Abstract
Bacillus cereus is a rare cause of serious human infection but, paradoxically, causes one of the most severe posttraumatic or endogenous infections of the eye, endophthalmitis, which frequently results in blindness. The virulence of B. cereus endophthalmitis historically has been attributed to toxin production. We therefore sought to examine the contribution of the dermonecrotic toxin, hemolysin BL, to the pathogenesis of B. cereus infection in an endophthalmitis system that is highly amenable to study. The pathogenesis of infection resulting from intravitreal injection of 102 CFU of either a clinical ocular isolate of B. cereus producing hemolysin BL (HBL+) or an isogenic mutant in this trait (HBL−) was assessed bacteriologically and by slit lamp biomicroscopy, electroretinography, histology, and inflammatory cell enumeration. Both HBL+ and HBL− strains evoked severe intraocular inflammatory responses as early as 12 h postinfection, with complete loss of retinal responsiveness by 12 h. The infections caused by both strains spread of the infection to adjacent tissues by 18 h. No significant differences in intraocular bacterial growth (P ≥ 0.21) or inflammatory changes (P ≥ 0.21) were observed in eyes infected with either HBL+ or HBL− strains during the course of infection. The level of retinal responsiveness was greater in HBL− infected eyes than in HBL+-infected eyes at 6 h only (P = 0.01). These results indicate that hemolysin BL makes no essential contribution to the severe and rapid course of infection in the endophthalmitis model.
Bacillus cereus is associated primarily with cases of food-borne gastrointestinal illnesses that are usually self-limiting and are rarely life-threatening. In that context, B. cereus has not generally been regarded as an important pathogen and is commonly dismissed as a laboratory contaminant. In the past several years, however, nongastrointestinal B. cereus infections have been reported with increasing frequency, perhaps because of an increasing awareness of the pathogenic potential of this saprophyte. B. cereus has been isolated as the causative agent of severe cases of septicemia, endocarditis, pneumonia, cutaneous infections, orthopedic infections, meningitis, and traumatic wound infections (2, 21, 23, 24, 34, 37, 39). The majority of reported cases of B. cereus nongastrointestinal disease occurred in immunocompromised individuals, patients with indwelling or prosthetic devices, or patients with traumatic injury (21, 34, 37, 39).
B. cereus ranks as a leading cause of posttraumatic endophthalmitis, a potentially blinding infection of the tissues of the interior of the eye, resulting from intraocular contamination during surgery or penetrating injury (1, 7, 30, 36, 41). B. cereus is also a leading cause of endogenous endophthalmitis, usually as a complication of high-grade bacteremia in patients with prolonged indwelling devices or intravenous drug abusers (11, 32). In contrast to its limited virulence at other anatomical sites, the course of B. cereus endophthalmitis is extremely explosive; it is characterized by the destruction of the posterior segment of the eye, with severe pain and a rapid decline in visual acuity within 1 to 2 days (25). A characteristic corneal ring abscess occurs in most cases. Severe edema and spread of the infection into adjacent tissues are common findings in severe cases. B. cereus endophthalmitis usually results in loss of all useful vision (12, 17, 25).
Toxin production has been hypothesized to be central to the severity of B. cereus endophthalmitis (11, 12, 32, 40). B. cereus produces a number of cytotoxins and enzymes that could contribute to the rapid course and severity of endophthalmitis, including hemolysins, lipases, enterotoxins, and proteases (12, 13, 38). Initial interest in B. cereus as a diarrheal food poisoning agent led to the characterization of a vascular permeability factor, termed hemolysin BL. Hemolysin BL is a tripartite enterotoxin, consisting of a binding component (B) and two lytic components (L1 and L2), encoded by the hblA, hblD, and hblC genes, respectively (16, 28). The hemolytic, vascular permeability, and enterotoxic activities of hemolysin BL require all three components for maximum activity, with initial binding of the component B and subsequent addition of L1 and L2 (4). Characterization of hemolysin BL led to its implication in the pathogenesis of B. cereus endophthalmitis (4, 5). Using an in vitro retinal button toxicity assay and in vivo vitreal injections of sterile culture supernatants and purified hemolysin BL, Beecher et al. (5) demonstrated that B. cereus exotoxins, including hemolysin BL, can cause ocular toxicity.
To study the host-parasite interactions in infections caused by organisms of considerable public health importance that do not fit paradigms established for traditional pathogens, such as Enterococcus faecalis and Staphylococcus aureus, we developed a rabbit endophthalmitis system (8, 9, 18, 19). This system is exquisitely sensitive, as infections can be established routinely with 10 to 100 organisms. Moreover, because of the clarity of the visual tract, repeated microscopic and electrophysiologic measurements can be made nondestructively, allowing differences in the pathogenesis of infection by nontraditional pathogens to be assessed noninvasively with precision. Because of the importance of eye infections due to B. cereus, this system for analysis provided a particularly appropriate launching point for examining the contribution of known and putative B. cereus toxins to the pathogenesis of nongastrointestinal infection.
In this report, we describe the generation of a hemolysin BL-deficient allelic replacement mutant of B. cereus and comparison of its virulence with that of the wild-type parental strain, using the endophthalmitis system. The results show that hemolysin BL is not required for the fulminant and destructive course of infection, as assessed with this in vivo system.
(This work was presented in part at the 99th General Meeting of the American Society for Microbiology, 30 May to 3 June 1999, Chicago, Ill.)
MATERIALS AND METHODS
Bacterial strains and plasmids.
The B. cereus strain used in these studies was isolated locally from a pediatric case of posttraumatic endophthalmitis that resulted in the ultimate surgical removal of the eye. This strain (MGBC145) produces hemolysin BL (5). Unless otherwise specified, bacterial strains were grown in brain heart infusion medium (BHI; Difco, Detroit, Mich.) with appropriate antibiotic selection. The relevant properties and sources of all bacterial strains and plasmids used in this study are summarized in Table 1.
TABLE 1.
Bacterial strains and plasmids used
| Strain or plasmid | Relevant properties | Reference or source |
|---|---|---|
| Strains | ||
| B. cereus | ||
| MGBC145 | Wild type, produces hemolysin BL | 5 |
| CJ145-1 | Isogenic mutant of MGBC145, contains pKRX-hbl::ermR insertion into hemolysin BL operon (Ermr Kanr) | This work |
| CJ145-1.1 | Isogenic mutant of MGBC145, contains ermAM replacement of a 1.6-kb internal fragment of the hemolysin BL operon (Ermr Kans) | This work |
| E. coli | ||
| XL1-Blue | supE44 hsdR17 recA1 endA1 gyrA46 thi-1 relA1 lac, F′ [proAB+lacIqlacZΔM15 Tn10 (Tetr)] | 29 |
| JM110 | dam dcm supE44 hsdR17 thi leu thr rpsL lacY galK galT ara tonA tsx Δ(lac-proAB), F′ [traD36 proAB+lacIqlacZΔM15] | 29 |
| Plasmids | ||
| pKRX | T vector derivative of pBluescript II SK (+/−) containing XcmI cloning site | 31 |
| pKRX-hbl | 3.4-kb hblCDA PCR fragment inserted into the EcoRI/PstI site of pKRX | This work |
| pUC18-ermAM | E. faecalis pAMβ1 Ermr determinant cloned into the SmaI site of pUC18 | J. Ferretti |
| pKRX-hbl::ermR | ermAM replacement of the internal 1.6-kb PacI/Eco47III deletion fragment of hblCDA | This work |
| p3ERM | Derivative of pSF15235 with ermAM replacing the spectinomycin resistance marker | J. Ferretti |
| pCASPER | Temperature-sensitive vector containing an inverse PCR fragment of pTV1OK15 [containing the repA(Ts) origin of pWVO1 and the aphA3 gene for kanamycin resistance] and the p3ERM multicloning region | This work |
| pΔHBL | Hemolysin BL-containing EcoRI/PstI fragment of pKRX-hbl::ermR ligated into multicloning region of pCASPER | This work |
DNA techniques.
B. cereus MGBC145 chromosomal DNA was isolated by phenol-chloroform extraction and CsCl gradient purification (29), and an internal fragment of the hemolysin BL operon was amplified by PCR as follows. PCR mixtures of 100 μl contained 25 ng of genomic DNA, 0.2 mM deoxynucleoside triphosphates Mg-free thermophilic DNA polymerase buffer (10×; Promega Corp., Madison, Wis.), 2.0 mM MgCl2, 0.5 mM of each primer (listed below), and 0.025 U of TaKaRa LA Taq polymerase (PanVera Corp., Madison, Wis.). Primers for amplification of the internal hemolysin BL fragment were derived from published sequences (GenBank accession no. L20441 and U63928) as follows: hblC forward primer with an EcoRI restriction site (underlined) incorporated into the 5′ end, GAATTCCAGCTAGAGGAAGTCCCAGC; and hblA reverse primer with a PstI restriction site (underlined) incorporated into the 5′ end, CTGCAGCAATATGCCCTAGAACGCCCG (Integrated DNA Technologies, Coralville, Iowa) (16, 28). The hemolysin BL fragment was amplified from B. cereus genomic DNA under the following conditions: hot-start denaturation for 1 min at 94°C, then 35 cycles of 1 min at 95°C, 1 min at 60°C, and 3 min at 72°C, followed by 10 min at 72°C. The amplified hemolysin BL fragment was resolved on a 1.0% low-melting-temperature agarose gel and purified by using GeneClean (BIO 101, Inc., Vista, Calif.).
DNA manipulations and Southern blot analyses were performed essentially as described by Sambrook et al. (29). Unless otherwise specified, all plasmids were propagated in E. coli strain JM110 (dam dcm). Plasmid DNA was prepared with the Wizard Mini-Prep DNA purification kit (Promega) according to the manufacturer’s instructions.
B. cereus transformation was performed essentially as described by Masson et al. (22), with minor modifications. Briefly, B. cereus MGBC145 was rendered electrocompetent by growth to mid-logarithmic phase (optical density at 650 nm [OD650] of 0.3 to 0.6) in BHI and stepwise concentration of 1010 CFU/ml in chilled 10% glycerol. Electrocompetent cells were stored at −70°C for up to 1 month. For electroporation, 40-μl aliquots of cells were thawed on ice, plasmid DNA (10 μg) was added, and the suspension was immediately transferred to a chilled 0.2-cm-gap electroporation cuvette. Cells were pulsed at 2.0 kV for 4.7 s (Bio-Rad [Hercules, Calif.] Escherichia coli Pulser). After pulsing, 1.0 ml of SOC medium (29) was added to the cells for recovery (2 h, 28°C), and 500-μl aliquots were plated onto BHI supplemented with 400 erythromycin (μg/ml) and kanamycin (75 μg/ml). Transformants were subcultured onto BHI supplemented with 2.5% (vol/vol) sheep erythrocytes and erythromycin plus kanamycin. Erythromycin- and kanamycin-resistant (Ermr Kanr) colonies identified with a loss of the discontinuous hemolytic zone (6) were chosen for further analysis.
Phenotypic assessment of B. cereus strains.
Phenotypic profiles of the parental (hemolysin BL-expressing [HBL+]) and hemolysin BL-deficient (HBL−) strains were assessed biochemically. Cytolytic activities of culture supernatants were assessed based on sheep erythrocytes and a hemolysis readout. Briefly, twofold serial dilutions of overnight culture supernatants were incubated with an equal volume of 4% (vol/vol) sheep erythrocytes in phosphate-buffered saline for 30 min, and the OD540 was measured. The hemolytic titer was determined as the dilution of supernatant exhibiting 50% hemolysis.
Hemolysin BL production was detected by the formation of a discontinuous zone of hemolysis surrounding isolated colonies on 2.5% sheep erythrocyte agar (6). The dermonecrotic ability of hemolysin BL was also tested in the vascular permeability assay (38). Briefly, late-logarithmic-phase culture supernatants were concentrated by ammonium sulfate precipitation, and 50-μl aliquots were injected intradermally. At 3 h postinjection, Evans blue dye (1 ml of a 2% solution/kg of body weight) was injected intravenously. Intradermal injection sites were observed every 15 min for 4 h; an area of bluing surrounding a necrotic center at the injection site indicated vascular permeability (38).
Phospholipase C and sphingomyelinase activities, indicating cereolysin AB production, were assessed by egg yolk agar turbidity and the chromogenic trinitrophenolaminolauroyl (TNPAL)-sphingomyelinase assay, respectively (14). Proteolytic activity was identified by lytic zones surrounding isolated colonies on casein agar.
Experimental B. cereus endophthalmitis.
New Zealand White rabbits (2 to 3 kg) were maintained in accordance with institutional guidelines and the Association for Research in Vision and Ophthalmology Statement on the Use of Laboratory Animals in Ophthalmic Research. Prior to intravitreal injection, eyes were dilated with topical 1% tropicamide and 2.5% phenylephrine HCl. Rabbits were anesthetized by intramuscular injection of ketamine (Ketaved; Phoenix Scientific Inc., St. Joseph, Mo.; 35 mg/kg of body weight) and xylazine Rompun; Bayer Corp., Shawnee Mission, Kans.; 5 mg/kg of body weight). Topical anesthesia (0.5% proparacaine HCl [Ophthetic; Allergan, Hormigueros, Puerto Rico]) was also applied.
With the aid of a binocular surgical microscope, eyes were immobilized with iris forceps, and 100 μl of aqueous humor withdrawn to relieve intraocular pressure before injection. Inocula (102 CFU of either the HBL+ or HBL− strain in 100 μl of BHI with or without 25 μg of erythromycin per ml) were delivered by slow infusion into the midvitreous via a 30-gauge needle attached to a 1.0-ml syringe introduced through the pars plana, approximately 3 mm from the limbus of one eye of each rabbit. The contralateral eye was injected with either BHI or BHI supplemented with 25 μg of erythromycin per ml (surgical controls) or was left undisturbed (absolute control) (8–10, 18). At various times after infection, the following parameters of infection and organ function were quantified: (i) slit lamp biomicroscopy, (ii) electroretinography (ERG), (iii) intraocular bacterial quantitation, (iv) quantitation of inflammatory cells and total protein in aqueous humor, and (v) thin-section histology. The inoculum was quantified retrospectively by plating triplicate serial 10-fold dilutions on BHI (20).
Slit lamp examination.
Rabbits were observed in a Topcon SL-5D slit lamp biomicroscope (Kogaku Kikai K.K., Tokyo, Japan) prior to injection and again following injection at 3, 6, 12, and 18 h. Ocular inflammation was scored by masked independent observers, using scoring criteria for progressive inflammation of the cornea, anterior chamber, vitreous, and retina as described in Table 2.
TABLE 2.
Slit lamp examination and histopathological scoring systemsa
| Grade | Clinical parameters
|
Histological parameters
|
||||||
|---|---|---|---|---|---|---|---|---|
| Anterior chamber | Fundus reflex | Vitreous | Retinal clarity | Cornea | Anterior chamber | Vitreous | Retina | |
| 0 | No inflammatory cells observed | Normal | Clear | Details clear | No infiltration of inflammatory cells | Normal | No inflammation | Normal |
| 1 | 5–10 inflammatory cells/field | Slightly diminished | Slight haze | Details distinct | Partial-thickness infiltration of inflammatory cells | Partially filled with fibrin, no inflammatory cells | Inflammatory cells visible, no focal abscesses | Partially infiltrated and necrotic, some normal retina visible |
| 2 | 10–20 inflammatory cells/field | Diminished | Progressive haze | Decreased clarity | Segmental full-thickness infiltration of inflammatory cells | Partially filled with fibrin and inflammatory cells visible | Partially filled with abscesses and infiltrate | Totally infiltrated and partially necrotic, no normal retina |
| 3 | 20–50 inflammatory cells/field | Moderately diminished | Fibrin formation; dense cellular reaction | Obscured details | Total full-thickness infiltration of inflammatory cells | Completely filled with fibrin and inflammatory cells visible | Completely filled with infiltrate | Totally necrotic, no retinal layer intact |
| 4 | >50 inflammatory cells/field | White | Fibrin deposits or large clumps of exudate | Completely obscured | ||||
ERG.
ERG was used to measure the ability of the retina to respond to a single low-intensity flash (one flash per second), and the resulting B-wave response (in millivolts) was measured. After dilation and 30 min of dark adaptation, baseline B-wave amplitude was recorded for each eye, using scotopic bright-flash ERG (EPIC-2000; LKC Technologies, Inc., Gaithersburg, Md.) at time points 24 h prior to injection and again following injection at 3, 6, 12, and 18 h. B-wave amplitude for each time point represented the average of 14 repeated measures. Percent loss of retinal function was calculated as [1 − (experimental B-wave amplitude/baseline B-wave amplitude)] × 100 (8–10, 18).
Bacterial enumeration.
To enumerate organisms in the vitreous, globes were enucleated, rinsed with sterile phosphate-buffered saline and placed cornea side up on sterile gauze. The cornea, iris, and lens were removed aseptically. The vitreous was then removed with a sterile pipette, its volume was measured, and the tissue was homogenized (60 s, 5,000 rpm; Mini-BeadBeater, Biospec Products, Bartlesville, Okla.) to reduce viscosity. Bacteria in the vitreous were quantified by plating serial 10-fold dilutions in triplicate on BHI. Colonies (50 or more) recovered from vitreous were replica plated onto 2.5% sheep blood agar to confirm the hemolysin BL phenotype of the infecting strains.
Anterior segment inflammation.
Anterior segment inflammation was analyzed by (i) enumeration of infiltrating inflammatory cells in anterior chamber fluid, using a hemocytometer, and (ii) quantification of total protein in anterior chamber fluid, using a bicinchoninic acid protein assay kit (Pierce Co., Rockford, Ill.).
Thin-section histopathology.
All globes recovered for histopathological analysis were fixed in 10% formalin for 24 h. Eyes were sectioned and stained with either hematoxylin and eosin or Brown & Hopps tissue Gram’s stain following standard procedures (33). Stained tissue sections were analyzed by masked observers, using scoring criteria as summarized in Table 2.
Statistical analysis.
All values represent the mean ± standard deviation for ≥4 eyes per time point assayed unless otherwise specified. Wilcoxon’s rank sum test was used for statistical comparison between groups. A P value of <0.05 was considered significant.
RESULTS
Construction of pΔHBL.
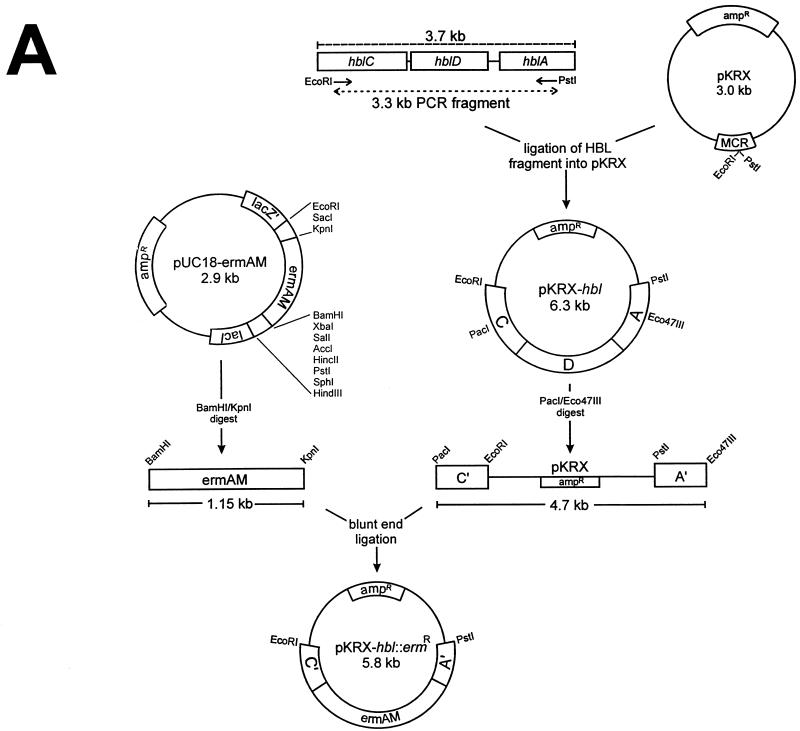
The construction of pΔHBL is illustrated in Fig. 1. PCR amplification of B. cereus MGBC145 chromosomal DNA with hemolysin BL-specific primers (hblC and hblA) generated a 3.3-kb DNA fragment containing 966 bp from the 5′ end of hblC, the entire hblD gene (1,154 bp), and 760 bp of the 3′ end of hblA, with synthetic EcoRI and PstI restriction sites at its 5′ and 3′ ends, respectively (Fig. 1A). The amplified fragment was ligated into the multicloning region of pKRX at the EcoRI and PstI sites, resulting in a 6.3-kb plasmid (pKRX-hbl). Sequencing of pKRX-hbl confirmed the identity of the hemolysin BL fragment (3).
FIG. 1.
Construction of pΔHBL for insertional mutagenesis of B. cereus hemolysin BL. Relevant restriction sites are shown. (A) A 3.3-kb PCR fragment of hemolysin BL was ligated into the multicloning region of pKRX, creating pKRX-hbl. The ermAM gene of pUC-ermAM was ligated to the 4.7-kb Eco47III/PstI fragment of pKRX-hbl to create pKRX-hbl::ermR. (B) The multicloning region of p3ERM was ligated to an inverse PCR fragment of pTV1OK containing the temperature-sensitive replicon from pWVO1 and a Kanr marker, to create pCASPER. (C) The EcoRI/PstI fragment of pKRX-hbl::ermR containing the disrupted hemolysin BL operon was ligated into the multicloning region of pCASPER to create pΔHBL.
Disruption of the hemolysin BL fragment of pKRX-hbl with the ermAM gene of pUC18-ermAM (provided by J. Ferretti, University of Oklahoma Health Sciences Center, Oklahoma City) was then performed. pUC18-ermAM was restricted with BamHI/KpnI, and a 1.1-kb fragment containing ermAM was recovered. In parallel, pKRX-hbl was restricted with Eco47III and PacI, resulting in a 4.7-kb fragment containing the 3′ end of hblC, an intact pKRX, and the 5′ end of hblA (truncated forms of hblC and hblA are designated as C′ and A′, respectively, in Fig. 1A). The recovered fragments were then ligated via blunt-end ligation, creating pKRX-hbl::ermR (5.8 kb) (Fig. 1A).
The temperature-sensitive vector pCASPER (3.5 kb) was created by ligating an inverse PCR fragment of pTV1OK (15) containing the repA(Ts) gene (for conditional replication in gram-positive organisms and in E. coli) and the Kanr marker (aphA3) into the multicloning region of p3ERM (Fig. 1B). The temperature-sensitive hemolysin BL knockout plasmid (pΔHBL) was created by digesting both pCASPER and pKRX-hbl::ermR with EcoRI/PstI and ligating the appropriate fragments (3.3 kb of pCASPER and 2.9 kb of pKRX-hbl::ermR) to generate pΔHBL (6.2 kb) (Fig. 1C).
Generation of the hemolysin BL-deficient allelic replacement mutant.
Transformation of B. cereus MGBC145 with pΔHBL and incubation for 24 h at 28°C yielded two Ermr Kanr colonies. Resistance to erythromycin and kanamycin indicated integration of the entire plasmid into the chromosome of these strains. Replica plating onto sheep blood agar with and without selective antibiotics and incubation at 37°C revealed that neither strain exhibited the discontinuous zone of hemolysis associated with the Hbl− phenotype and that this mutation was stable at 37°C. The first mutant (strain CJ145-1) was chosen for further analysis.
To generate an HBL− mutant containing the erm marker specifically within the hemolysin BL operon (instead of insertion of the entire pΔHBL plasmid), CJ145-1 was cultured in BHI supplemented with 100 μg of erythromycin per ml to maintain selection for the interrupted hbl operon, at elevated temperatures to facilitate plasmid curing (18 h at 42°C) (15). The resulting cultures were serially diluted and plated for isolation on BHI agar supplemented with 100 μg of erythromycin per ml and then cultured for an additional 18 h at 42°C. Isolated colonies (approximately 100) were replica plated on BHI agar supplemented with either erythromycin (100 μg/ml) or kanamycin (50 μg/ml) and incubated overnight at 37°C. A single Ermr Kans clone (strain CJ145-1.1) was recovered for further analysis. Kanamycin susceptibility indicated excision of the pCASPER vector by a second recombination and loss by temperature-induced curing.
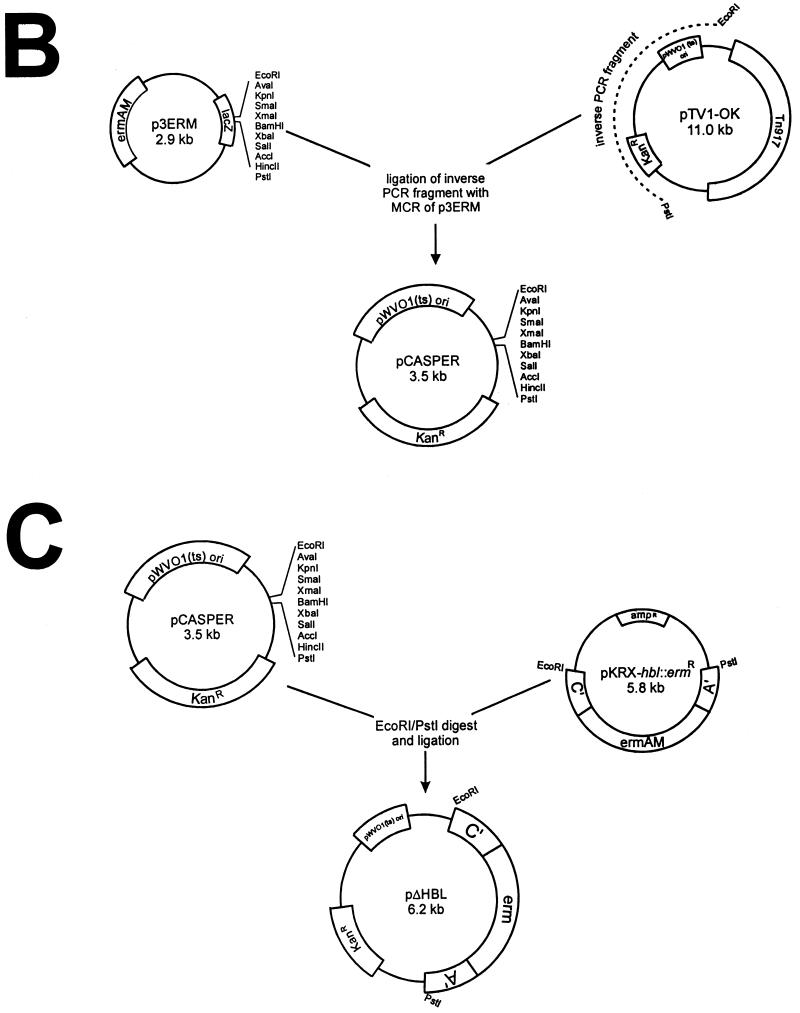
PCR amplification of the hemolysin BL genes in chromosomal DNA preparations of B. cereus MGBC145 and CJ145-1.1 revealed that the HBL− mutant contained the deleted 2.8-kb hemolysin BL PCR fragment, compared to a 3.3-kb fragment from the wild-type strain. Since construction of pKRX-hbl::ermR involved deletion of a 1.6 kb Eco47III/PacI fragment and insertion of the 1.1-kb erythromycin marker, the isogenic HBL− mutant had a corresponding amplified hemolysin BL fragment that was 0.5 kb smaller than that in the wild type (Fig. 2A).
FIG. 2.
(A) PCR analysis of wild-type and hemolysin BL-deficient strains. Samples of chromosomal DNA from strains MGBC145 (lane 1) and CJ145-1.1 (lane 2) were used to amplify hemolysin BL. PCR products were of the predicted sizes (indicated by lines on the left). (B) Chromosomal DNA from strains MGBC145 (lanes 1 and 3) and CJ145-1.1 (lanes 2 and 4) were digested with EcoRI, and fragments were separated on a 0.8% agarose gel. Lanes 1 and 2 were probed with 33P-labeled pKRX-hbl. Lanes 3 and 4 were probed with 33P-labeled pUC18-ermAM. MW, molecular weight markers.
Southern blot hybridization of EcoRI-digested chromosomal DNA from strains MGBC145 and CJ145-1.1 was performed with 33P-labeled pKRX-hbl and pUC18-ermAM as probes (Fig. 2B). The hbl probe hybridized to chromosomal fragments of approximately 5.7, 2.5, and 1.3 kb in the wild-type strain. The 5.7-kb hbl-hybridizing fragments observed in the present study corroborates previous findings (16). An additional 4.0-kb hbl-hybridizing fragment has been reported for a different B. cereus strain (20), which may correlate with the 2.5- and 1.3-kb hbl-hybridizing fragments observed. These results suggest that MGBC145 may contain two EcoRI sites within hbl, accounting for the three hbl-hybridizing EcoRI fragments observed. The erm probe did not hybridize to chromosomal DNA from the wild-type strain. A single approximately 9.3-kb fragment of CJ145-1.1 hybridized to both the hbl and erm probes, indicating an insertion event resulting from replacement of the internal hbl fragment with ermAM. The two smaller hbl-hybridizing fragments observed in the wild type were lost in CJ145-1.1, indicating replacement of two EcoRI restriction sites with the ermAM marker. Strain CJ145-1.1 was used for subsequent in vivo studies and will be designated the HBL− mutant.
Phenotypic assessment.
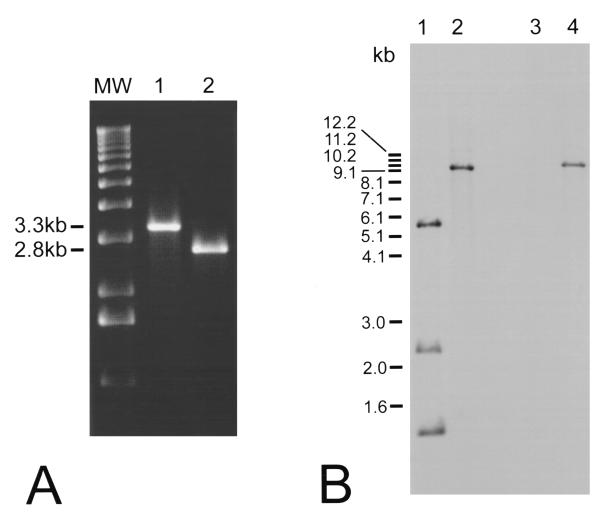
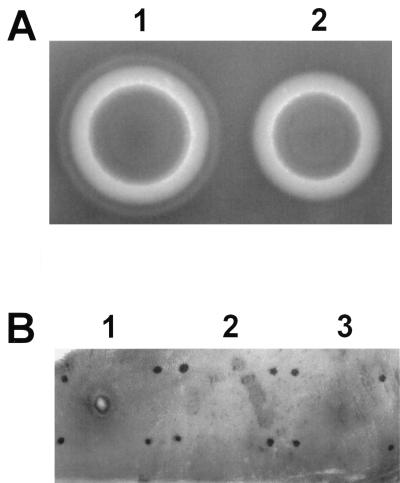
Results of the phenotypic assessment of the wild-type HBL+ strain and the HBL− mutant are summarized in Table 3. The two strains differed only in hemolysin BL activities, as measured by several criteria. The hemolytic titer of the HBL− mutant for sheep erythrocytes was significantly less than that observed for the HBL+ strain. Isolated colonies of both strains exhibited comparable hemolytic zones adjacent to each colony on sheep erythrocyte agar. However, the characteristic hemolysin BL-mediated discontinuous zone of hemolysis surrounding isolated HBL− colonies was absent (Fig. 3A). Similarly, concentrated supernatants of the HBL− mutant did not induce the vascular permeability reaction observed with supernatants of the HBL+ strain (Fig. 3B). These results indicated that hemolysin BL was indeed not functional in the HBL− mutant. The cereolysin AB and proteolytic activities of the HBL+ and HBL− strains were not different (Table 3).
TABLE 3.
Phenotypic assessment of the wild-type HBL+ strain (MGBC145) and the isogenic HBL− mutant (CJ145-1.1)
| Strain | Phenotypic assay
|
|||||
|---|---|---|---|---|---|---|
| Hemolytic titera | Discontinuous hemolytic zoneb | Vascular permeabilityc | Sphingomyelinased (mean ± SD) | Phospholipase Ce | Proteasef | |
| HBL+ | 1:8 | Yes | Yes | 0.023 ± 0.007 | Yes | Yes |
| HBL− | 1:32 | No | No | 0.036 ± 0.003 | Yes | Yes |
Hemolytic assay of filtered supernatants of 18-h B. cereus cultures. A twofold difference in titer was considered significant.
Presence or absence of a discontinuous hemolytic zone surrounding isolated B. cereus colonies on 2.5% sheep erythrocyte agar (6). The results of this assay are depicted in Fig. 3A.
Presence or absence of bluing, necrotic foci following intradermal injection of 50 μl of concentrated B. cereus culture supernatants. The results of this assay are depicted in Fig. 3B.
Chromogenic TNPAL-sphingomyelinase assay (14). Values represent the OD330 of filtered supernatants of 18-h B. cereus cultures (values represent two separate duplicate assays). Values were not significantly different (P = 0.154, Student’s t test).
Presence or absence of turbidity surrounding isolated colonies of B. cereus on egg yolk agar (14).
Presence or absence of a proteolytic zone surrounding isolated colonies on casein agar.
FIG. 3.
Phenotypic analysis of wild-type and hemolysin BL-deficient strains. (A) Single colonies of MGBC145 (plate 1) and CJ145-1.1 (plate 2) were isolated on 2.5% sheep erythrocyte agar. Presence of the discontinuous zone of hemolysis (as seen in plate 1) indicated hemolysin BL activity. (B) Concentrated supernatants of MGBC145 (sample 1), uninoculated BHI (sample 2), or CJ145-1.1 (sample 3) were injected intradermally. Presence of a necrotic center surrounded by a blue (dark) zone (as seen in sample 1) indicated hemolysin BL activity.
Experimental B. cereus endophthalmitis.
Reproducible endophthalmitis was achieved by intravitreal injection of 215 ± 27 CFU of the wild-type HBL+ B. cereus strain. A very rapid and intense inflammatory response beginning as early as 3 h was observed. Mild to moderate conjunctival inflammation and 10 to 20 inflammatory cells per microscopic field were present in the anterior chamber. All eyes had a normal fundus reflex. At 6 h postinfection, inflammatory symptoms progressed, with a haze developing in the vitreous and a decrease in fundus reflex. From 12 to 18 h, inflammatory symptoms were severe in all animals, with anterior chamber hyphema, severe iritis, significant inflammatory cell infiltration into the vitreous, and no fundus reflex. Gross examination of enucleated globes and surrounding tissues showed severe inflammation of periorbital tissues at 12 and 18 h postinfection. Because of the extensive panophthalmitis evident at 18 h, infections were not allowed to progress further. Sham-injected eyes were indistinguishable from uninjected eyes by slit lamp biomicroscopy.
Experimental hemolysin BL-deficient B. cereus endophthalmitis.
The inflammatory changes observed with eyes infected with 195 ± 30 CFU of the HBL− mutant were similar to those observed in eyes infected with the HBL+ strain at all time points except 6 h. Eyes infected with the HBL− mutant showed minimal conjunctival inflammation and no anterior chamber inflammatory cells present in four of six eyes at 6 h. The remaining two eyes exhibited infiltration of 5 to 10 inflammatory cells per microscopic field present in the anterior chamber, and a slight reduction of the fundus reflex. After 12 h, however, eyes infected with the HBL− mutant were indistinguishable from eyes infected with the HBL+ strain. Sham-injected eyes were indistinguishable from uninjected eyes by slip lamp biomicroscopy.
In vitro and in vivo growth of HBL+ and HBL− strains.
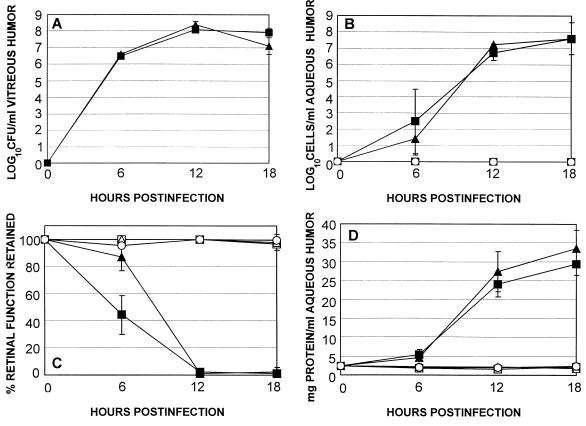
Growth of the HBL+ strain and that of the HBL− mutant in BHI were similar. The patterns of intraocular growth of the HBL+ and HBL− strains were also similar at each time point assayed (P ≥ 0.21) (Fig. 4A). Each strain grew logarithmically until approximately 12 h, after which a stationary phase of growth was maintained at 108 CFU/ml until the termination of the experiment.
FIG. 4.
Comparison of experimental B. cereus endophthalmitis initiated by wild type (HBL+) and isogenic HBL− strains. Inocula of 102 CFU of either the HBL+ (■) or HBL− (▴) strain were injected intravitreally, and infections were assessed at various times by bacterial enumeration (A), inflammatory cell enumeration in aqueous humor (B), ERG (C), and total protein concentration in aqueous humor (D). Also included were sham-injected (BHI [□] or BHI supplemented with 25 μg of erythromycin per ml [▵]) and uninjected (○) controls. All values represent the mean ± standard deviation for ≥4 eyes per group.
Anterior segment inflammation.
There were no significant differences in anterior segment inflammation in terms of inflammatory cells per milliliter of aqueous humor (P ≥ 0.21) (Fig. 4B) or total protein concentration per milliliter of aqueous humor (P ≥ 0.38) (Fig. 4D) throughout the course of infection. Aqueous humor samples from both sham-injected and uninjected control eyes were free of inflammatory cells (Fig. 4B) and maintained only baseline levels of total protein (Fig. 4D) throughout the duration of the experiment.
ERG.
The results of ERG of eyes infected with the wild-type HBL+ strain or HBL− mutant are summarized in Fig. 4C. Values at 3 h were not included in the study because the retinal responses of injected, surgical control, and absolute control eyes were each diminished at this time. This generalized loss of retinal response in eyes with little to no inflammation was likely the result of the recent anesthetization of the animals, causing a general unresponsiveness to light stimuli.
The retinal responsiveness of sham-injected and uninjected control eyes remained preoperative levels throughout the duration of the experiment (Fig. 4C). The retinal responsiveness of eyes injected with the HBL− mutant was significantly greater than that of eyes injected with the HBL+ strain at 6 h only (P = 0.01). At 12 h, retinal responses diminished completely in each infection group (P = 0.91).
Histological assessment.
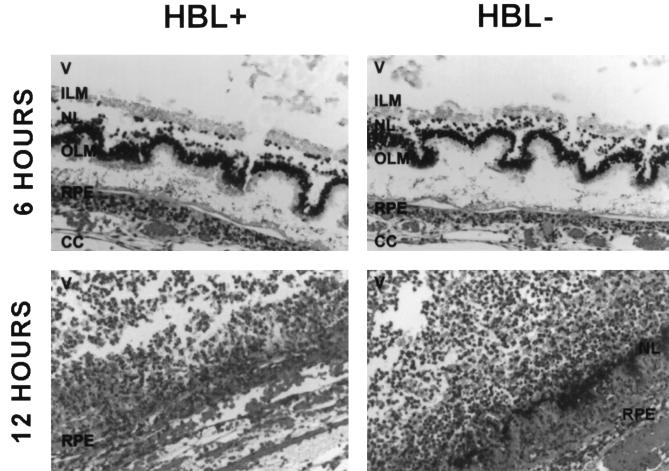
Immediately following intravitreal injection and at 3 h postinfection, eyes from both infection groups possessed intact retinal architecture, no observable inflammation, and few bacilli in the vitreous. At 6 h, eyes injected with the HBL+ strain exhibited a mild to moderate inflammatory response in the anterior segment and the vitreous. Bacilli were observed throughout the vitreous but occurred in greatest numbers at vitreous interfaces and on the posterior surface of the ciliary body. Retinal architecture was disrupted, with retinal detachment, photoreceptor layer folding, and bacilli located within the retinal layers (Fig. 5). Eyes injected with the HBL− mutant exhibited inflammation and B. cereus localization similar to that observed with the HBL+ strain (Fig. 5). Some normal retinal tissue was seen. Disruption of the retinal architecture and bacilli within the retinal layers was also observed. In general, these eyes were slightly, but not significantly, less inflamed than those infected with the HBL+ strain.
FIG. 5.
Retinal sections of eyes intravitreally infected with B. cereus MGBC145 (HBL+) or CJ145-1.1 (HBL−) at 6 and 12 h postinfection. All sections were stained with hematoxylin and eosin. Photoreceptor layer folding and B. cereus residing between retinal layers were observed at 6 h. Retinal layers were virtually indistinguishable at 12 h. V, vitreous; ILM, inner limiting membrane; NL, nuclear layer; OLM, outer limiting membrane; RPE, retinal pigment epithelium; CC, choriocapillaris. Magnification of all panels, ×200.
At 12 h, eyes injected with the HBL+ strain exhibited moderate inflammation in both the anterior and posterior segments. Inflammatory cells were interspersed with fibrin in the anterior chamber. Inflammatory cells were observed migrating from the optic nerve head into the vitreous and into the cornea from the limbal vessels. Bacilli were localized in large clusters on the posterior surface of the ciliary body. Moderate disruption of the retinal architecture and severe retinal inflammation were observed. Bacilli remained in clusters within disrupted retinal layers. Eyes injected with the HBL− strain were histopathologically indistinguishable from those infected with the HBL+ strain (Fig. 5).
At 18 h, eyes injected with either strain exhibited severe inflammation in all parts of the eye. Inflammatory cells migrated in more significant numbers into the corneal periphery, forming a corneal ring abscess. Inflammatory cells, fibrin, and erythrocytes filled the anterior segment. Retinal structures were indistinguishable, and the posterior chamber was filled with inflammatory cells and fibrin.
DISCUSSION
Isogenic mutants deficient in single or multiple virulence determinants are used routinely to assess the relative contributions of such factors to the pathogenesis of infection. To our knowledge, this is the first report of the generation of an allelic replacement mutant of B. cereus and its use in assessing the relative contribution of a specific protein to B. cereus virulence.
B. cereus endophthalmitis is unique in its fulminance and invariably devastating outcome. The experimental model used in these studies mimicked several findings of clinical B. cereus endophthalmitis, namely, photoreceptor layer folding, retinal epithelial disruption, ciliary body damage, corneal ring abscess formation, and severe vitritis. Comparison of an isogenic B. cereus mutant deficient in hemolysin BL with its parental strain in this model specifically addressed the contribution of this toxin to natural infection pathogenesis. Previous studies of the contribution of hemolysin BL to endophthalmitis relied on in vitro retinal toxicity studies using purified toxins at concentrations of unknown physiological relevance (5). The results of the present study show that B. cereus endophthalmitis results in complete destruction of organ function by 12 h, irrespective of hemolysin BL production. Comparable levels of intraocular inflammation, retinal detachment, and photoreceptor layer folding occurred in the absence of hemolysin BL, highlighting the importance of inflammogenic and toxic factors other than hemolysin BL in intraocular virulence.
The present study suggests that while hemolysin BL may contribute to retinal toxicity during the earliest stage of infection (i.e., at 6 h), its role is limited, and the unique virulence of B. cereus for the eye results from other host/parasite interactions. A recent study from our laboratory demonstrated the inflammogenic potential of B. cereus cell walls (10). Metabolically inactive B. cereus and cell wall sacculus preparations did not affect retinal architecture or neuroresponsiveness but did elicit significant intraocular inflammation. B. cereus supernatants resulted in both retinal toxicity and intraocular inflammation, indicating that secreted toxins or other proteins contribute to retinal toxicity, as previously observed (5), and that intraocular inflammation results from the combination of toxins and cell wall constituents. The present study unambiguously demonstrates that in the multifactorial pathogenesis of extraintestinal B. cereus infection, hemolysin BL does not play an essential role. Continuing efforts are directed toward identifying the principle interactions resulting in this unusually explosive infection of the eye and toward defining precisely the role of hemolysin BL and other toxins in gastroenteritis and other diseases caused by B. cereus.
ACKNOWLEDGMENTS
We thank William J. Moar (Auburn University, Auburn, Ala.) for providing transformation protocols. The technical assistance of Judy Hanna (DMEI Pathology), Russ Burris, Carolyn Thompson, and Jan Sullivan (DMEI Photography), and Mark Dittmar (DMEI Animal Facility) is greatly appreciated. We also thank Mary C. Booth, James Chodosh, Viswanathan Shankar, Wolfgang Haas, Brett Shepard, and Phillip Coburn for critical reading and stimulating discussions and Willis Owen (OUHSC Biostatistics and Epidemiology) for statistical expertise.
This work was supported in part by National Research Service Award EY06813 (to M.C.C.), grant EY08289 (to M.S.G.), and an unrestricted grant from Research to Prevent Blindness, Inc., New York, N.Y.
REFERENCES
- 1.Affeldt J C, Flynn H W, Forster R K, Mandelbaum S, Clarkson J G, Jarus G D. Microbial endophthalmitis resulting from ocular trauma. Ophthalmology. 1987;94:407–413. doi: 10.1016/s0161-6420(87)33447-5. [DOI] [PubMed] [Google Scholar]
- 2.Akesson A, Hedstrom S A, Ripa T. Bacillus cereus: a significant pathogen in postoperative and post-traumatic wounds on orthopaedic wards. Scand J Infect Dis. 1991;23:71–77. doi: 10.3109/00365549109023377. [DOI] [PubMed] [Google Scholar]
- 3.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 4.Beecher D J, Macmillan J D. Characterization of the components of hemolysin BL from Bacillus cereus. Infect Immun. 1991;59:1778–1784. doi: 10.1128/iai.59.5.1778-1784.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beecher D J, Pulido J S, Barney N P, Wong A C L. Extracellular virulence factors in Bacillus cereus endophthalmitis: methods and implication of involvement of hemolysin BL. Infect Immun. 1995;63:632–639. doi: 10.1128/iai.63.2.632-639.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beecher D J, Wong A C L. Tripartite hemolysin BL from Bacillus cereus: hemolytic analysis of component interactions and a model for its characteristic paradoxical zone phenomenon. J Biol Chem. 1997;272:233–239. doi: 10.1074/jbc.272.1.233. [DOI] [PubMed] [Google Scholar]
- 7.Boldt H C, Pulido J S, Blodi C F, Folk J C, Weingeist T A. Rural endophthalmitis. Ophthalmology. 1989;96:1722–1726. doi: 10.1016/s0161-6420(89)32658-3. [DOI] [PubMed] [Google Scholar]
- 8.Booth M C, Atkuri R V, Nanda S K, Iandolo J J, Gilmore M S. Accessory gene regulator controls Staphylococcus aureus virulence in endophthalmitis. Invest Ophthalmol Visual Sci. 1995;36:1828–1836. [PubMed] [Google Scholar]
- 9.Booth M C, Cheung A L, Hatter K L, Jett B D, Callegan M C, Gilmore M S. Staphylococcal accessory regulator (sar) in conjunction with agr contributes to Staphylococcus aureus virulence in endophthalmitis. Infect Immun. 1997;65:1550–1556. doi: 10.1128/iai.65.4.1550-1556.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Callegan M C, Booth M C, Jett B D, Gilmore M S. Pathogenesis of gram-positive bacterial endophthalmitis. Infect Immun. 1999;67:3348–3356. doi: 10.1128/iai.67.7.3348-3356.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cowan C L, Madden W M, Hatem G F, Merritt J C. Endogenous Bacillus cereus panophthalmitis. Ann Ophthalmol. 1987;19:65–68. [PubMed] [Google Scholar]
- 12.Davey R T, Tauber W B. Post-traumatic endophthalmitis: the emerging role of Bacillus cereus infection. Rev Infect Dis. 1987;9:110–123. doi: 10.1093/clinids/9.1.110. [DOI] [PubMed] [Google Scholar]
- 13.Drobniewski F A. Bacillus cereus and related species. Clin Microbiol Rev. 1993;6:324–338. doi: 10.1128/cmr.6.4.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilmore M S, Cruz-Rodz A L, Leimeister-Wachter M, Kreft J, Goebel W. A Bacillus cereus cytolytic determinant, cereolysin AB, which comprises the phospholipase C and sphingomyelinase genes: nucleotide sequence and genetic linkage. J Bacteriol. 1989;171:744–753. doi: 10.1128/jb.171.2.744-753.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gutierrez J A, Crowley P J, Brown D P, Hillman J D, Youngman P, Bleiweis A S. Insertional mutagenesis and recovery of interrupted genes of Streptococcus mutans by using transposon Tn917: preliminary characterization of mutants displaying acid sensitivity and nutritional requirements. J Bacteriol. 1996;178:4166–4175. doi: 10.1128/jb.178.14.4166-4175.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heinrichs J H, Beecher D J, Macmillan J D, Zilinskas B A. Molecular cloning and characterization of the hblA gene encoding the B component of hemolysin BL from Bacillus cereus. J Bacteriol. 1993;175:6760–6766. doi: 10.1128/jb.175.21.6760-6766.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ho P C, O’Day D M, Head W S. Fulminating panophthalmitis due to exogenous infection with Bacillus cereus: a report of 4 cases. Br J Ophthalmol. 1982;66:205–208. doi: 10.1136/bjo.66.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jett B D, Jensen H G, Nordquist R E, Gilmore M S. Contribution of the pAD1-encoded cytolysin to the severity of experimental Enterococcus faecalis endophthalmitis. Infect Immun. 1992;60:2445–2452. doi: 10.1128/iai.60.6.2445-2452.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jett B D, Parke D W, Booth M C, Gilmore M S. Host/parasite interactions in bacterial endophthalmitis. Zentrbl Bakteriol. 1997;285:341–367. doi: 10.1016/s0934-8840(97)80002-3. [DOI] [PubMed] [Google Scholar]
- 20.Jett B D, Hatter K L, Huycke M M, Gilmore M S. Simplified agar plate method for quantifying viable bacteria. BioTechniques. 1997;23:648–650. doi: 10.2144/97234bm22. [DOI] [PubMed] [Google Scholar]
- 21.Marley E F, Saini N K, Venkatraman C, Orenstein J M. Fatal Bacillus cereus meningoencephalitis in an adult with acute myelogenous leukemia. South J Med. 1995;88:969–972. doi: 10.1097/00007611-199509000-00017. [DOI] [PubMed] [Google Scholar]
- 22.Masson L, Prefontaine G, Brousseau R. Transformation of Bacillus thuringiensis vegetative cells by electroporation. FEMS Microbiol Lett. 1989;60:273–278. doi: 10.1016/0378-1097(89)90409-6. [DOI] [PubMed] [Google Scholar]
- 23.Meredith F T, Fowler V G, Gautier M, Corley G R, Reller L B. Bacillus cereus necrotizing cellulitis mimicking clostridial myonecrosis: case report and review of the literature. Scand J Infect Dis. 1997;29:528–529. doi: 10.3109/00365549709011872. [DOI] [PubMed] [Google Scholar]
- 24.Miller J M, Hair J G, Hebert M, Hebert L, Roberts F J. Fulminating bacteremia and pneumonia due to Bacillus cereus. J Clin Microbiol. 1997;35:504–507. doi: 10.1128/jcm.35.2.504-507.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Day D M, Smith R S, Gregg C R, Turnbull P C B, Head W S, Ives J A, Ho P C. The problem of Bacillus species infection with special emphasis on the virulence of Bacillus cereus. Ophthalmology. 1981;88:833–838. doi: 10.1016/s0161-6420(81)34960-4. [DOI] [PubMed] [Google Scholar]
- 26.Park S S, Samiy N, Ruoff K, D’Amico D J, Baker A S. Effect of intravitreal dexamethasone in treatment of pneumococcal endophthalmitis in rabbits. Arch Ophthalmol. 1995;113:1324–1329. doi: 10.1001/archopht.1995.01100100112040. [DOI] [PubMed] [Google Scholar]
- 27.Peyman G A, Pague J T, Meisels H I, Bennett T O. Postoperative endophthalmitis: a comparison of methods for treatment and prophylaxis with gentamicin. Ophthalmic Surg. 1975;6:45–55. [PubMed] [Google Scholar]
- 28.Ryan P A, Macmillan J D, Zilinskas B A. Molecular cloning and characterization of the genes encoding the L1 and L2 components of hemolysin BL from Bacillus cereus. J Bacteriol. 1997;179:2551–2556. doi: 10.1128/jb.179.8.2551-2556.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 30.Schemmer G R, Driebe W T. Post-traumatic Bacillus cereus endophthalmitis. Arch Ophthalmol. 1987;105:342–344. doi: 10.1001/archopht.1987.01060030062026. [DOI] [PubMed] [Google Scholar]
- 31.Schutte B C, Ranade K, Pruessner J, Dracopoli N. Optimized conditions for cloning PCR products into an XcmI T-vector. BioTechniques. 1997;22:40–44. doi: 10.2144/97221bm06. [DOI] [PubMed] [Google Scholar]
- 32.Shamsuddin D, Tuazon C U, Levy C, Curtin J. Bacillus cereus panophthalmitis: source of the organism. Rev Infect Dis. 1982;4:97–103. doi: 10.1093/clinids/4.1.97. [DOI] [PubMed] [Google Scholar]
- 33.Sheehan D C, Hrapchak B B. Theory and practice of histotechnology. Columbus, Ohio: Battelle Press; 1987. [Google Scholar]
- 34.Sliman R, Rehm S, Shlaes D M. Serious infections caused by Bacillus species. Medicine. 1987;66:218–223. doi: 10.1097/00005792-198705000-00005. [DOI] [PubMed] [Google Scholar]
- 35.Tao L, LeBlanc D J, Ferretti J J. Novel streptococcal-integration shuttle vectors for gene cloning and inactivation. Gene. 1992;120:105–110. doi: 10.1016/0378-1119(92)90016-i. [DOI] [PubMed] [Google Scholar]
- 36.Thompson S T, Parver L M, Enger C L, Meiler W F, Liggett P E. Infectious endophthalmitis after penetrating injuries with retained intraocular foreign bodies. Ophthalmology. 1993;100:1468–1474. doi: 10.1016/s0161-6420(93)31454-5. [DOI] [PubMed] [Google Scholar]
- 37.Tuazon C U, Murray H W, Levy C, Solny M N, Curtin J A, Sheagren J N. Serious infections from Bacillus sp. JAMA. 1979;241:1137–1140. [PubMed] [Google Scholar]
- 38.Turnbull P C B. Bacillus cereus toxins. Pharmacol Ther. 1981;13:453–505. doi: 10.1016/0163-7258(81)90026-7. [DOI] [PubMed] [Google Scholar]
- 39.Turnbull P C B, Jorgensen K, Kramer J M, Gilbert R J, Parry J M. Severe clinical conditions associated with Bacillus cereus and the apparent involvement of exotoxins. J Clin Pathol. 1979;32:289–293. doi: 10.1136/jcp.32.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turnbull P C B, Kramer J M. Non-gastrointestinal Bacillus cereus infections: an analysis of exotoxin production by strains isolated over a two-year period. J Clin Pathol. 1983;36:1091–1096. doi: 10.1136/jcp.36.10.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Williams D R, Meiler W F, Abrams G W, Lewis H. Results and prognostic factors in penetrating ocular injuries with retained intraocular foreign bodies. Ophthalmology. 1988;95:911–916. doi: 10.1016/s0161-6420(88)33069-1. [DOI] [PubMed] [Google Scholar]