Abstract
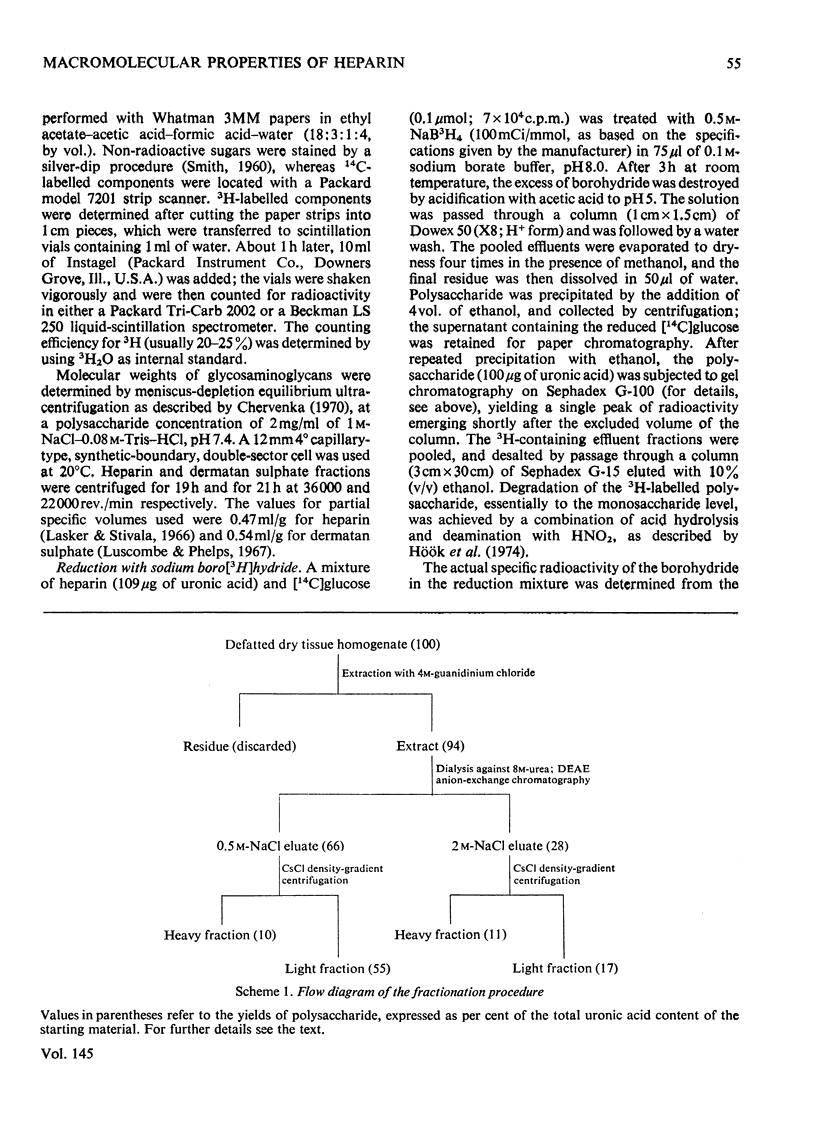
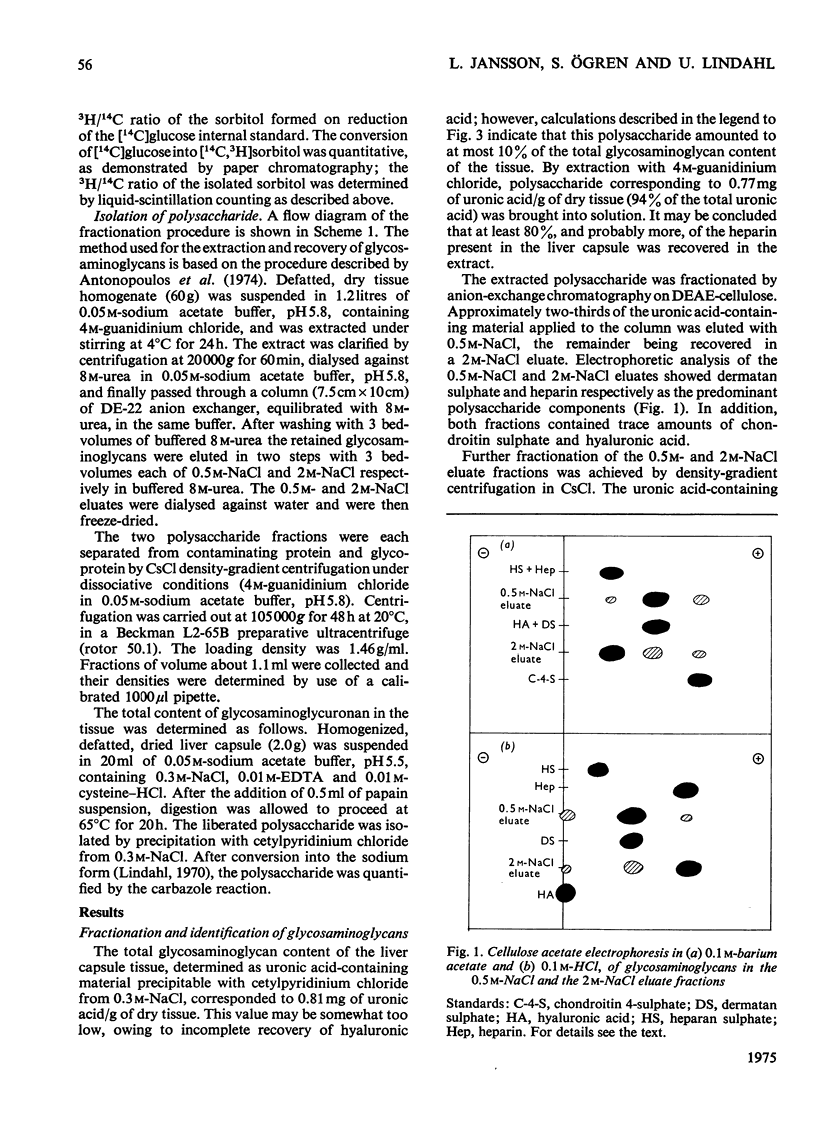
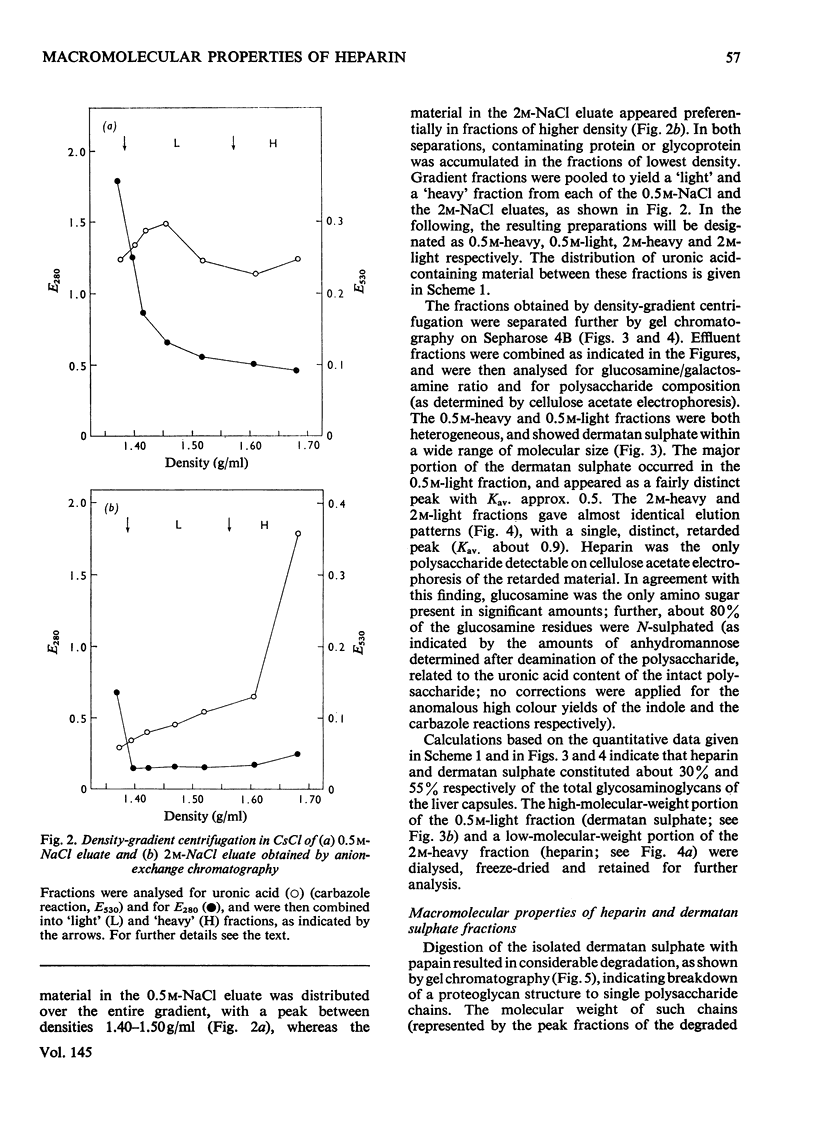
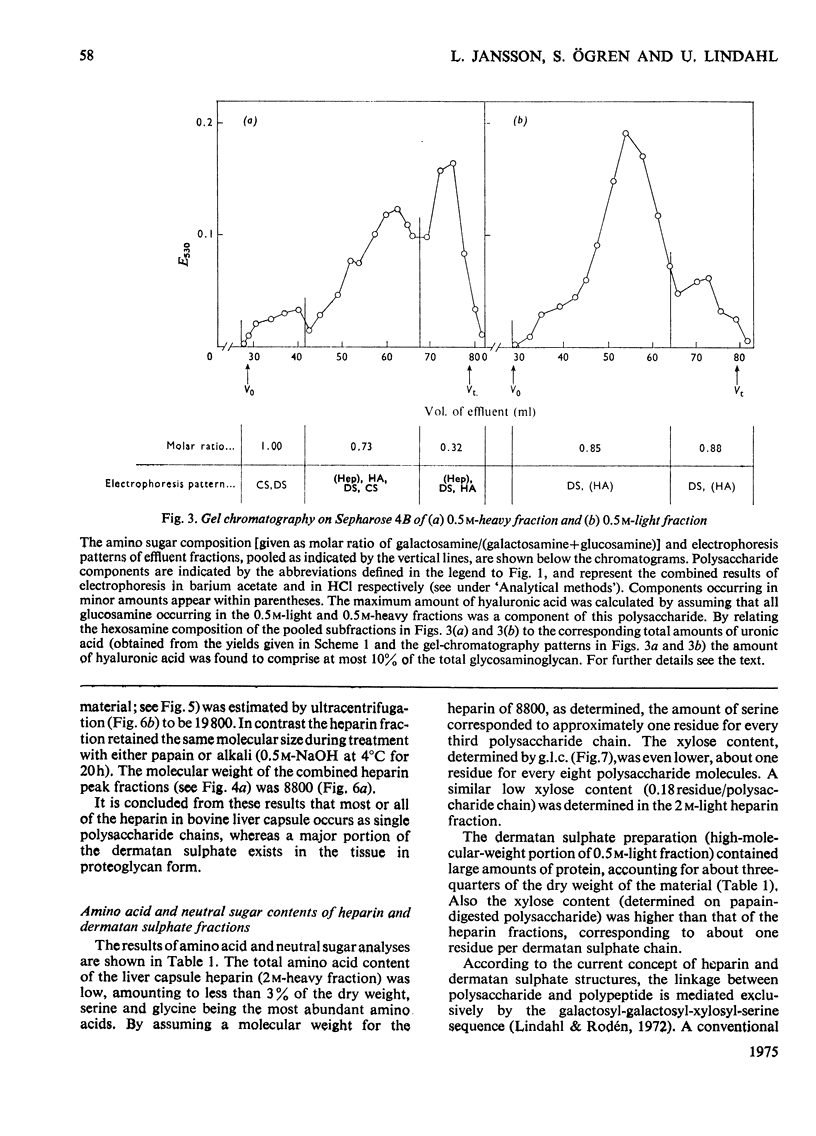
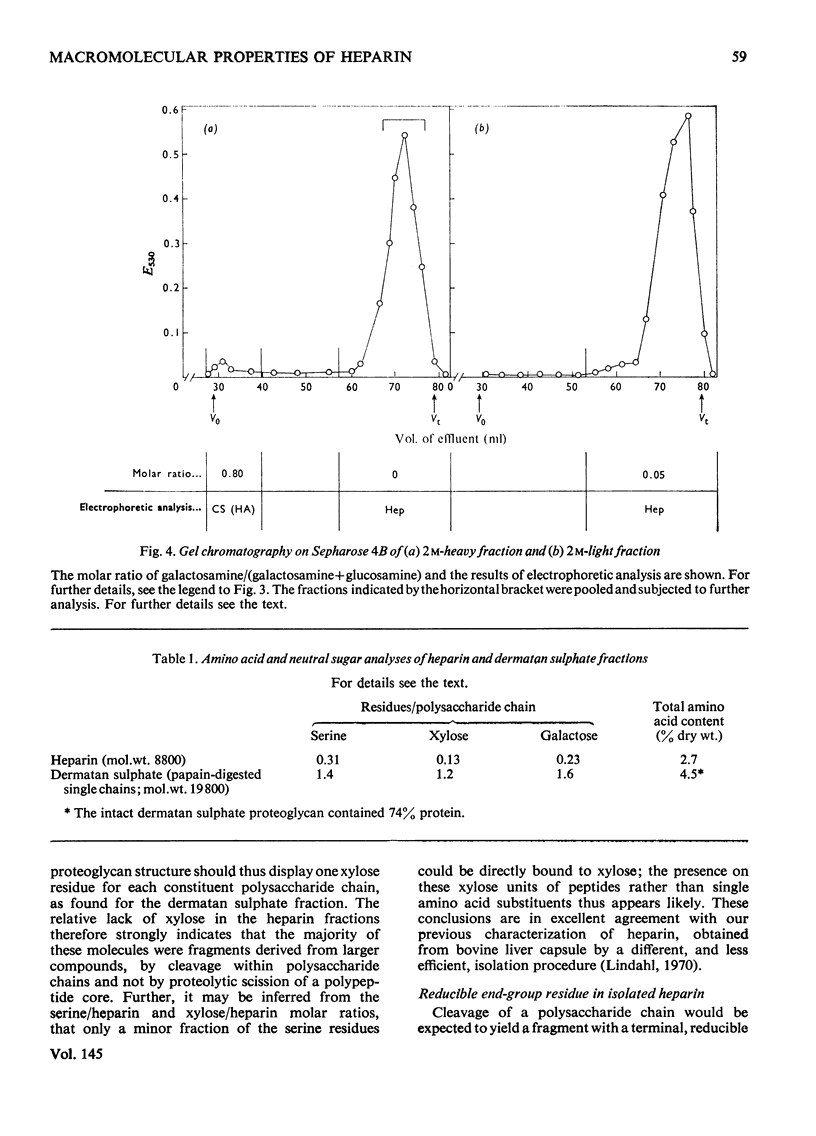
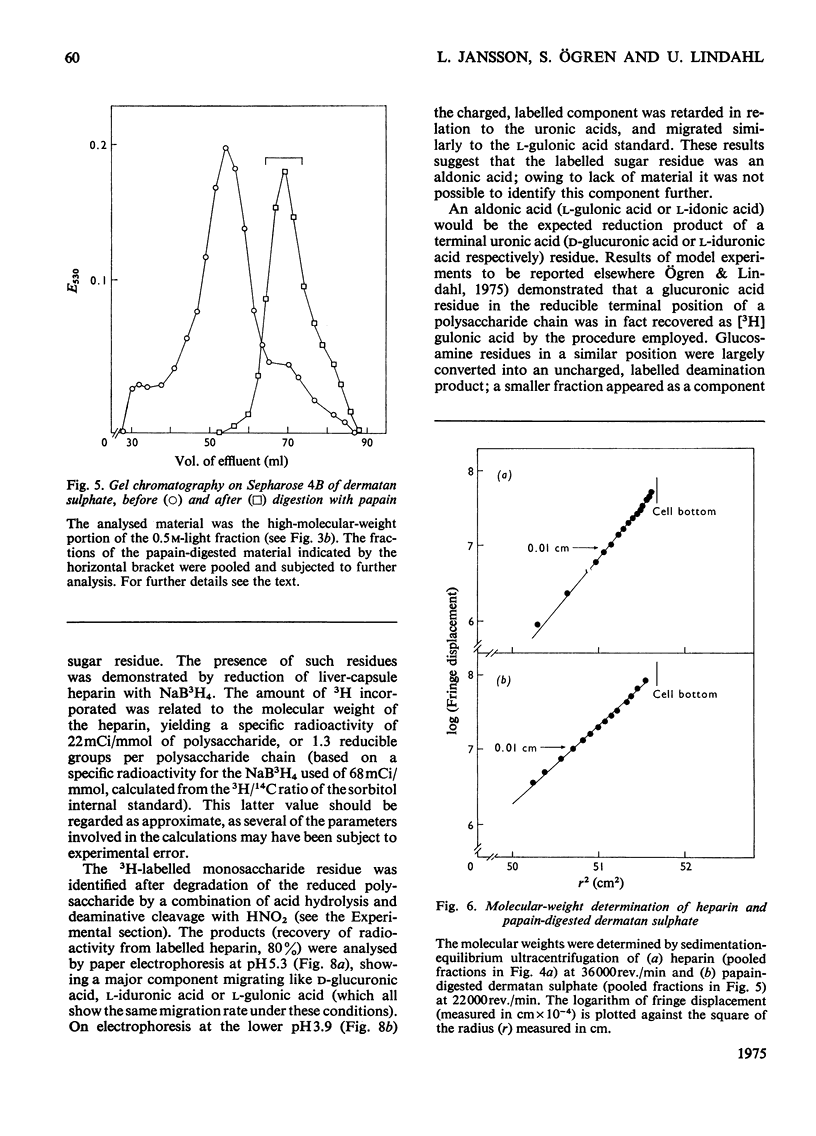
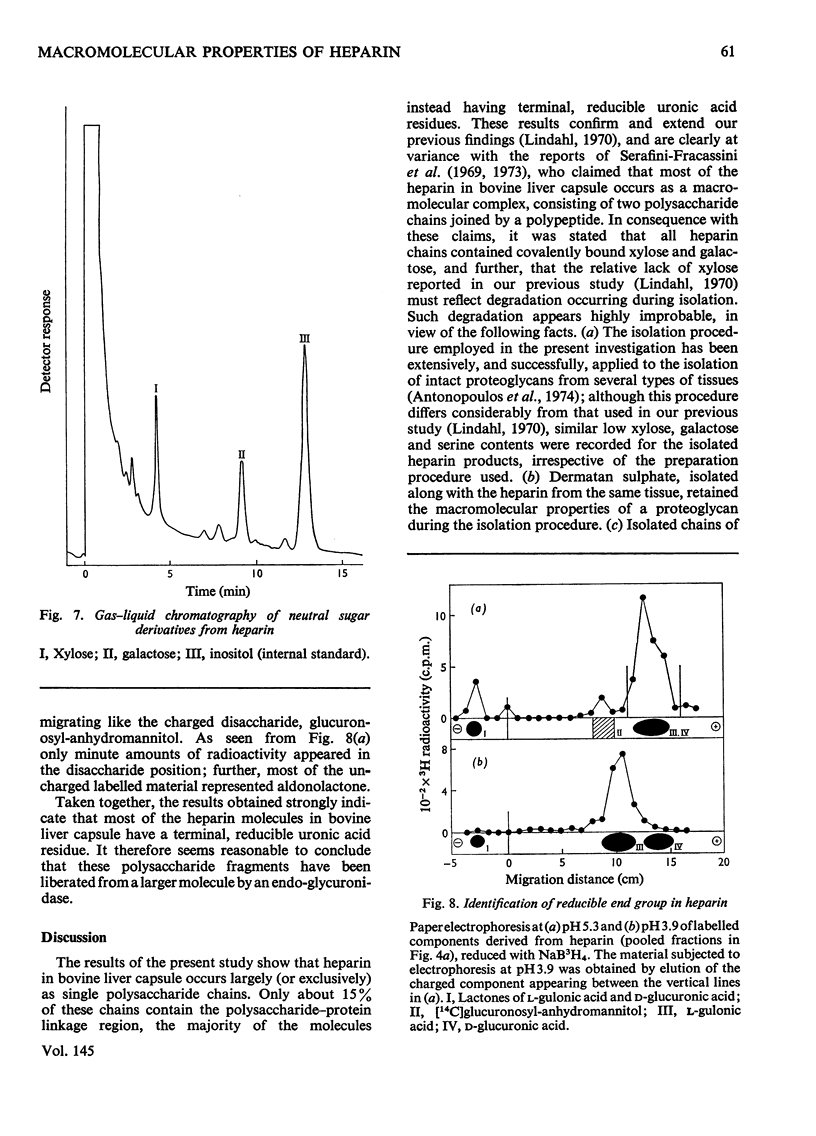
Glycosaminoglycans were extracted from bovine liver capsule with 4 M-guanidinium chloride, resulting in solubilization of approx. 90% of the total uronic acid-containing polysaccharide of the tissue. The extracted polysaccharide was purified and fractionated by anion-exchange chromatography on DEAE-cellulose, density-gradient ultracentrifugation in CsCl and finally gel chromatography on Sepharose 4B. By using these procedures, the two major polysaccharide components, dermatan sulphate and heparin, which constituted 55 and 30% respectively of the total glycosaminoglycan content of the tissue, were separated from each other. Analysis of the macromolecular properties of the two polysaccharides showed that heparin existed exclusively as single polysaccharide chains, whereas dermatan sulphate occurred largely as a proteoglycan (protein content, 74% dry wt.). The purified heparin preparation was subjected to sedimentation-equilibrium ultracentrifugation, indicating a molecular weight of 8800. Analysis for neutral sugars (by g.l.c.) showed 0.1 residue of xylose and 0.2 residue of galactose/polysaccharide chain; serine amounted to 0.3 residue/polysaccharide chain. Reduction of the heparin with NaB3H4 resulted in incorporation of 3H, approximately corresponding to one reducible group/polysaccharide chain. The 3H-labelled sugar residue was liberated by a combination of acid hydrolysis and deaminative cleavage of the polysaccharide with HNO2; it was subsequently identified as an aldonic acid by paper electrophoresis. Most of the heparin chains thus contained a uronic acid residue in reducing position. It is suggested that heparin isolated from bovine liver capsule is a degradation product released from larger molecules by an endo-glycuronidase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Chervenka C. H. Long-column meniscus depletion sedimentation equilibrium technique for the analytical ultracentrifuge. Anal Biochem. 1970 Mar;34:24–29. doi: 10.1016/0003-2697(70)90082-5. [DOI] [PubMed] [Google Scholar]
- Horner A. A. Enzymic depolymerization of macromolecular heparin as a factor in control of lipoprotein lipase activity. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3469–3473. doi: 10.1073/pnas.69.11.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner A. A. Macromolecular heparin from rat skin. Isolation, characterization, and depolymerization with ascorbate. J Biol Chem. 1971 Jan 10;246(1):231–239. [PubMed] [Google Scholar]
- Hök M., Lindahl U., Iverius P. H. Distribution of sulphate and iduronic acid residues in heparin and heparan sulphate. Biochem J. 1974 Jan;137(1):33–43. doi: 10.1042/bj1370033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson L., Lindahl U. Evidence for the existence of a multichain proteoglycan of heparan sulphate. Biochem J. 1970 May;117(4):699–702. doi: 10.1042/bj1170699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIMMEL J. R., SMITH E. L. Crystalline papain. I. Preparation, specificity, and activation. J Biol Chem. 1954 Apr;207(2):515–531. [PubMed] [Google Scholar]
- Koeltzow D. E., Epley J. D., Conrad H. E. The lipopolysaccharides of Aerobacter aerogenes strains A3(S1) and NCTC 243. Biochemistry. 1968 Aug;7(8):2920–2928. doi: 10.1021/bi00848a032. [DOI] [PubMed] [Google Scholar]
- Kraemer P. M., Smith D. A. High molecular-weight heparan sulfate from the cell surface. Biochem Biophys Res Commun. 1974 Jan 23;56(2):423–430. doi: 10.1016/0006-291x(74)90859-6. [DOI] [PubMed] [Google Scholar]
- LINDAHL U., CIFONELLI J. A., LINDAHL B., RODEN L. THE ROLE OF SERINE IN THE LINKAGE OF HEPARIN TO PROTEIN. J Biol Chem. 1965 Jul;240:2817–2820. [PubMed] [Google Scholar]
- Lasker S. E., Stivala S. S. Physicochemical studies of fractionated bovine heparin. I. Some dilute solution properties. Arch Biochem Biophys. 1966 Aug;115(2):360–372. doi: 10.1016/0003-9861(66)90286-4. [DOI] [PubMed] [Google Scholar]
- Lindahl U. Attempted isolation of a heparin proteoglycan from bovine liver capsule. Biochem J. 1970 Jan;116(1):27–34. doi: 10.1042/bj1160027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl U., Bäckström G., Jansson L., Hallén A. Biosynthesis of heparin. II. Formation of sulfamino groups. J Biol Chem. 1973 Oct 25;248(20):7234–7241. [PubMed] [Google Scholar]
- Lloyd A. G., Bloom G. D., Balazs E. A., Haegermark O. Combined biochemical and morphological ultrastructure studies on mast-cell granules. Biochem J. 1967 Jun;103(3):75P–76P. [PMC free article] [PubMed] [Google Scholar]
- Lohmander S. Ion exchange chromatography of glucosamine and galactosamine in microgram amounts with quantitative determination and specific radioactivity assay. Biochim Biophys Acta. 1972 May 16;264(3):411–417. doi: 10.1016/0304-4165(72)90003-7. [DOI] [PubMed] [Google Scholar]
- Luscombe M., Phelps C. F. Action of degradative enzymes on the light fraction of bovine septa protein polysaccharide. Biochem J. 1967 Apr;103(1):103–109. doi: 10.1042/bj1030103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogren S., Lindahl U. Degradation of heparin in mouse mastocytoma tissue. Biochem J. 1971 Dec;125(4):1119–1129. doi: 10.1042/bj1251119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini-Fracassini A., Durward J. J., Floreani L. The heparin-protein complex of ox liver capsule. Isolation and chemical characterization. Biochem J. 1969 Apr;112(2):167–172. doi: 10.1042/bj1120167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini-Fracassini A., White C. J., Hunter J. C. The characterisation of heparin in bovine liver capsule. FEBS Lett. 1973 May 15;32(1):116–118. doi: 10.1016/0014-5793(73)80751-3. [DOI] [PubMed] [Google Scholar]
- Wessler E. Analytical and preparative separation of acidic glycosaminoglycans by electrophoresis in barium acetate. Anal Biochem. 1968 Dec;26(3):439–444. doi: 10.1016/0003-2697(68)90205-4. [DOI] [PubMed] [Google Scholar]
- Wessler E. Electrophoresis of acidic glycosaminoglycans in hydrochloric acid: a micro method for sulfate determination. Anal Biochem. 1971 May;41(1):67–69. doi: 10.1016/0003-2697(71)90192-8. [DOI] [PubMed] [Google Scholar]


