncanonical NF-κB pathway in the pathogenesis of common variable immunodeficiency. Am J Hum Genet. 2013;93(5):812–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brue T, Quentien MH, Khetchoumian K, Bensa M, Capo-Chichi JM, Delemer B, Balsalobre A, Nassif C, Papadimitriou DT, Pagnier A, Hasselmann C, Patry L, Schwartzentruber J, Souchon PF, Takayasu S, Enjalbert A, Van Vliet G, Majewski J, Drouin J, Samuels ME. Mutations in NFKB2 and potential genetic heterogeneity in patients with DAVID syndrome, having variable endocrine and immune deficiencies. BMC Med Genet. 2014;15:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liu Y, Hanson S, Gurugama P, Jones A, Clark B, Ibrahim MA. Novel NFKB2 mutation in early-onset CVID. J Clin Immunol. 2014;34(6):686–690. [DOI] [PubMed] [Google Scholar]
- 5. Shi C, Wang F, Tong A, Zhang XQ, Song HM, Liu ZY, Lyu W, Liu YH, Xia WB. NFKB2 mutation in common variable immunodeficiency and isolated adrenocorticotropic hormone deficiency: a case report and review of literature. Medicine (Baltimore). 2016;95(40):e5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee CE, Fulcher DA, Whittle B, Chand R, Fewings N, Field M, Andrews D, Goodnow CC, Cook MC. Autosomal-dominant B-cell deficiency with alopecia due to a mutation in NFKB2 that results in nonprocessable p100. Blood. 2014;124(19):2964–2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lindsley AW, Qian Y, Valencia CA, Shah K, Zhang K, Assa’ad A. Combined immune deficiency in a patient with a novel NFKB2 mutation. J Clin Immunol. 2014;34(8):910–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ricciuti A, De Remigis A, Landek-Salgado MA, De Vincentiis L, Guaraldi F, Lupi I, Iwama S, Wand GS, Salvatori R, Caturegli P. Detection of pituitary antibodies by immunofluorescence: approach and results in patients with pituitary diseases. J Clin Endocrinol Metab. 2014;99(5):1758–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Andrioli M, Pecori Giraldi F, Cavagnini F. Isolated corticotrophin deficiency. Pituitary. 2006;9(4):289–295. [DOI] [PubMed] [Google Scholar]
- 10. Pulichino A-M, Vallette-Kasic S, Couture C, Gauthier Y, Brue T, David M, Malpuech G, Deal C, Van Vliet G, De Vroede M, Riepe FG, Partsch C-J, Sippell WG, Berberoglu M, Atasay B, Drouin J. Human and mouse TPIT gene mutations cause early onset pituitary ACTH deficiency. Genes Dev. 2003;17(6):711–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kühnen P, Clément K, Wiegand S, Blankenstein O, Gottesdiener K, Martini LL, Mai K, Blume-Peytavi U, Grüters A, Krude H. Proopiomelanocortin deficiency treated with a melanocortin-4 receptor agonist. N Engl J Med. 2016;375(3):240–246. [DOI] [PubMed] [Google Scholar]
- 12. Sun SC. The noncanonical NF-κB pathway. Immunol Rev. 2012;246(1):125–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Paciolla M, Pescatore A, Conte MI, Esposito E, Incoronato M, Lioi MB, Fusco F, Ursini MV. Rare mendelian primary immunodeficiency diseases associated with impaired NF-κB signaling. Genes Immun. 2015;16(4):239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]