Abstract
The severity of endophthalmitis has been associated generally with the virulence of the offending pathogen. However, precisely what constitutes the virulence in intraocular infections remains ill defined. We therefore sought to identify the basis for virulence for three common ocular pathogens (Bacillus cereus, Enterococcus faecalis, and Staphylococcus aureus) in terms of intraocular growth rates, bacterial localization patterns, and the contribution of cell walls and secreted products to the pathogenesis of endophthalmitis. Rabbit eyes were injected intravitreally with (i) viable B. cereus, E. faecalis, or S. aureus, (ii) metabolically inactive B. cereus, E. faecalis, or S. aureus, (iii) sacculus preparations from each strain, or (iv) culture fluid containing products secreted by each strain. Eyes were assessed at various times following injection by slit lamp biomicroscopy, electroretinography (ERG), bacterial and inflammatory cell enumeration, and histology. B. cereus endophthalmitis followed a more rapid and virulent course than E. faecalis or S. aureus endophthalmitis, eliminating retinal responsiveness, as measured by ERG, by 12 h. Analysis of bacterial localization revealed that B. cereus uniquely migrated rapidly from posterior to anterior segment during infection. Although injection of neither metabolically inactive bacteria nor cell wall sacculi greatly affected ERG, significant intraocular inflammation was observed. Injection of B. cereus or S. aureus culture fluids caused both significant reductions in retinal responsiveness and significant intraocular inflammation, paralleling that seen in natural infections. The results demonstrate that toxins, intraocular localization, and, to a lesser extent, the intraocular host response to cell walls all contribute to the pathogenesis of B. cereus, S. aureus, and E. faecalis endophthalmitis in a pathogen-specific manner. The key pathophysiologic differences in these intraocular diseases highlight opportunities for optimizing conventional therapies and deriving new ones.
Endophthalmitis is a vision-threatening disease that usually results from microbial infection of the interior of the eye. The course of bacterial endophthalmitis varies widely depending on the etiologic agent involved, ranging from relatively avirulent and therapeutically responsive infections caused by Staphylococcus epidermidis to the therapeutically challenging and often sight-threatening infections caused by more virulent pathogens such as Bacillus cereus, Enterococcus faecalis, and Staphylococcus aureus (1, 7, 16, 21, 28, 50, 52, 58, 66). Although the outcome of severe endophthalmitis cases has been associated broadly with the virulence of particular bacterial species, precisely what constitutes virulence in these infections remains to be defined. Specific bacterial components that trigger aggressive intraocular inflammatory responses may represent candidate therapeutic targets for limiting visual loss in endophthalmitis. The emergence of multidrug-resistant organisms (24, 25, 35, 37, 57) further highlights the importance of developing new therapeutic strategies.
Toward defining bacterial virulence in endophthalmitis, recent studies have centered on the specific contributions of bacterial toxins to disease severity. Attenuation of an organism’s ability to produce a single toxin (the E. faecalis cytolysin [27, 55]) or several toxins (S. aureus pore-forming toxins [8, 9]) resulted in significant reductions in infection severity, demonstrating that the production of toxins in situ in these two infection models measurably contributes to the course of endophthalmitis. The specific mechanisms by which these toxins induce intraocular tissue damage and inflammation are unclear. Despite attenuation resulting from insertional inactivation of toxin genes, substantial intraocular inflammation was observed (8, 9, 27, 55), indicating that bacterial components other than exotoxins contribute to endophthalmitis pathogenesis. Furthermore, attenuation of the B. cereus dermonecrotic toxin, hemolysin BL, did little to alter the course of experimental B. cereus endophthalmitis (11), suggesting that additional factors likely contribute to this highly virulent infection. Evidence from a number of experimental systems indicates the likelihood that a multitude of proteolytic or superantigenic proteins, chemoattractants, or other inflammatory mediators secreted by the bacterium during infection can contribute to an aggressive inflammatory response (5, 45, 49, 51).
There exists a modest but rapidly emerging body of evidence highlighting the importance of gram-positive cell wall components in inflammation. Metabolically inactive organisms, cell walls, and individual envelope components (peptidoglycan, lipoteichoic acid, and capsular polysaccharide) stimulated inflammatory cell chemotaxis, cytokine production, and cellular toxicity in several ocular (17, 18, 32, 38, 39) and nonocular (4, 13, 19, 20, 23, 29–31, 33, 36, 46, 47, 54, 59, 61, 62) experimental systems. In the single report documenting the intraocular inflammogenicity of gram-positive cell walls, Fox et al. (17) noted that peptidoglycan provoked chronic inflammation and retinal necrosis similar to that observed in eyes injected with lipopolysaccharide. However, due to the crude nature of the cell wall extracts injected, the specific basis for cell wall-induced inflammation was not determined.
Evidence suggests that the tertiary configuration of peptidoglycan and its association with the cell wall or with lipoteichoic acid can directly affect the degree of inflammation (23, 59). Enzymatic depolymerization or sonic shearing significantly reduced the inflammogenicity of peptidoglycan. Furthermore, whole cells were more inflammogenic than purified intact peptidoglycan (23, 59). Together, these results indicate that whole cells contain components which act either alone or synergistically with peptidoglycan to stimulate inflammation. With respect to the role of peptidoglycan, the extent of inflammatory stimulation may depend on the extent of intrachain cross-linking of peptidoglycan layers of a particular bacterial species. Comparisons of the inflammogenicity of cell walls from several gram-positive species showed that Bacillus sp. was the most stimulatory, while Propionibacterium sp. was the least stimulatory (23, 56, 65). Since peptidoglycan from gram-positive species are cross-linked to various degrees (Bacillus spp. possess minimal cross-linkage, while Propionibacterium and Staphylococcus spp. are highly cross-linked [48]), the extent to which the unique tertiary configurations of these cell walls account for the clinical variability observed with these infections is an open question.
Because the secretory products and cell wall structural components of gram-positive ocular pathogens differ in terms of both toxigenicity and inflammogenicity, it was of interest to ascertain the degree to which these components contributed to the observed variability in endophthalmitis. It was also of interest to determine whether intraocular growth rates or localization patterns were associated with developing pathology during infection.
(This work was presented in part at the Association of Research in Vision and Ophthalmology annual meeting, 9 to 14 May 1999, Fort Lauderdale, Fla.).
MATERIALS AND METHODS
Strains used and preparation of inocula.
The strains used in the experimental bacterial endophthalmitis model included B. cereus MGBC145, a clinical strain isolated from a pediatric posttraumatic endophthalmitis case that progressed to enucleation (2, 11); E. faecalis JH2SS(pAM714), a laboratory strain harboring the pAD1-encoded cytolysin (10) and used previously (27) in an experimental rabbit endophthalmitis model (D. B. Clewell, University of Michigan, Ann Arbor); and S. aureus RN6390, a toxin-producing laboratory strain used previously (8, 9) in an experimental rabbit endophthalmitis model (A. L. Cheung, The Rockefeller University, New York, N.Y.).
Inocula for each organism were prepared by propagating cultures in brain heart infusion (BHI; Difco Laboratories, Detroit, Mich.) at 37°C for 18 h. Cultures were then serially diluted in phosphate-buffered saline (PBS; pH 7.4). Retrospective quantitation of inocula was done by plating triplicate serial 10-fold dilutions on BHI (26).
Preparation of metabolically inactive bacteria and cell wall sacculi.
Metabolically inactive bacterial suspensions were prepared by propagating cultures overnight in BHI, as noted above, washing the cultures three times in PBS, and diluting the cultures to approximately 109 CFU/ml in PBS. Pairs of 1.0-ml aliquots of each bacterial suspension were pipetted into the center of each section of a sterile bisectional petri dish and were irradiated at minimum conditions found experimentally to render all cultures sterile (120 mJ for 40 min). One 1.0-ml aliquot was plated on BHI agar and incubated at 37°C for 72 h to ensure sterility; the second aliquot was stored at 4°C prior to injection.
Cell wall sacculi were prepared by using a modification of the method of De Jonge et al. (12). Briefly, overnight cultures were harvested by centrifugation, resuspended in 4% sodium dodecyl sulfate and boiled for 30 min. Cell walls were then pelleted by centrifugation, resuspended in 100 mM Tris-Cl (pH 7.5) supplemented with DNase (10 μg/ml, final concentration), RNase (50 μg/ml, final concentration), and 20 mM (final concentration) MgSO4, and incubated for 2 h at 37°C. Trypsin (100 μg/ml, final concentration) and CaCl2 (10 mM, final concentration) were added, and the cell wall suspensions were incubated for an additional 16 h at 37°C. Enzymes were then inactivated by boiling in 1% sodium dodecyl sulfate for 15 min. Cell walls were pelleted by centrifugation, washed four times in sterile H2O, and stored at 4°C prior to injection. Suspensions were examined by light microscopy to ensure that cell walls remained intact following treatment, and aliquots of each suspension were plated onto BHI agar and incubated at 37°C for 72 h to ensure sterility. Both metabolically inactive bacterial and sacculus suspensions were prepared in PBS, and whole-cell equivalents were enumerated by light microscopy prior to injection.
Preparation of bacterium-free supernatants.
Overnight cultures of each organism were diluted 1:100 in sterile BHI and incubated with aeration for 8 h at 37°C to an early stationary phase of growth. Cultures were centrifuged, and cell-free supernatants filter sterilized (Acrodisc; 0.2-μm pore size; Gelman Sciences, Ann Arbor, Mich.), adjusted to equivalent protein concentrations, and stored at −70°C prior to injection.
Experimental endophthalmitis.
New Zealand White rabbits (2 to 3 kg) were maintained in accordance with institutional guidelines and the Association for Research in Vision and Ophthalmology Statement on the Use of Laboratory Animals in Ophthalmic Research. Prior to intravitreal injections, eyes were dilated with topical 1% tropicamide and 2.5% phenylephrine HCl. Rabbits were anesthetized generally by intramuscular injection of ketamine (Ketaved; Phoenix Scientific Inc., St. Joseph, Mo.); 35 mg/kg of body weight) and xylazine Rompun; Bayer Corp., Shawnee Mission, Kans; 5 mg/kg body weight) and topically in each eye with proparacaine HCl (Ophthetic; Allergan, Hormigueros, Puerto Rico; 0.5%).
Prior to intravitreal injection, aqueous humor (100 μl) was aspirated to relieve intraocular pressure. Bacterial suspensions or cell-free supernatants (100 μl) were injected into the midvitreous via a 30-gauge needle attached to a 1.0-ml syringe introduced through the pars plana approximately 3 mm from the limbus. The contralateral eye was injected with PBS or sterile BHI (surgical controls) or was left undisturbed (absolute control) (8, 9, 11, 27, 55). At various times postinfection, the course of infection and inflammation were assessed by (i) slit lamp biomicroscopy, (ii) electroretinography (ERG), (iii) intraocular bacterial growth, (iv) anterior chamber inflammatory cell enumeration, and (v) thin-section histology, as described below. Quantities of bacteria injected and times of tissue recovery for analysis are listed in Results.
Slit lamp examination.
To quantify intraocular inflammation, rabbits were observed by slit lamp biomicroscopy (Topcon SL-5D; Kogaku Kikai K.K., Tokyo, Japan) before and at various times during infection. Ocular inflammation was scored by masked observers based on the scoring of progressive inflammation in the cornea, anterior chamber, vitreous, and retina (11, 44). Each area of the eye was scored independently on a scale of 0 (no inflammation) to 4 (maximal inflammation). With regard to the anterior chamber, intraocular inflammation was measured in parameters termed cell and flare. “Cell” corresponds to inflammatory cells appearing as flecks within the slit lamp beam, while “flare” corresponds to protein leakage into to anterior chamber, giving a dusty appearance to the slit lamp beam (40). The fundus reflex was assessed by the extent of red reflex observed when the eye was exposed to an open slit-lamp beam. Vitreous and retinal clarity were each scored based on the extent of progressive haze, exudate and fibrin clump formation, and cellular reactions observed under thin slit-lamp beam conditions.
Analysis of retinal function.
ERG was used to measure the extent of retinal function during the course of infection. Retinal responses are generally divided into A-wave maximum and B-wave minimum values, with the B-wave amplitude being the difference between the two responses. After dilation and 30 min of dark adaptation, a ground electrode is placed on the rabbit’s ear, while bipolar contact lens electrodes that record the flash response are placed in each eye. A monopolar electrode is placed on the forehead. Retinas are illuminated with a single, low-intensity flash (one flash per second), and the resulting B-wave amplitude is measured (in millivolts). Baseline B-wave amplitude was established for each eye, using scotopic bright-flash ERG (EPIC-2000; LKC Technologies, Gaithersburg, Md.), 24 h before intravitreal injection and again following injection at the time points depicted in Fig. 1. B-wave amplitude at a particular time point was the average of 14 repeated measures. Percent loss of retinal function (B-wave amplitude) was calculated as [1 − (experimental B-wave amplitude/baseline B-wave amplitude)] × 100 (8, 9, 11, 27, 55).
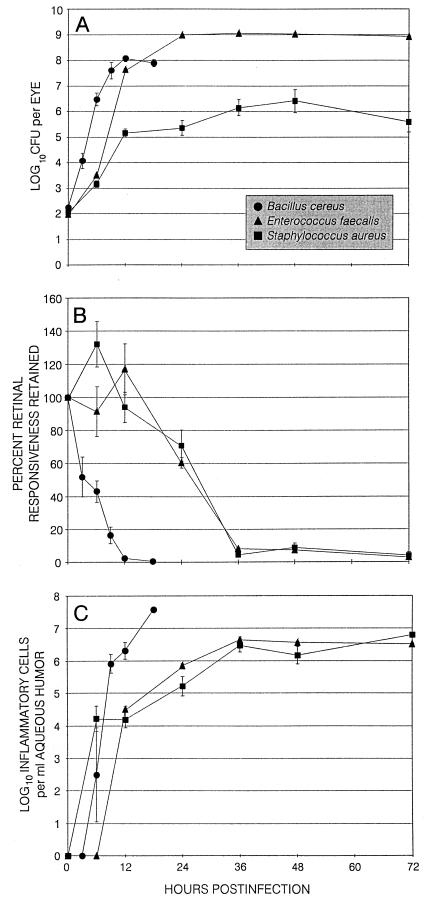
FIG. 1.
Quantification of viable bacteria per eye (A), ERG (B), and enumeration of inflammatory cells in the aqueous humor (C) during experimental bacterial endophthalmitis. Eyes were injected midvitreally with 102 CFU of B. cereus, S. aureus, or E. faecalis. (A) Ocular tissues were recovered at various times postinfection for quantification of cumulative bacteria per eye. (B) ERG was performed at various times postinfection for quantification of B-wave activity. (C) Aqueous humor was aspirated at various times postinfection for enumeration of inflammatory cells per milliliter of aqueous humor. All values represent the mean ± SEM for ≥4 eyes per group.
Analysis of anterior segment inflammation.
Anterior segment inflammation was assessed by counting infiltrating inflammatory cells in aspirated anterior chamber fluid, using a hemocytometer.
Analysis of bacterial growth.
To enumerate organisms in specific ocular tissues, whole globes were surgically removed, rinsed with sterile PBS, and placed cornea side up on sterile surgical dressing. Aqueous humor was recovered from the anterior chamber by aspiration. The cornea, iris, lens, and vitreous were each dissected away and placed separately into sterile tubes. The remaining outer tunic was minced into small pieces and placed into a sterile tube. After each solid tissue was weighed 0.5 ml of sterile PBS was added. All samples were then homogenized with 1.0-mm-diameter glass beads in a Mini-BeadBeater (5,000 rpm, 30 s; Biospec Products, Bartlesville, Okla.). Bacterial CFU were quantified by plating triplicate serial 10-fold dilutions on BHI.
Histopathological analysis.
All eyes recovered for thin-section histopathology were enucleated and fixed in 10% formalin for 24 h. Eyes were sectioned and stained with either hematoxylin and eosin or tissue Gram’s stain (53). Stained tissue sections were analyzed by masked observers based on the extent of inflammation in the cornea, anterior chamber, vitreous, and retina (11, 43).
Statistical analysis.
All values represent the mean ± standard error of the mean (SEM) for ≥4 eyes per time point and inflammatory parameters assayed. Wilcoxon’s rank sum test was used for statistical comparisons between groups. A P value of ≤0.05 was considered significant.
RESULTS
Strain-to-strain variation in natural experimental endophthalmitis.
Experimental infections with viable organisms were initiated by injection of the following inocula (log10): B. cereus, 2.06 ± 0.04 CFU; E. faecalis, 1.99 ± 0.07 CFU; and S. aureus, 2.07 ± 0.07 CFU (mean ± SEM, p≥0.08). The natural courses of experimental endophthalmitis were then analyzed as described in Materials and Methods. Sham-injected and uninjected control eyes were all normal, as measured by all inflammatory parameters.
(i) Intraocular inflammation.
In B. cereus-infected eyes, intraocular inflammation was observed as early as 3 h, with mild to moderate conjunctival edema and minimal cell and flare in the anterior chamber. At 6 h, inflammatory symptoms progressed, with moderate anterior chamber cell and flare, vitreous haze, and a significant decrease in fundus reflex. From 12 to 18 h, inflammatory symptoms were severe in all animals, with anterior chamber hyphema, severe iritis, and peripheral corneal ring abscesses present. Fundus reflex was absent. Gross examination of enucleated globes and surrounding tissues showed severe periorbital inflammation, indicating a developing panophthalmitis. No B. cereus infections were allowed to progress further.
The evolution of experimental E. faecalis and S. aureus endophthalmitis occurred over a slower time course than that of B. cereus. Briefly, anterior and posterior chamber inflammatory changes and a significant loss of fundus reflex were evident by 24 h in both infection groups. Moderate to severe inflammation (severe anterior chamber cell and flare, vitreous opacities, white fundus reflex) was observed by 36 h in both infection groups. No additional evolution of disease occurred from 36 to 72 h in either infection group.
(ii) Bacterial growth and distribution in the eye.
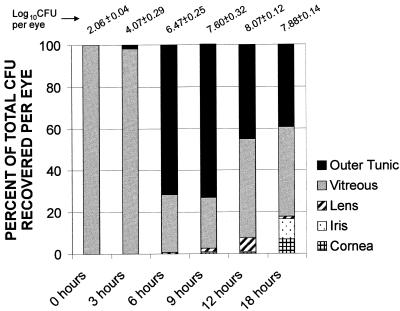
The numbers of viable B. cereus per eye increased steadily from 0 to 12 h. After reaching maximal levels (B. cereus, 8.07 ± 0.12 log10 CFU), bacterial numbers were sustained until the termination of infection. The numbers of B. cereus per eye were significantly greater than those of E. faecalis and S. aureus per eye at 6 and 12 h (P ≤ 0.03) (Fig. 1A). B. cereus was recovered in both posterior and anterior segment tissues. At 3 h, the majority of viable bacilli were recovered from the vitreous (Fig. 2). At 6 and 9 h, greater numbers of bacilli were recovered from the outer tunic tissues than from the isolated vitreous. After 9 h, B. cereus populations recovered demonstrated a migration of organisms toward and into the anterior segment. Microscopic examination of aqueous humor samples recovered after 9 h showed bacilli moving about vigorously and clinging to fibrin clots within the sample.
FIG. 2.
Ocular tissue distribution of B. cereus during experimental endophthalmitis. Ocular tissues (cornea, iris, lens, vitreous, and outer tunic) were recovered at various times postinfection for quantification of B. cereus. The charted values represent the percentage of organisms recovered from each tissue compared with the cumulative number of organisms recovered per eye (listed at the top; data from Fig. 1A). Because E. faecalis and S. aureus were recovered exclusively from posterior segment tissues during all stages of infection, those data are not included.
The numbers of viable E. faecalis per eye increased from 0 to 24 h to 8.98 ± 0.03 log10 CFU, with no significant intraocular growth thereafter. The numbers of viable S. aureus per eye increased from 0 to 36 h to approximately 6.14 ± 0.32 log10 CFU, with no significant intraocular growth observed thereafter. Bacterial counts were higher in eyes infected with E. faecalis than in S. aureus-infected eyes from 12 to 72 h (P ≤ 0.04) (Fig. 1A). E. faecalis and S. aureus were recovered exclusively from posterior segment tissues.
(iii) ERG.
The significant reductions in retinal responses of B. cereus-infected eyes observed as early as 3 h evolved rapidly to a >97% loss of B-wave activity by 12 h (Fig. 1B). Supernormal ERG responses were observed in E. faecalis-infected eyes and S. aureus-infected eyes at 12 and 6 h, respectively. Significant reductions in B-wave responses were observed at 24 h, with >90% loss of B-wave activity by 36 h in both E. faecalis- and S. aureus-infected eyes (Fig. 1B).
(iv) Anterior segment inflammation.
Inflammatory cells were recovered from the aqueous humor of B. cereus-infected eyes as early as 6 h (Fig. 1C). By 12 h, the numbers of inflammatory cells recovered from B. cereus-infected eyes were greater than that recovered from E. faecalis- or S. aureus-infected eyes (P ≤ 0.02). Inflammatory cells were recovered from the aqueous humor of E. faecalis- and S. aureus-infected eyes as early as 12 and 6 h, respectively (Fig. 1C), with similar inflammatory cell numbers recovered from infected eyes in both groups from 36 to 72 h (P ≥ 0.08).
(v) Histology.
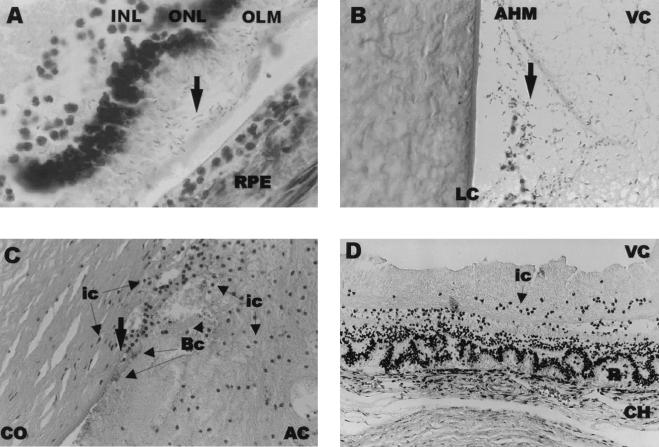
At 6 h, bacilli were observed primarily at posterior segment structural interfaces. Retinal detachment and photoreceptor layer folding and disruption were observed as early as 9 h. Bacilli were observed in the spaces between the outer limiting membrane and the retinal pigment epithelium (Fig. 3A) and at the posterior/anterior segment interface (Fig. 3B). Marked cell infiltration advanced from the optic nerve head into the vitreous. Bacilli were observed in the anterior segment at the posterior corneal surface. By 12 h, greater numbers of infiltrating cells and bacilli were interspersed within fibrin in the anterior chamber, and migration of cells into the cornea from the limbus and anterior chamber was observed. Bacilli appeared to invade the corneal endothelium and stroma (Fig. 3C). A similar but more severe inflammatory reaction was observed in the vitreous, with moderate disruption of the retinal architecture. At 18 h, eyes demonstrated maximal inflammation in all parts of the eye, including periocular tissues.
FIG. 3.
Histological analysis of experimental B. cereus endophthalmitis. B. cereus is designated by arrows, unless otherwise indicated. All sections were stained with hematoxylin and eosin. (A) B. cereus residing between the outer limiting membrane and retinal pigment epithelium of the retina at 9 h postinfection. Magnification, ×1,000. INL, inner limiting membrane; ONL, outer nuclear layer; OLM, outer limiting membrane; RPE, retinal pigment epithelium. (B) B. cereus migrating toward the iris at 9 h postinfection. Magnification, ×400. LC, lens capsule; AHM, anterior hyaloid membrane; VC, vitreous chamber. (C) B. cereus in the anterior chamber at 12 h postinfection. Magnification, ×400. CO, cornea; ic, inflammatory cells; Bc, B. cereus; AC, anterior chamber. (D) Photoreceptor layer folding of the retina 6 h following injection of B. cereus supernatant. Magnification, ×200. VC, vitreous chamber; ic, inflammatory cells; R, retina; CH, choroid.
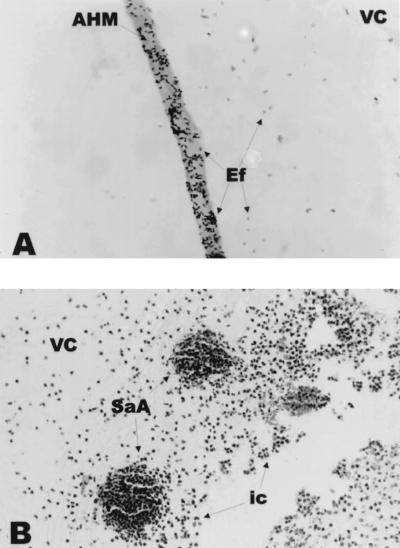
Histological changes of eyes infected with E. faecalis and S. aureus were broadly similar to previous findings (8, 9, 27, 55). At 6 and 12 h, retinal and anterior segment tissues appeared histologically normal, and organisms were observed in the midvitreous in both infection groups. By 24 h, marked inflammatory cell infiltration advanced from the optic nerve head into the vitreous, and the outermost retinal layers were slightly inflamed in both infection groups. Enterococci associated with vitreous structures, including the anterior hyaloid membrane (Fig. 4A), while staphylococci adhered primarily to midvitreous fibrinous exudate and within small inflammatory cell clusters (Fig. 4B). The cornea, iris, and anterior chamber of E. faecalis-infected eyes were histologically normal, while inflammatory cells were observed in the anterior chamber and cornea of S. aureus-infected eyes.
FIG. 4.
Histological analysis of experimental E. faecalis (A) and S. aureus (B) endophthalmitis. (A) E. faecalis adhering to the anterior hyaloid membrane at 24 h postinfection. Brown & Hopps tissue Gram’s stain; magnification, ×1,000. AHM, anterior hyaloid membrane; VC, vitreous chamber; Ef, E. faecalis. (B) Clusters of inflammatory cells in the vitreous during S. aureus endophthalmitis (48 h). Hematoxylin-and-eosin stain; magnification, ×400. VC, vitreous chamber; SaA, staphylococcal abscess; ic, inflammatory cells.
At 36 h, the number of inflammatory cells in the vitreous increased, and fibrin and inflammatory cells were observed in the anterior chamber in both infection groups. Enterococci were associated with fibrinous exudate and vitreous structures, and significant retinal layer disruption was observed. The few staphylococci visualized were associated with fibrinous exudate or with inflammatory cell clusters in the vitreous. Anterior segment changes of S. aureus-infected eyes also included inflammatory cells in the cornea. By 48 h, retinal layers were indistinguishable in E. faecalis-infected eyes, and infiltrating cells filled the vitreous cavity. In S. aureus-infected eyes, significant retinal layer disruption and extensive abscess formation in the vitreous were observed. Anterior segment inflammation was more severe, with erythrocyte infiltration into the anterior chamber in both infection groups. The histological changes observed at 48 and 72 h were similar. Neither enterococci nor staphylococci were observed in the anterior segment in these histological sections.
Contribution of secreted bacterial products to intraocular inflammation.
To assess the relative contributions of secreted bacterial products to intraocular inflammogenicity, supernatants of early-stationary-phase cultures of B. cereus, E. faecalis, and S. aureus were each injected intravitreally, and inflammation was assessed as described in Materials and Methods. Results are shown in Fig. 5 and 6. For clarity, in the discussion of results that follows, the individual data sets are designated by bracketed numbers which correspond to those in Fig. 5 and 6.
FIG. 5.
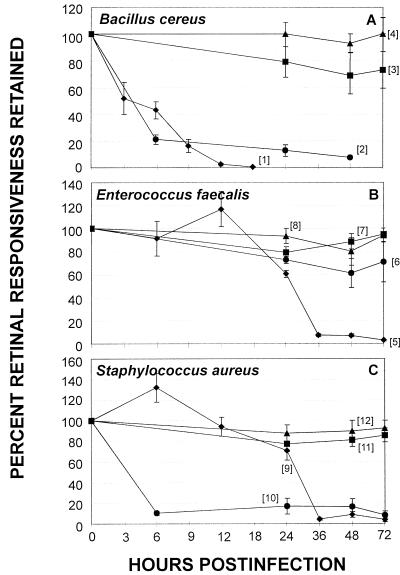
Comparative retinal toxicity of the natural infection, metabolically inactive organisms, cell wall sacculi, and bacterial supernatants for the rabbit eye. ERG was performed at various times following midvitreal injection of the following preparations of B. cereus (A), E. faecalis (B), and S. aureus (C): live organisms (⧫), cell-free supernatant (●), metabolically inactive organisms (■), and sacculus preparations (▴). Values represent the mean ± SEM of the percent retinal responsiveness retained for ≥4 eyes per group. Individual data sets are designated by bracketed numbers that correspond to text in Results.
FIG. 6.
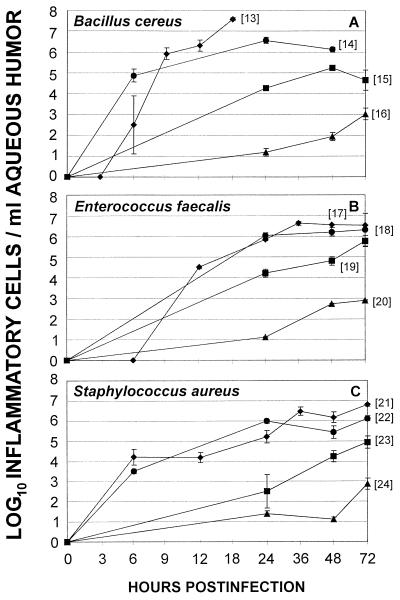
Comparative intraocular inflammogenicity of the natural infection, metabolically inactive organisms, cell wall sacculi, and bacterial supernatants for the rabbit eye. Anterior chamber paracentesis and enumeration of inflammatory cells per milliliter of aqueous humor was performed at various times following midvitreal injection of the following preparations of B. cereus (A), E. faecalis (B), and S. aureus (C): live organisms (⧫), cell-free supernatant (●), metabolically inactive organisms (■), and sacculus preparations (▴). Values represent the mean ± SEM of the log10 inflammatory cells per milliliter of aqueous humor for ≥4 eyes per group. Individual data sets are designated by bracketed numbers that correspond to text in Results.
(i) B. cereus.
In general, intravitreal injection of B. cereus supernatant significantly reduced ERG values and induced influx of inflammatory cells into the aqueous humor in numbers approaching that of the natural infection (live organisms [1] to supernatants [2] [Fig. 5A and live organisms [13] to supernatants [14] [Fig. 6A]). Histological examination of eyes injected with B. cereus supernatant exhibited retinal photoreceptor layer folding similar to that observed in the early stages of natural B. cereus infection (Fig. 3D).
(ii) E. faecalis.
Intravitreal injection of E. faecalis supernatant significantly reduced ERG values compared to controls at 24 and 48 h (P ≤ 0.03), but not to the extent of that observed during the natural infection (live organisms [5] to supernatants [6] [Fig. 5B]). Histological analysis demonstrated transient inflammatory cells in the vitreous and a normal retina. The numbers of aqueous humor inflammatory cells recovered were similar to that induced by the natural infection at 24, 48, and 72 h (P ≥ 0.29) (Fig. 6B, live organisms [17] to supernatants [18]).
(iii) S. aureus.
Intravitreal injection of S. aureus supernatant significantly reduced retinal responsiveness similar to that of the natural infection at 48 and 72 h (P ≥ 0.22) (Fig. 5C, live organisms [9] to supernatants [10]). Histological analysis demonstrated transient inflammatory cells in the vitreous and a normal retina. Aqueous humor inflammatory cell numbers approached that induced by the natural infection (Fig. 6C, live organisms [21] to supernatants [22]).
(iv) Strain-to-strain variation in supernatant inflammogenicity and retinal toxicity.
ERG values of eyes injected with B. cereus and S. aureus supernatants were similar (P = 0.43) and were lower than those of eyes injected with E. faecalis supernatants at 24 and 48 h (P ≤ 0.04) (Fig. 5 [2, 6, 10]). ERG values of eyes injected with S. aureus supernatant were lower than those injected with E. faecalis supernatant at 72 h (P = 0.02) (Fig. 5 [10, 6]).
Intravitreal injection of B. cereus supernatant caused an influx of greater numbers of inflammatory cells into the aqueous humor compared to that of E. faecalis or S. aureus supernatant at 24 h. (P ≤ 0.03) (Fig. 6 [14, 18, 22]). Aqueous humor inflammatory cell numbers recovered from eyes injected with E. faecalis and S. aureus supernatants were similar at 48 and 72 h (P ≥ 0.08) (Fig. 6 [18, 22]).
Contribution of cell wall constituents to virulence.
To assess the relative inflammogenicity of gram-positive cell walls in endophthalmitis, metabolically inactive cells and sacculi of B. cereus, E. faecalis, and S. aureus were each injected intravitreally, and inflammation was assessed as described in Materials and Methods. Sacculi were tested to specifically assess potential differences in the inflammogenicity of the cell wall structural components in the absence of surface proteins to intraocular inflammation.
(i) Metabolically inactive organisms.
The numbers of metabolically inactive bacteria injected were chosen based on the number of viable organisms present when early signs of inflammation were observed in the natural infection. The following inocula (log10 CFU) were used: B. cereus, 6.07 ± 0.15; E. faecalis, 7.96 ± 0.07; and S. aureus, 6.07 ± 0.12 (mean ± SEM; E. faecalis significantly greater [P ≤ 0.01]).
Metabolically inactive E. faecalis and S. aureus caused slight but significant reductions in ERG values at 24 h (P ≤ 0.01) (Fig. 5 [8, 12]), which returned to preoperative levels by 48 h (P ≥ 0.59). ERG values of eyes injected with metabolically inactive B. cereus remained at preoperative levels at all time points (P ≥ 0.08) (Fig. 5A [3]). Significant increases in the numbers of aqueous humor inflammatory cells following intravitreal injection of metabolically inactive B. cereus, E. faecalis, or S. aureus were observed (P ≤ 0.01) (Fig. 6 [15, 19, 23]). At 72 h, the numbers of inflammatory cells induced by metabolically inactive E. faecalis were greater than those induced by either metabolically inactive S. aureus or B. cereus (P ≤ 0.04) (Fig. 6 [15, 19, 23]).
(ii) Sacculi.
The numbers of sacculi injected were chosen based on the inflammogenicity observed in assays using metabolically inactive organisms and to compare the inflammogenic potential of the three organisms to themselves. The following inocula (log10 CFU) were used: B. cereus, 5.92 ± 0.08; E. faecalis, 6.02 ± 0.19; and S. aureus, 6.14 ± 0.21 (mean ± SEM, (P ≥ 0.42).
ERG values of eyes injected with sacculi of B. cereus, E. faecalis, or S. aureus remained at preoperative levels throughout the assay period (P ≥ 0.39) (Fig. 5 [3, 7, 11]). There were, however, increases in the numbers of aqueous humor inflammatory cells from 24 to 72 h that were similar among the three infection groups throughout the assay period (P ≥ 0.13).
(iii) Comparative analyses: whole bacteria versus sacculi.
ERG values of eyes injected with metabolically inactive organisms were similar to those of eyes injected with sacculi, regardless of the strain tested (P ≥ 0.07) (Fig. 5). Overall, the numbers of aqueous humor inflammatory cells recovered from eyes injected with metabolically inactive organisms were greater than those recovered from eyes injected with their sacculi (P ≤ 0.03) (Fig. 6).
(iv) Comparative analyses: whole bacteria versus supernatants and natural infections.
ERG values of eyes injected with metabolically inactive B. cereus or S. aureus or their respective sacculi were greater than those of eyes injected with supernatants (P ≤ 0.03) (Fig. 5A and C). ERG values of eyes injected with metabolically inactive E. faecalis, sacculi, or supernatant were similar at all time points (P ≥ 0.06) (Fig. 5B). In general, ERG values of metabolically inactive S. aureus and E. faecalis and their sacculi were greater than those of the natural infections (P ≤ 0.01) (Fig. 5B). In general, the numbers of aqueous inflammatory cells recovered from eyes injected with supernatants were greater than the numbers recovered from eyes injected with metabolically inactive organisms or sacculi preparations (P ≤ 0.03) (Fig. 6) but were lower than cell numbers recovered from natural infections (P ≤ 0.02) (Fig. 6).
DISCUSSION
Intraocular infection following the introduction of B. cereus, S. aureus, or E. faecalis into the posterior segment of the eye can follow one of two courses: (i) a highly inflammatory infection that is treated with aggressive therapy and in many cases salvages useful vision, or (ii) a highly inflammatory infection that is refractory to treatment, resulting in permanent vision loss, if not loss of the eye itself. The visual consequences of severe endophthalmitis cases caused by these ocular pathogens have been linked with virulence traits of the particular bacterial species. In most cases, however, the specific factors associated with virulence in these infections have not been characterized. It was, therefore, of interest to determine what cellular components (i.e., secretory products and/or cell wall constituents) and the intraocular behavior (i.e., growth patterns and tissue localization) of each organism contributed to the pathogenesis of endophthalmitis.
Reports correlating the intraocular virulence of these pathogens with visual outcome regularly list toxins as the proponents of tissue destruction and inflammation (28). The specific toxins active during an intraocular infection, at least for S. aureus and B. cereus, have not been identified. In the present study, assessment of the intraocular inflammogenicity of secreted bacterial products demonstrates that, at least for S. aureus and B. cereus, secreted products are toxic for the retina, are highly inflammogenic, and approach the virulence observed during a natural infection. Recently, we observed that culture supernatants of a B. cereus strain attenuated in hemolysin BL production resulted in significant retinal toxicity and intraocular inflammation (data not shown). In contrast, intravitreal injection of the supernatant of an S. aureus strain attenuated in pore-forming toxin production resulted in little retinal toxicity but a significant inflammatory response (data not shown). These results suggest that pore-forming toxins are of primary importance in terms of retinal function in S. aureus endophthalmitis, while secreted products other than hemolysin BL may be more significant in B. cereus endophthalmitis.
In contrast, E. faecalis culture supernatant was not toxic for the retina but was highly inflammogenic, eliciting an inflammatory response similar to that of the natural infection. We have previously reported that the E. faecalis cytolysin is a primary virulence factor causing retinal tissue destruction at the earliest stages of experimental endophthalmitis (27). In the present study, we used a cytolysin-producing strain of E. faecalis known to cause a virulent infection in this model (27, 55). It has been long established, however, that cytolysin is not produced in detectable levels in vitro (60), and retrospective analysis of the E. faecalis supernatant used for intravitreal injection for hemolytic activity revealed that no cytolytic activity was detectable. These results highlight an important limitation of experiments involving in vitro bacterial growth; while useful in identifying many inflammatory and toxic bacterial products, those factors dependent on in vivo cues or localized expression may evade detection.
With regard to the intraocular inflammogenicity of cell walls, neither the metabolically inactive pathogens nor purified sacculi caused significant reductions in retinal responsiveness, they but evoked significant inflammation in both the posterior and anterior segments of the eye. The inflammogenicity of metabolically inactive organisms was greater than that of purified sacculi, indicating that the association of structural components (peptidoglycan and teichoic acid) with an intact cell wall is necessary for significant intraocular inflammation. Previous studies demonstrated that whole cell walls are more inflammogenic than individual components (23, 59). Significant differences in the relative inflammogenicity of S. aureus, B. cereus, and E. faecalis cell walls were not identified in this model; however, these results indicate that whole cells contain additional components that act either alone or synergistically with peptidoglycan or lipoteichoic acid to stimulate inflammation.
With reference to the intraocular behavior of these pathogens during infection, the course and severity of infections caused by S. aureus or E. faecalis generally correlated with intraocular growth rates. Maximal inflammation was observed shortly after these organisms reached a stationary phase of intraocular growth (Fig. 1). The natural courses of S. aureus and E. faecalis endophthalmitis were significantly slower than that of B. cereus infection. Reports correlating the virulence of these organisms and visual outcome suggest that S. aureus and E. faecalis ocular infections have a greater likelihood of useful visual outcome than does B. cereus infection (28). The prolonged interval between intraocular inoculation of S. aureus or E. faecalis and detection of inflammatory symptoms could allow sufficient time for the initiation of successful therapeutic intervention. The aggressively fulminant course of B. cereus endophthalmitis would likely render delayed therapy ineffective.
Eyes infected with B. cereus exhibited an almost immediate and aggressive inflammatory response despite the low number of organisms at the earliest stages of infection (Fig. 1). These infections caused severe inflammation several hours before the growing organisms reached maximal numbers in the eye. Like S. aureus, B. cereus produces several membrane-destabilizing toxins (other than hemolysin BL [11]) that could contribute to intraocular tissue damage. The relative contributions of B. cereus toxins to intraocular virulence are presently being investigated. However, relatively nonpathogenic bacilli, such as Bacillus subtilis, can produce a highly virulent intraocular infection (1, 25a, 50, 58, 64). These results suggest that the presence and/or growth of bacilli could be an important mediator of the aggressive host inflammatory response.
Intraocular localization of organisms may play a role virulence. Studies using primate models of experimental endophthalmitis demonstrated the barrier effect of the posterior capsule, contributing to the overall resistance of the anterior chamber to infections from the posterior segment (3). No organisms were recovered from the anterior segment during S. aureus and E. faecalis infections. This was not the case for B. cereus infection. Histological analysis and bacterial enumeration revealed a larger proportion of bacilli within retinal and outer tunic tissues than in the vitreous body itself (Fig. 2 and 4) and confirmed the migration of bacilli from the posterior to anterior segment. Viable bacilli were also recovered from the aqueous humor as early as 6 h and were observed by light microscopy to be motile and adherent to fibrin deposits in the anterior chamber. Rapid dissolution of posterior segment tissues (including the posterior capsule [41, 42]) by B. cereus toxins or resulting from the aggressive inflammatory response, coupled with the motile nature of B. cereus (22, 34), may promote the migration of organisms into the anterior segment.
A marked polymorphonuclear migration into the cornea from 12 to 18 h with subsequent corneal ring abscess formation was a hallmark of B. cereus infection (15). Corneal ring abscess formation was not observed in S. aureus or E. faecalis infection, even during the most inflammatory stages of infection (i.e., after 36 h). Bacilli were shown associating with and penetrating into the cornea, suggesting that corneal ring abscess formation may be attributed to the induction of a corneal inflammatory response to the bacterial invasion itself or to locally produced inflammatory mediators generated in response to the tissue invasion. Questions remain as to the cause of such an aggressive inflammatory response and almost certain loss of vision accompanying B. cereus endophthalmitis. B. cereus, unlike S. aureus and E. faecalis, is a soil saprophyte and therefore is not likely to have adapted to commensal existence within or on the surface of the human body. The intense inflammatory reaction observed with B. cereus endophthalmitis may therefore be attributable to a lack of coevolutionary history and therefore a lack of evolved tolerance.
The results of these studies revealed key differences in the pathophysiology of gram-positive bacterial endophthalmitis caused by different organisms. If B. cereus, S. aureus, and E. faecalis possessed similar general biochemical traits (i.e., cell wall composition and toxin production), one would expect such infections to be similar. B. cereus and S. aureus each produce multiple toxins, both pore forming and cell membrane destabilizing, that have been implicated as virulence factors in various animal models of infection (6, 14, 63). The cytolysin is the only toxin of E. faecalis reported to contribute to its virulence (27, 28, 55). Yet S. aureus and E. faecalis infections were clinically similar to one another, and B. cereus intraocular infections were more severe. Another important difference observed was that of B. cereus motility and migration into retinal tissues and into the anterior segment, a phenomenon observed for neither S. aureus nor E. faecalis. In any case, the ocular pathogenesis of these organisms has been demonstrated to be an organism-dependent contribution of toxins, intraocular localization and behavior, and, to a lesser extent, the host response to bacterial cell walls. The results suggest that conventional therapeutic approaches may not be adequate for all types of endophthalmitis due to key differences in the pathophysiology of each infection. Understanding the basis for the inherent differences in these virulent infections will provide unique insights into endophthalmitis pathogenesis and advance the goal of developing new therapeutic strategies for these diseases.
ACKNOWLEDGMENTS
The technical assistance of Trish Mulden (DMEI Pathology), Russ Burris, Carolyn Thompson, and Jan Sullivan (DMEI Photography), and Mark Dittmar (DMEI Animal Facility) is greatly appreciated. We also thank Diana Locher and Scott Seigler for their histological interpretations, James Chodosh for insightful discussions, and Willis Owen (OUHSC Biostatistics and Epidemiology) for statistical expertise.
This work was supported in part by an NIH National Research Service Award (EY06813, to M.C.C.), an NIH new investigator award (EY10867, to M.C.B.), and NIH grant EY08289 (to M.S.G.) and also by grant HN6-040 from the Oklahoma Center for the Advancement of Science and Technology (to B.D.J.) and an unrestricted grant from Research to Prevent Blindness, Inc., New York, N.Y.
REFERENCES
- 1.Affeldt J C, Flynn H W, Forster R K, Mandelbaum S, Clarkson J G, Jarus G D. Microbial endophthalmitis resulting from ocular trauma. Ophthalmology. 1987;94:407–413. doi: 10.1016/s0161-6420(87)33447-5. [DOI] [PubMed] [Google Scholar]
- 2.Beecher D J, Pulido J S, Barney N P, Wong A C L. Extracellular virulence factors in Bacillus cereus endophthalmitis: methods and implication of involvement of hemolysin BL. Infect Immun. 1995;63:632–639. doi: 10.1128/iai.63.2.632-639.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beyer T L, O’Donnell F E, Goncalves V, Singh R. Role of the posterior capsule in the prevention of postoperative bacterial endophthalmitis: experimental primate studies and clinical implications. Br J Ophthalmol. 1985;69:841–846. doi: 10.1136/bjo.69.11.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhakdi S, Klonisch T, Nuber P, Fischer W. Stimulation of monokine production by lipoteichoic acids. Infect Immun. 1991;59:4614–4620. doi: 10.1128/iai.59.12.4614-4620.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blancquaert A M, Colgan S P, Bruyninckx W J. Chemotactic and chemokinetic activity of Streptococcus faecalis culture supernatant for equine neutrophils. Vet Immunol Immunopathol. 1988;19:285–297. doi: 10.1016/0165-2427(88)90115-8. [DOI] [PubMed] [Google Scholar]
- 6.Bohach G A, Dinges M M, Mitchell D T, Ohlendorf D H, Schlievert P M. Exotoxins. In: Crossley K B, Archer G L, editors. The staphylococci in human disease. New York, N.Y: Churchill Livingstone; 1997. pp. 83–111. [Google Scholar]
- 7.Boldt H C, Pulido J S, Blodi C F, Folk J C, Weingeist T A. Rural endophthalmitis. Ophthalmology. 1989;96:1722–1726. doi: 10.1016/s0161-6420(89)32658-3. [DOI] [PubMed] [Google Scholar]
- 8.Booth M C, Atkuri R V, Nanda S K, Iandolo J J, Gilmore M S. Accessory gene regulator controls Staphylococcus aureus virulence in endophthalmitis. Investig Ophthalmol Visual Sci. 1995;36:1828–1836. [PubMed] [Google Scholar]
- 9.Booth M C, Cheung A L, Hatter K L, Jett B D, Callegan M C, Gilmore M S. Staphylococcal accessory regulator (sar) in conjunction with agr contributes to Staphylococcus aureus virulence in endophthalmitis. Infect Immun. 1997;65:1550–1556. doi: 10.1128/iai.65.4.1550-1556.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brock T D, Davie J M. Probable identity of a group D hemolysin with a bacteriocine. J Bacteriol. 1963;86:708–712. doi: 10.1128/jb.86.4.708-712.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Callegan M C, Jett B D, Hancock L E, Gilmore M S. Role of hemolysin BL in the pathogenesis of extraintestinal Bacillus cereus infection assessed in an endophthalmitis model. Infect Immun. 1999;67:3348–3356. doi: 10.1128/iai.67.7.3357-3366.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeJonge B D J, Chang Y S, Gage D, Tomasz A. Peptidoglycan composition of a highly methicillin-resistant Staphylococcus aureus strain. J Biol Chem. 1992;267:11248–11254. [PubMed] [Google Scholar]
- 13.DeKimpe S J, Kengatharan M, Thiemermann C, Vane J R. The cell wall components peptidoglycan and lipoteichoic acid from Staphylococcus aureus act in synergy to cause shock and multiple organ failure. Proc Natl Acad Sci USA. 1995;92:10359–10363. doi: 10.1073/pnas.92.22.10359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drobniewski F A. Bacillus cereus and related species. Clin Microbiol Rev. 1993;6:324–338. doi: 10.1128/cmr.6.4.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duke-Elder S, Leigh A G. Diseases of the outer eye. Part 2. Cornea and sclera. In: Duke-Elder S, editor. System of ophthalmology. VIII. St. Louis, Mo: Mosby; 1965. pp. 854–855. [Google Scholar]
- 16.Forster R K. Experimental postoperative endophthalmitis. Trans Am Ophthalmol Soc. 1992;90:505–559. [PMC free article] [PubMed] [Google Scholar]
- 17.Fox A, Hammer M E, Lill P, Burch T G, Burrish G. Experimental uveitis elicited by peptidoglycan-polysaccharide complexes, lipopolysaccharide, and muramyl dipeptide. Arch Ophthalmol. 1984;102:1063–1067. doi: 10.1001/archopht.1984.01040030857033. [DOI] [PubMed] [Google Scholar]
- 18.Giese M J, Adamu S A, Pitchekian-Halabi H, Ravindranath R M, Mondino B J. The effect of Staphylococcus aureus phage lysate vaccine on a rabbit model of staphylococcal blepharitis, phlyctenulosis, and catarrhal infiltrates. Am J Ophthalmol. 1996;122:245–254. doi: 10.1016/s0002-9394(14)72016-1. [DOI] [PubMed] [Google Scholar]
- 19.Goldman W E, Cookson B T. Structure and functions of the Bordetella tracheal cytotoxin. Tokai J Exp Clin Med. 1988;13:187–191. [PubMed] [Google Scholar]
- 20.Gupta D, Jin Y P, Dziarski R. Peptidoglycan induces transcription and secretion of TNF-alpha and activation of lyn, extracellular signal-regulated kinase, and rsk signal transduction proteins in mouse macrophages. J Immunol. 1995;155:2620–2630. [PubMed] [Google Scholar]
- 21.Han D, Wisniewski S, Wilson L, Barza M, Vine A K, Doft B H, Kelsey S F. Spectrum and susceptibilities of microbiologic isolates in the Endophthalmitis Vitrectomy Study. Am J Ophthalmol. 1996;122:1–17. doi: 10.1016/s0002-9394(14)71959-2. [DOI] [PubMed] [Google Scholar]
- 22.Harshey R M, Toguchi A. Spinning tails: homologies among bacterial flagellar systems. Trends Microbiol. 1996;4:226–231. doi: 10.1016/0966-842X(96)10037-8. [DOI] [PubMed] [Google Scholar]
- 23.Heumann D, Barras C, Severin A, Glauser M P, Tomasz A. Gram-positive cell walls stimulate synthesis of tumor necrosis factor alpha and interleukin-6 by human monocytes. Infect Immun. 1994;62:2715–2721. doi: 10.1128/iai.62.7.2715-2721.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hiramatsu K. The emergence of Staphylococcus aureus with reduced susceptibility to vancomycin in Japan. Ann J Med. 1998;104:7S–10S. doi: 10.1016/s0002-9343(98)00149-1. [DOI] [PubMed] [Google Scholar]
- 25.Huycke M M, Sahm D F, Gilmore M S. Multiple-drug resistant enterococci: the nature of the problem and an agenda for the future. Emerg Infect Dis. 1998;4:239–249. doi: 10.3201/eid0402.980211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25a.Jett, B. D. Unpublished results.
- 26.Jett B D, Hatter K L, Huycke M M, Gilmore M S. Simplified agar plate method for quantifying viable bacteria. BioTechniques. 1997;23:648–650. doi: 10.2144/97234bm22. [DOI] [PubMed] [Google Scholar]
- 27.Jett B D, Jensen H G, Nordquist R E, Gilmore M S. Contribution of the pAD1-encoded cytolysin to the severity of experimental Enterococcus faecalis endophthalmitis. Infect Immun. 1992;60:2445–2452. doi: 10.1128/iai.60.6.2445-2452.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jett B D, Parke D W, Booth M C, Gilmore M S. Host/parasite interactions in bacterial endophthalmitis. Zentrbl Bakteriol. 1997;285:341–367. doi: 10.1016/s0934-8840(97)80002-3. [DOI] [PubMed] [Google Scholar]
- 29.Keller R, Fischer W, Keist R, Bassetti S. Macrophage response to bacteria: induction of marked secretory and cellular activities by lipoteichoic acids. Infect Immun. 1992;60:3664–3672. doi: 10.1128/iai.60.9.3664-3672.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim Y S, Kennedy S, Tauber M G. Toxicity of Streptococcus pneumoniae in neurons, astrocytes, and microglia in vitro. J Infect Dis. 1995;171:1363–1368. doi: 10.1093/infdis/171.5.1363. [DOI] [PubMed] [Google Scholar]
- 31.Kim Y S, Tauber M G. Neurotoxicity of glia activated by gram-positive bacterial products depends on nitric oxide production. Infect Immun. 1996;64:3148–3153. doi: 10.1128/iai.64.8.3148-3153.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kufoy E A, Fox K, Fox A, Parks C, Pakalnis V A. Modulation of the blood-aqueous barrier by gram positive and gram negative bacterial cell wall components in the rat and rabbit. Exp Eye Res. 1990;50:189–195. doi: 10.1016/0014-4835(90)90230-r. [DOI] [PubMed] [Google Scholar]
- 33.Kusunoki T, Halman E, Juan T, Lichenstein H, Wright S D. Molecules from Staphylococcus aureus that bind and stimulate innate immune responses. J Exp Med. 1995;182:1673–1682. doi: 10.1084/jem.182.6.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laszlo D J, Niwano M, Goral W W, Taylor B L. Bacillus cereus electron transport and proton motive force during aerotaxis. J Bacteriol. 1984;159:820–824. doi: 10.1128/jb.159.3.820-824.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ligozzi M, LoCascio G, Fontana R. vanA gene cluster in a vancomycin-resistant clinical isolate of Bacillus circulans. Antimicrob Agents Chemother. 1998;42:2055–2059. doi: 10.1128/aac.42.8.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mattson E, Verhage L, Rollof J, Fleer A, Verhoef J, VanDijk H. Peptidoglycan and teichoic acid from Staphylococcus epidermidis stimulate human monocytes to release tumor necrosis factor-alpha, interleukin-1 beta and interleukin-6. FEMS Immunol Med Microbiol. 1993;7:281–287. doi: 10.1111/j.1574-695X.1993.tb00409.x. [DOI] [PubMed] [Google Scholar]
- 37.Moellering R C. Antibiotic resistance: Lessons for the future. Clin Infect Dis. 1998;27:S135–S140. doi: 10.1086/514902. [DOI] [PubMed] [Google Scholar]
- 38.Mondino B J, Adamu S A, Pitchekian-Halabi H. Antibody studies in a rabbit model of corneal phlyctenulosis and catarrhal infiltrates related to Staphylococcus aureus. Investig Ophthalmol Visual Sci. 1991;32:1854–1863. [PubMed] [Google Scholar]
- 39.Mondino B J, Caster A I, Dethlefs B. A rabbit model of staphylococcal blepharitis. Arch Ophthalmol. 1987;105:409–412. doi: 10.1001/archopht.1987.01060030129042. [DOI] [PubMed] [Google Scholar]
- 40.Newell F W. Physical examination of the eye. In: Newell F W, editor. Ophthalmology: principles and concepts. New York, N.Y: Mosby; 1992. pp. 151–162. [Google Scholar]
- 41.O’Day D M, Smith R S, Andrews J S, Head W S, Ives J. Pathogenic mechanisms in Bacillus cereus panophthalmitis. Investig Ophthalmol Visual Sci. 1980;21:111–112. . (Abstr. 4.) [Google Scholar]
- 42.O’Day D M, Smith R S, Gregg C R, Turnbull P C B, Head W S, Ives J A, Ho P C. The problem of Bacillus species infection with special emphasis on the virulence of Bacillus cereus. Ophthalmology. 1981;88:833–838. doi: 10.1016/s0161-6420(81)34960-4. [DOI] [PubMed] [Google Scholar]
- 43.Park S S, Samiy N, Ruoff K, D’Amico D J, Baker A S. Effect of intravitreal dexamethasone in treatment of pneumococcal endophthalmitis in rabbits. Arch Ophthalmol. 1995;113:1324–1329. doi: 10.1001/archopht.1995.01100100112040. [DOI] [PubMed] [Google Scholar]
- 44.Peyman G A, Pague J T, Meisels H I, Bennett T O. Postoperative endophthalmitis: a comparison of methods for treatment and prophylaxis with gentamicin. Ophthalmic Surg. 1975;6:45–55. [PubMed] [Google Scholar]
- 45.Ras G, Wilson R, Todd H, Taylor G, Cole P. Effect of bacterial products on neutrophil migration in vitro. Thorax. 1990;45:276–280. doi: 10.1136/thx.45.4.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Riber U, Espersen F, Wilkinson B J, Kharazmi A. Neutrophil chemotactic activity of peptidoglycan. A comparison between Staphylococcus aureus and Staphylococcus epidermidis. APMIS. 1990;98:881–886. [PubMed] [Google Scholar]
- 47.Riesenfeld-Orn I, Wolpe S, Garcia-Bustos J F, Hoffmann M K, Tuomanen E. Production of interleukin-1 but not tumor necrosis factor by human monocytes stimulated with pneumococcal cell surface components. Infect Immun. 1989;57:1890–1893. doi: 10.1128/iai.57.7.1890-1893.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rogers H J, Perkins H R, Ward J B. Structure of peptidoglycan. In: Rogers H J, Perkins H R, Ward J B, editors. Microbial cell walls and membranes. New York, N.Y: Chapman and Hall; 1980. pp. 191–214. [Google Scholar]
- 49.Rot A, Henderson L E, Sowder R, Leonard E J. Staphylococcus aureus tetrapeptide with high chemotactic potency and efficacy for human leukocytes. J Leukoc Biol. 1989;45:114–120. doi: 10.1002/jlb.45.2.114. [DOI] [PubMed] [Google Scholar]
- 50.Rowsey J J, Newsom D L, Sexton D J, Harms W K. Endophthalmitis: current approaches. Ophthalmology. 1982;89:1055–1066. doi: 10.1016/s0161-6420(82)34691-6. [DOI] [PubMed] [Google Scholar]
- 51.Sannomiya P, Craig R A, Clewell D B, Suzuki A, Fujino M, Till G O, Marasco W A. Characterization of a class of nonformylated Enterococcus faecalis-derived neutrophil chemotactic peptides: the sex pheromones. Proc Natl Acad Sci USA. 1990;87:66–70. doi: 10.1073/pnas.87.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schemmer G R, Driebe W T. Post-traumatic Bacillus cereus endophthalmitis. Arch Ophthalmol. 1987;105:342–344. doi: 10.1001/archopht.1987.01060030062026. [DOI] [PubMed] [Google Scholar]
- 53.Sheehan D C, Hrapchak B B. Theory and practice of histotechnology. Columbus, Ohio: Battelle Press; 1987. [Google Scholar]
- 54.Standiford T J, Arenberg D A, Danforth J M, Kunkel S L, VanOtteren G M, Strieter R M. Lipoteichoic acid induces secretion of interleukin-8 from human blood monocytes: a cellular and molecular analysis. Infect Immun. 1994;62:119–125. doi: 10.1128/iai.62.1.119-125.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stevens S X, Jensen H G, Jett B D, Gilmore M S. A hemolysin-encoding plasmid contributes to bacterial virulence in experimental Enterococcus faecalis endophthalmitis. Investig Ophthalmol Visual Sci. 1992;33:94–100. [PubMed] [Google Scholar]
- 56.Stimpson S A, Brown R R, Anderle S K, Klapper D G, Clark R L, Cromartie W J, Schwab J H. Arthropathic properties of cell wall polymers from normal flora bacteria. Infect Immun. 1986;51:240–249. doi: 10.1128/iai.51.1.240-249.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tenover T C, Lancaster M V, Hill B C, Steward C D, Stocker S A, Hancock G A, O’Hara C M, McAllister S K, Clark N C, Hiramatsu K. Characterization of staphylococci with reduced susceptibilities to vancomycin and other glycopeptides. J Clin Microbiol. 1998;36:1020–1027. doi: 10.1128/jcm.36.4.1020-1027.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thompson S T, Parver L M, Enger C L, Meiler W F, Liggett P E. Infectious endophthalmitis after penetrating injuries with retained intraocular foreign bodies. Ophthalmology. 1993;100:1468–1474. doi: 10.1016/s0161-6420(93)31454-5. [DOI] [PubMed] [Google Scholar]
- 59.Timmerman C P, Mattsson E, Martinez-Martinez L, De Graaf L, Van Strijp J A, Verbrugh H A, Verhoef J, Fleer A. Induction of release of tumor necrosis factor from human monocytes by staphylococci and staphylococcal peptidoglycans. Infect Immun. 1993;61:4167–4172. doi: 10.1128/iai.61.10.4167-4172.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Todd E W. A comparative serological study of streptolysins derived from human and from animal infections, with notes on pneumococcal hemolysins, tetanolysin and Staphylococcus toxin. J Pathol Bacteriol. 1934;39:299–321. [Google Scholar]
- 61.Tuomanen E, Liu H, Hengstler B, Zak O, Tomasz A. The induction of meningeal inflammation by components of the pneumococcal cell wall. J Infect Dis. 1985;151:859–868. doi: 10.1093/infdis/151.5.859. [DOI] [PubMed] [Google Scholar]
- 62.Tuomanen E, Tomasz A, Hengstler B, Zak O. The relative role of bacterial cell wall and capsule in the induction of inflammation in pneumococcal meningitis. J Infect Dis. 1985;151:535–540. doi: 10.1093/infdis/151.3.535. [DOI] [PubMed] [Google Scholar]
- 63.Turnbull P C B. Bacillus cereus toxins. Pharmacol Ther. 1981;13:453–505. doi: 10.1016/0163-7258(81)90026-7. [DOI] [PubMed] [Google Scholar]
- 64.Vahey J B, Flynn H W. Results in the management of Bacillus endophthalmitis. Ophthalmic Surg. 1991;22:681–686. [PubMed] [Google Scholar]
- 65.Webster G F, Leyden J J, Musson R A, Douglas S D. Susceptibility of Propionibacterium acnes to killing and degradation by human neutrophils and monocytes in vitro. Infect Immun. 1985;49:116–121. doi: 10.1128/iai.49.1.116-121.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Williams D R, Meiler W F, Abrams G W, Lewis H. Results and prognostic factors in penetrating ocular injuries with retained intraocular foreign bodies. Ophthalmology. 1988;95:911–916. doi: 10.1016/s0161-6420(88)33069-1. [DOI] [PubMed] [Google Scholar]