Abstract
Supraspinous fossa is an important location in the periscapular region, which houses important structures such as the supraspinatus muscle and the suprascapular nerve. The supraspinous fossa can be affected by pathologies involving its contents (supraspinatus muscle and suprascapular nerve), osseous boundary (scapular body, distal clavicle, and spinous process), or superficial soft tissue covering it. In this pictorial review, we describe the detailed anatomy of the supraspinous fossa. We have also covered imaging of wide range of pathologies that can affect supraspinous fossa such as paralabral cyst, muscle edema/atrophy, malignancies (primary and secondary), and miscellaneous lesions (myositis ossificans, fibromatosis, nerve sheath tumor, etc.). An awareness of the imaging findings of these entities is essential for a radiologist to avoid misinterpretation and can aid a timely diagnosis.
Keywords: supraspinatus muscle, suprascapular nerve, ultrasonography, MRI
Introduction
Supraspinous fossa is an important location in the periscapular region, which houses important structures such as the supraspinatus muscle and the suprascapular nerve. Supraspinatus muscle atrophy, due to chronic tendon tear or any other reason, will lead to concavity or depression in this region. The supraspinous fossa can be affected by pathologies involving its contents, osseous boundary, or superficial soft tissue covering it. In this pictorial review, we describe the detailed anatomy of the supraspinous fossa with imaging of various pathologies affecting it. To the best of our knowledge, an imaging review of supraspinous fossa has not been published to date.
Imaging Anatomy
The supraspinous fossa, also known as the supraspinatous fossa, is a concave bony cavity in the posterior aspect of the scapula bordered by the spine of the scapula inferiorly, acromion process laterally, and superior angle of the scapula cranially. It is broader medially and narrower laterally. The medial two-thirds of this fossa give origin to the supraspinatus muscle ( Fig. 1 ). The suprascapular notch, located on the superior border of scapula, separates it from the coracoid process. The superior transverse scapular ligament covers the notch and attaches to the base of the coracoid process, converting the notch into a foramen. The suprascapular notch has been classified into six types by Rengachary et al with a completely ossified superior transverse scapular ligament noted in type VI ( Fig. 2 ). 1 The spinoglenoid notch is located between the lateral border of the scapula spine and the glenoid and is a connection between the supraspinous fossa and the infraspinous fossa. The spinoglenoid ligament covers the notch and converts it into foramen. 2
Fig. 1.
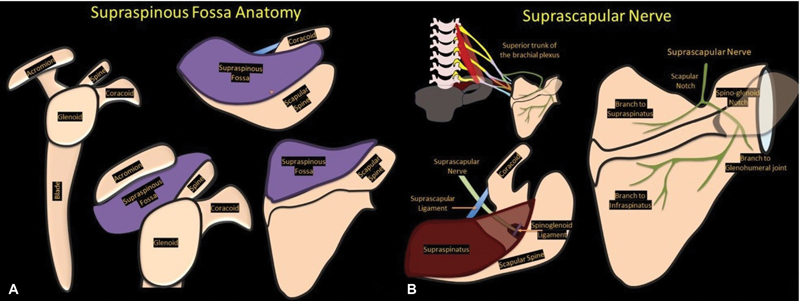
Graphic showing ( A ) supraspinous fossa anatomy and ( B ) the course of suprascapular nerve.
Fig. 2.
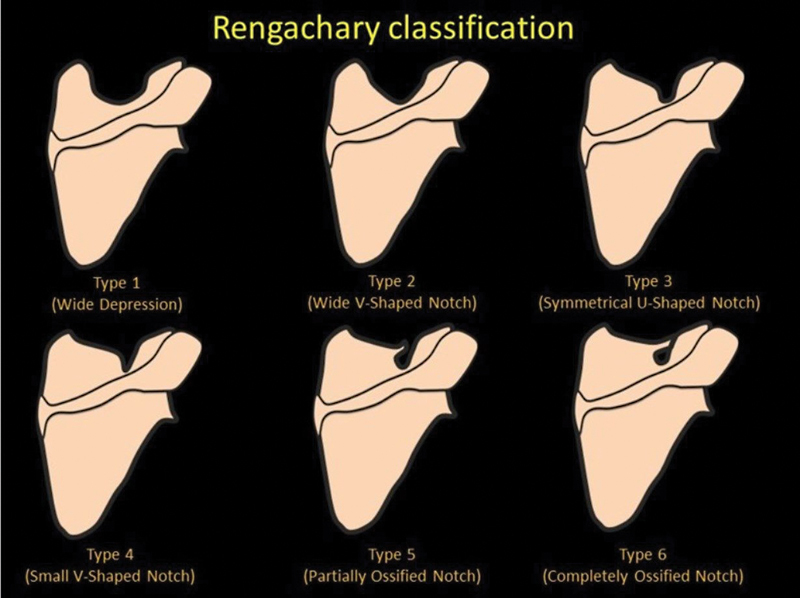
Graphic showing Rengachary classification.
The suprascapular nerve passes through the suprascapular notch while coursing over the scapula to reach the supraspinous fossa. It is accompanied by the suprascapular artery and vein. The suprascapular nerve passes through the foramen, while the vessels pass superficial to the ligament. The nerve then passes deep to the supraspinatus muscle and gives off two branches to it before passing through the spinoglenoid notch to pass into the infraspinous fossa, where it provides two terminal branches to the infraspinatus muscle. A superior labral tear can give rise to paralabral cyst formation, which can extend into suprascapular notch or spinoglenoid notch and can cause compression of the suprascapular nerve. A predictable pattern of muscle denervation can be seen depending on the location of the compression: supraspinatus and infraspinatus muscles with compression at the suprascapular notch, while only the infraspinatus is affected with compression at the spinoglenoid notch. 3
The supraspinous fossa can be imaged with the help of conventional plain radiography, ultrasonography, computed tomography (CT), or magnetic resonance imaging (MRI). Conventional radiography can only give information about the bony outline, presence of calcification or ossification. Ultrasonography is useful in evaluating the supraspinous fossa because of its superficial location. It is a quick and practical modality with no radiation exposure. CT is rarely needed and involves radiation. MRI is the best modality for the evaluation of the musculoskeletal system because of its three-dimensional capability and high spatial resolution, offering unparalleled tissue contrast.
Supraspinatus Muscle
The supraspinatus muscle is one of the rotator cuff muscles. It originates from the supraspinous fossa of the scapula and inserts over the superior facet of the greater tubercle of the humerus. Its tendon passes underneath the acromion process and blends with the glenohumeral joint capsule. It is innervated by the suprascapular nerve. Its functions include abduction of the arm, pulling the head of the humerus medially toward the glenoid cavity, and preventing the head of the humerus from slipping inferiorly. It works in coordination with the deltoid muscle during abduction of the arm. 2 Its tendon is the most frequently damaged tendon of the rotator cuff, including acute injury or chronic degeneration. Impingement from the acromion process is often a contributing factor. Both ultrasound and MRI can be used to evaluate the supraspinatus muscle and tendon. Normal muscle on ultrasonography appears as a hypoechoic structure with echogenic fibrous tissue, giving a starry sky appearance, whereas normal tendon appears as a fibrillar echogenic structure. 4 As muscle undergoes atrophy, it becomes more echogenic with loss of pennate pattern, and the volume also decreases. Muscle edema is relatively difficult to evaluate on ultrasound. MRI is an excellent modality for evaluating the supraspinatus muscle and tendon. Normal muscle on proton density-weighted sequence appears gray (intermediate signal intensity). A T2 hyperintense signal will be seen if there is muscle edema, whereas atrophy will give rise to a signal pattern equaling fat on the T1-weighted sequence along with a decrease in volume ( Fig. 3 ). Normal tendon appears as a hypointense structure because of tightly packed fibers. 5
Fig. 3.
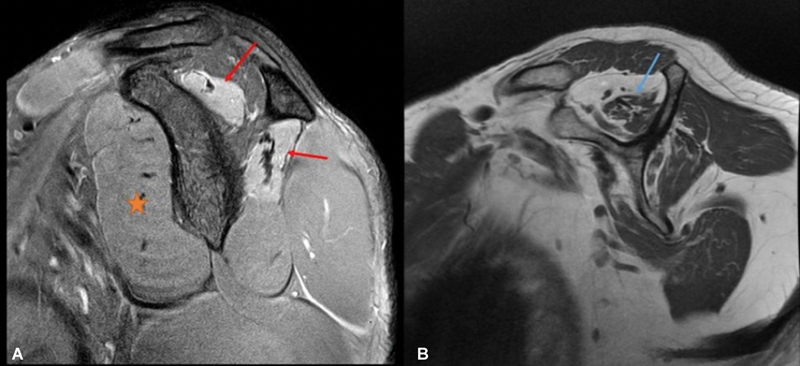
Muscle magnetic resonance imaging showing ( A ) normal (orange star) muscle with intermediate signal intensity on proton density-weighted sagittal sequence. Note that supraspinatus and infraspinatus muscles show increase signal intensity suggesting edema along with volume loss (red arrows in A ), and ( B ) T1-weighted sagittal sequence showing fatty atrophy of supraspinatus muscle (blue arrow).
Suprascapular Nerve
The suprascapular nerve originates from the ventral rami of the C5 and C6 and courses through the superior border of the scapula into the suprascapular notch and then spinoglenoid notch. The suprascapular nerve provides motor innervation to the supraspinatus and infraspinatus muscles and receives sensory innervation from the shoulder. 2 For evaluating suprascapular nerve pathologies, electromyography and nerve conduction studies are useful. However, ultrasonography and MRI can be used to visualize pathological entities in the soft and bony tissues surrounding the nerve, which can be useful to surgeons. On ultrasonography, a normal nerve appears as a fascicular structure that can be traced along its length. The suprascapular nerve can be easily identified in the suprascapular notch and spinoglenoid notch, and as it is accompanied by the suprascapular artery and vein, Doppler can help in localizing the nerve. On MRI, the suprascapular nerve can be assessed on T1 or proton density or heavily T2-weighted sequence. T1-weighted sequences are good for visualizing normal anatomy and the course of the nerve. Fatty infiltration of muscles is also best evaluated on the T1-weighted sequence. MR neurography involves typically heavy T2-weighted sequences, allowing the high-signal nerves to stand out from the darker fat-suppressed background soft tissues. With a wider field of view, MR neurography can help in evaluating the nerve along its complete course right from its origin at the cervical spinal nerve roots ( Fig. 4 ). 6 7
Fig. 4.
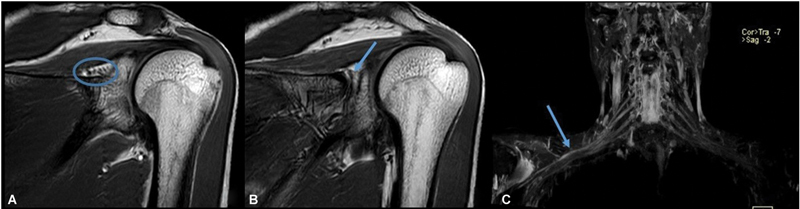
Magnetic resonance (MR) imaging showing normal suprascapular nerve in suprascapular notch (circle in A) and spinoglenoid notch (arrow in B), and MR neurography showing high-signal nerves (arrow in C) standing out from the darker fat-suppressed background soft tissues.
Pathologies
The supraspinous fossa can be affected by pathologies affecting its contents as well as its osseous boundary and superficial soft tissue covering it. Table 1 shows the list of pathologies affecting supraspinous fossa.
Table 1. Pathologies affecting supraspinous fossa.
| 1 | Pathologies affecting supraspinatus muscles | (a) Muscle atrophy (b) Delaminating tear with cyst (c) Denervation muscle edema (d) Parsonage–Turner's syndrome |
| 2 | Pathologies affecting suprascapular nerve (suprascapular notch) | (a) Idiopathic (b) Space occupying lesion such as paralabral cyst |
| 3 | Pathologies affecting scapular body, distal clavicle, and spinous process | (a) Tumor (b) Trauma |
| 4 | Glenohumeral pathology extending into supraspinous fossa | (a) Infective arthritis with extension of abscess (b) Paralabral cyst (c) Ganglion cyst (d) Synovial cyst |
| 5 | Acromioclavicular joint pathology extending into supraspinous fossa | (a) Ganglion cyst (b) Synovial cyst |
| 6 | Miscellaneous | (a) Soft tissue tumor (b) Hemangioma (c) Hematoma (d) Myositis ossificans (e) Fibromatosis (f) Myocysticercosis |
Supraspinatus Muscle
The supraspinatus muscle can be affected by a wide variety of pathologies ranging from trauma, infection, and denervation to tumors. The most frequent condition affecting the supraspinatus muscle is its involvement secondary to tendon degeneration and tear. 8 Tendon tears can range from partial thickness and partial width tears to full thickness, complete width tears. Disuse of the affected shoulder can affect muscle bulk and health. With chronicity, gradual atrophy of the supraspinatus muscle is a frequent finding. Fatty atrophy of the supraspinatus muscle can be assessed using ultrasonography or MRI. On ultrasound, the atrophic muscle will have reduced bulk and appear more echogenic with loss of pennate pattern. Khoury et al found a good correlation between ultrasound and MRI in the assessment of supraspinatus muscle atrophy and fatty infiltration. 9 There are different grading systems used in assessing supraspinatus atrophy on ultrasound and MRI. On MRI, Thomazeau et al proposed a method based on calculation of occupation ratio of supraspinatus muscle. The occupation ratio is the ratio between the cross section of the muscle belly and that of its fossa on the Y view. A ratio between 1 and 0.6 is considered as grade I, 0.4 to 0.6 as grade II, and less than 0.4 as grade III, with atrophy increasing from I to III. 10 Another method described by Goutallier et al is based on subjectively grading the proportion of fat in the supraspinatus muscle on the sagittal T1-weighted images. Using this method, grade 0 stands for normal muscle; grade 1: presence of fatty streaks; grade 2: muscle more than fat; grade 3: fat equals muscle; and grade 4: fat more than muscle 11 ( Fig. 5 ). Zanetti et al described a tangent sign for identifying supraspinatus muscle atrophy. On the Y view of a sagittal MRI, a normal supraspinatus muscle should cross superior to a line drawn through the superior borders of the scapular spine and the superior margin of the coracoid process. This finding is not present with atrophy 12 ( Fig. 6 ). On ultrasound, Khoury et al proposed a method similar to the previously described occupation ratio. Another method described by the authors on ultrasound is qualitative, based on comparison of echogenicity of the supraspinatus with that of the trapezius muscle. 9 Another pathology that can be secondary to tendon tear is cyst formation. It is seen in cases of delaminating tears extending up to the myotendinous junction with formation of intramuscular cyst. 13
Fig. 5.
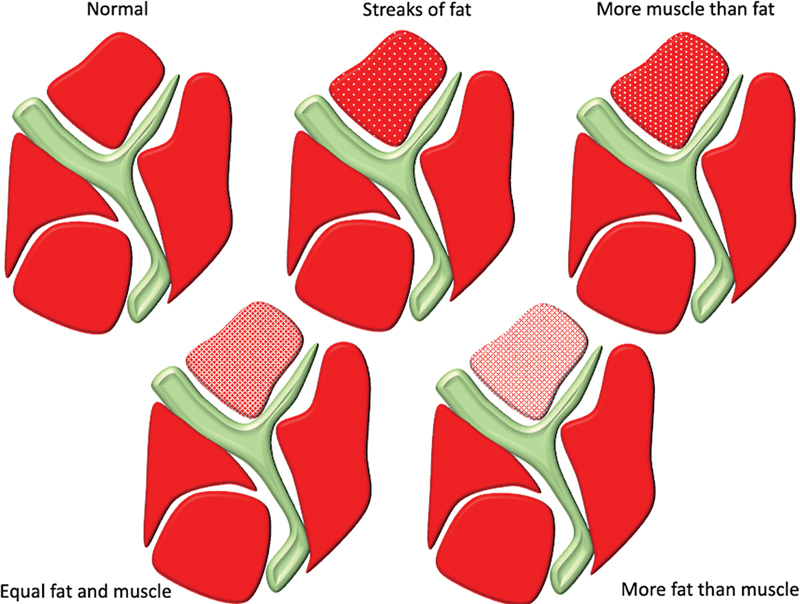
Goutallier classification of fatty atrophy of muscle.
Fig. 6.
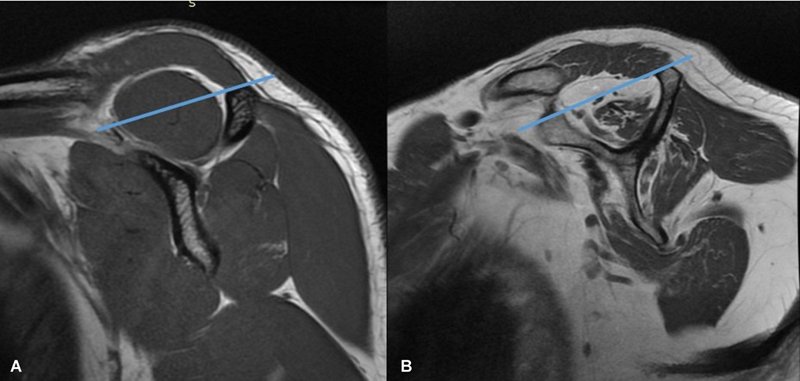
Sagittal magnetic resonance imaging (Y view) showing “tangent sign” for supraspinatus fatty atrophy; note that normal muscle ( A ) crosses the tangent, whereas atrophic muscle ( B ) does not cross it.
Denervation of the supraspinatus is generally due to involvement of suprascapular nerve in the suprascapular notch or proximal to it. Parsonage–Turner's syndrome, an idiopathic viral neuritis affecting the brachial plexus, can affect the suprascapular nerve resulting in shoulder pain and weakness ( Fig. 7 ). 14 Superior labral tears can give rise to paralabral cyst formation, which can extend into the suprascapular notch causing compression of the nerve and subsequent muscle denervation. In the acute phase, edema will be seen on T2 fat-suppressed sequences in the denervated muscle. Chronic pathology causes muscle atrophy.
Fig. 7.
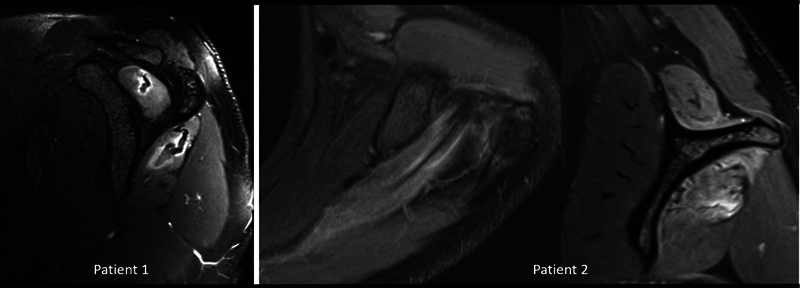
Parsonage–Turner's syndrome in two different patients showing edema in supraspinatus and infraspinatus muscles.
Suprascapular Nerve
Suprascapular nerve pathology is a rare diagnosis that is increasingly being recognized among the conditions that cause shoulder pain and dysfunction. Along its course, a wide variety of mechanisms can cause injury or compression. The nerve can be compressed or injured in the suprascapular notch and the spinoglenoid notch. As described previously, several anatomical variations of the suprascapular notch can cause compression of the nerve. Fractures of the suprascapular notch, or scar tissue around healing distal clavicle fracture can cause nerve entrapment symptoms. A hypertrophied inferior transverse scapular ligament and enlarged veins have been found as predisposing factors for nerve compression in the spinoglenoid notch leading to selective atrophy of the infraspinatus muscle. Al-Redouan et al performed a detailed topographical study of the suprascapular canal and organized the different types of suprascapular nerve entrapment according to the anatomical localization within the canal. They divided suprascapular canal as an osteofibrous canal composed of three segments: an entrance, a passage, and an exit. They further classified the suprascapular nerve entrapment into five subtypes (pre-entrance, entrance, passage, exit, and postexit) depending on the level. 15
Superior labral tears can give rise to paralabral cyst formation, which can extend into the suprascapular notch or spinoglenoid notch, causing compression of the suprascapular nerve. If the nerve is compressed in the suprascapular notch, denervation of both the supraspinatus and infraspinatus is seen, whereas if the compression is in the spinoglenoid notch, selective denervation of infraspinatus is resulted ( Fig. 8 ). Soft tissue and bony masses, such as lipomas and ganglion cysts, have been known to cause nerve compression. Recently, it has been documented that there is a possible association between suprascapular neuropathy and retracted rotator cuff tears, with an improvement in nerve function following repair of the tear. However, other studies have found that overlateralization of the supraspinatus and infraspinatus during a repair can place the motor branches of the suprascapular nerve on tension. Hematoma in the scapular region causing nerve compression has been reported. 16
Fig. 8.
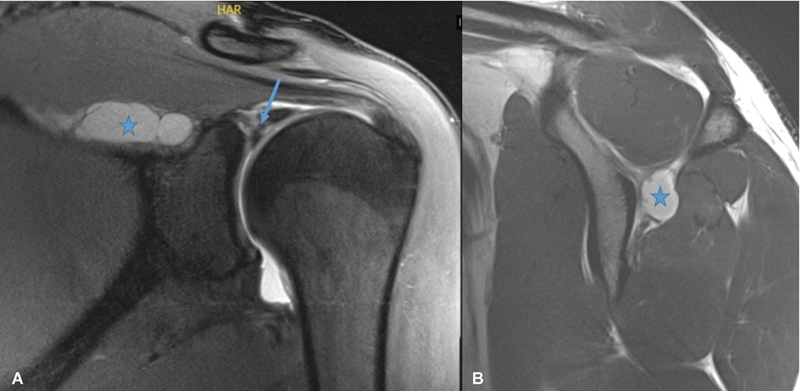
Magnetic resonance imaging of the shoulder showing superior labral tear (arrow in A ) with paralabral cyst (star in A ) and ( B ) formation extending into spinoglenoid notch causing compression of suprascapular nerve.
Overhead activities put the shoulder in external rotation and abduction. This can cause the supraspinatus and infraspinatus muscles to impinge on the scapular spine compressing the suprascapular nerve in the spinoglenoid notch resulting in isolated infraspinatus muscle weakness. Iatrogenic injury to the suprascapular nerve can also occur in cases of distal clavicle excision and posterior shoulder surgeries. Making a clinical diagnosis of suprascapular nerve pathology is very difficult as there are numerous conditions around the shoulder that can mimic symptoms of suprascapular neuropathy. A high degree of suspicion is required in overhead athletes who are at risk of suprascapular neuropathy. Imaging can help in identifying organic causes of suprascapular neuropathy which can help in taking management decisions. MRI is the best imaging modality and can visualize the degree of muscle edema/atrophy, labral tear, paralabral cyst, rotator cuff pathologies, and soft tissue masses around the shoulder joint. Nonsurgical management generally suits patients who suffer from neuropathy due to overuse or overhead athletic activities and when no focal compression of the nerve is identified. However, patients with massive retracted rotator cuff tears and/or space-occupying lesions causing nerve compressions benefit from open/arthroscopic surgeries. 17 18
Pathologies Affecting Scapular Body, Distal Clavicle, and Spinous Process
Neoplastic involvement of the scapular body, distal clavicle, and spinous process can be primary or metastatic in origin, with the latter being more common. As the scapula is a flat bone, chondrosarcoma is the most common primary malignant tumor in middle-aged and elderly individuals, whereas Ewing sarcoma is seen in the pediatric age group. 19 20 Osteochondroma can be seen in adolescent and young adults, more frequently in diaphyseal aclasis. 21 These tumors can extend into the supraspinous fossa ( Figs. 9 and 10 ).
Fig. 9.
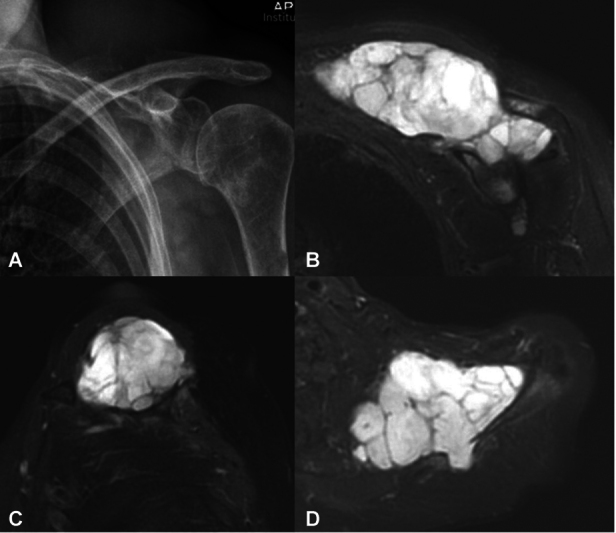
Conventional radiograph ( A ) and magnetic resonance imaging ( B – D ) of a 66-year-old man with chondrosarcoma affecting scapular spine with extension into supraspinous fossa.
Fig. 10.
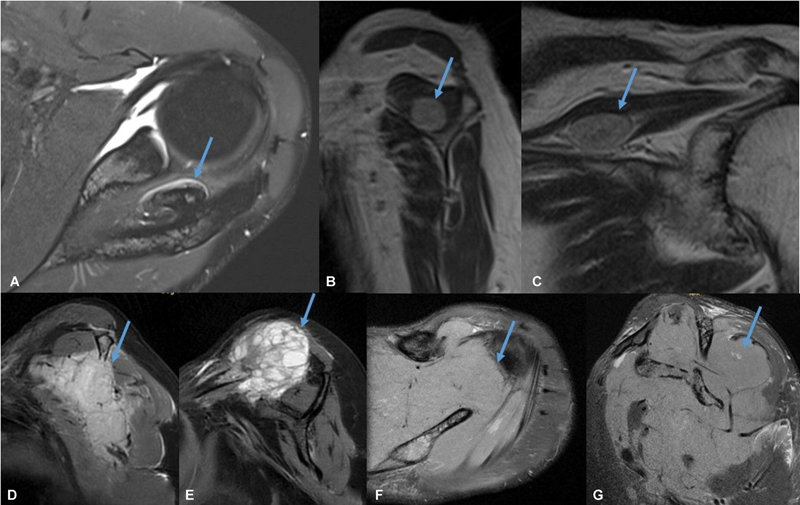
Magnetic resonance imaging in different patient showing osteochondroma (arrow in A ), nerve sheath tumor (arrow in B and C ), metastasis (arrow in D and E ), and lymphoma (arrow in F and G ) affecting supraspinous fossa.
Scapular body fracture is rare but can be seen in high-energy trauma, such as road traffic accidents. Scapular fractures are frequently associated with fractures in other parts of the body. The majority of scapular fractures are extra-articular affecting the body (most common), neck, and spine. Hematoma secondary to fracture can be seen in the supraspinous fossa. 22
Glenohumeral Pathology Extending into Supraspinous Fossa
Acute trauma, dislocation, or chronic repetitive trauma to the glenohumeral joint can affect the labrum with formation of a paralabral cyst, which can extend into the suprascapular notch, supraspinous fossa, and spinoglenoid notch with secondary effects as already described in the preceding paragraphs. Ganglion cyst or synovial cyst can also arise from the arthritic glenohumeral joint and extend into the supraspinous fossa. 23 Infective arthritis affecting the glenohumeral joint can lead to periarticular abscess formation, which can extend into the supraspinous fossa.
Acromioclavicular Joint Pathology Extending into Supraspinous Fossa
The acromioclavicular joint is frequently involved by osteoarthritis. Ganglion cysts or synovial cysts can arise from the arthritic acromioclavicular joint and extend into the supraspinous fossa. 24
Miscellaneous
Benign and malignant soft tissue tumors can arise in the region of the supraspinous fossa, similar to anywhere else in the body. The space can be affected by hemangioma, hematoma, fibromatosis, myocysticercosis, and myositis ossificans. The most common benign soft tissue tumor in this region is a lipoma, which is generally a well-defined and localized lesion with signal intensity equal to fat on all the sequences ( Fig. 11 ). Large size, septa, nodularity, and enhancing soft tissue component point toward an atypical lipomatous lesion or liposarcoma. Another soft tissue neoplastic lesion that can be seen in this region is fibromatosis, which is a locally aggressive, nonmetastasizing fibrous lesion that shows predominantly hypointense signal on T2-weighted sequence and, depending on cellularity, hyperintense signal and enhancement ( Fig. 12 ).
Fig. 11.
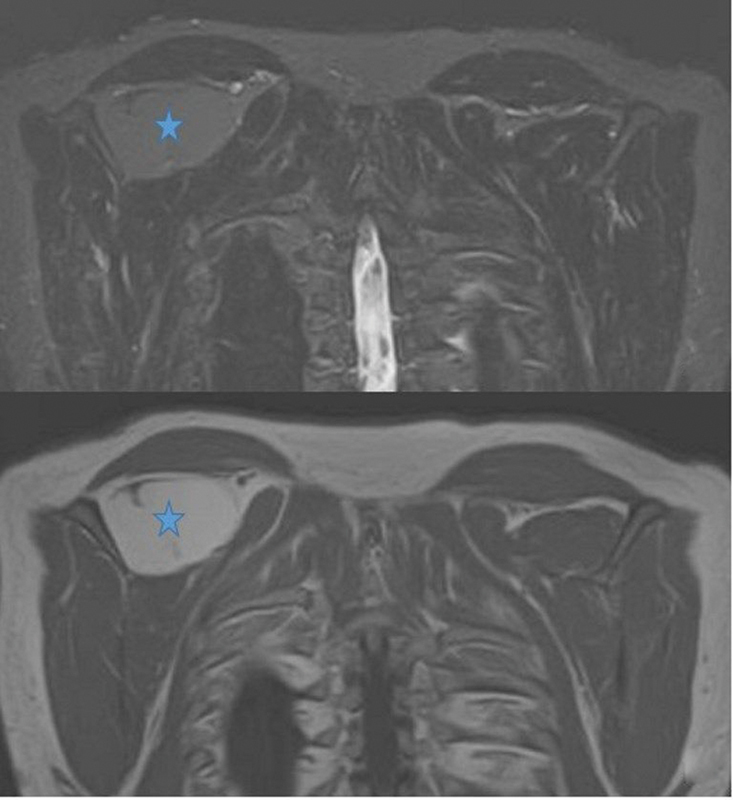
Magnetic resonance imaging showing lipoma (star) in supraspinous fossa following fat signal intensity.
Fig. 12.
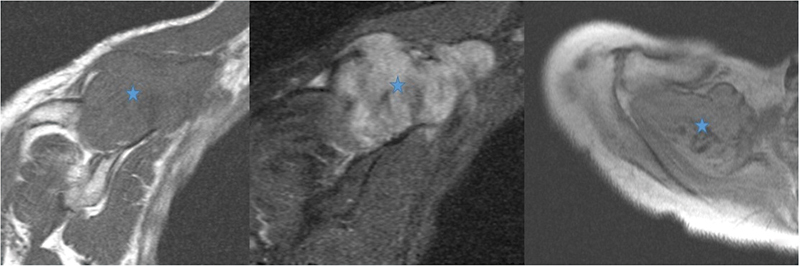
Magnetic resonance imaging showing fibromatosis (star) in supraspinous fossa showing heterogeneous signal intensity with areas of low signal intensity in it.
Myocysticercosis is prevalent in developing countries. The lesions are predominantly cystic with an eccentric nodule, which can be calcified at times. Perilesional edema or fluid can be seen depending on the stage. Myositis ossificans is another important entity that can be mistaken for malignancy on imaging. It is secondary to trauma and, contrary to its name, inflammation is not a feature. It is one of the skeletal “don't touch” lesions. Typical radiographic appearances include peripheral calcification with a lucent center. It is often separated from the adjacent bone by a radiolucent cleft ( Fig. 13 ). 25
Fig. 13.
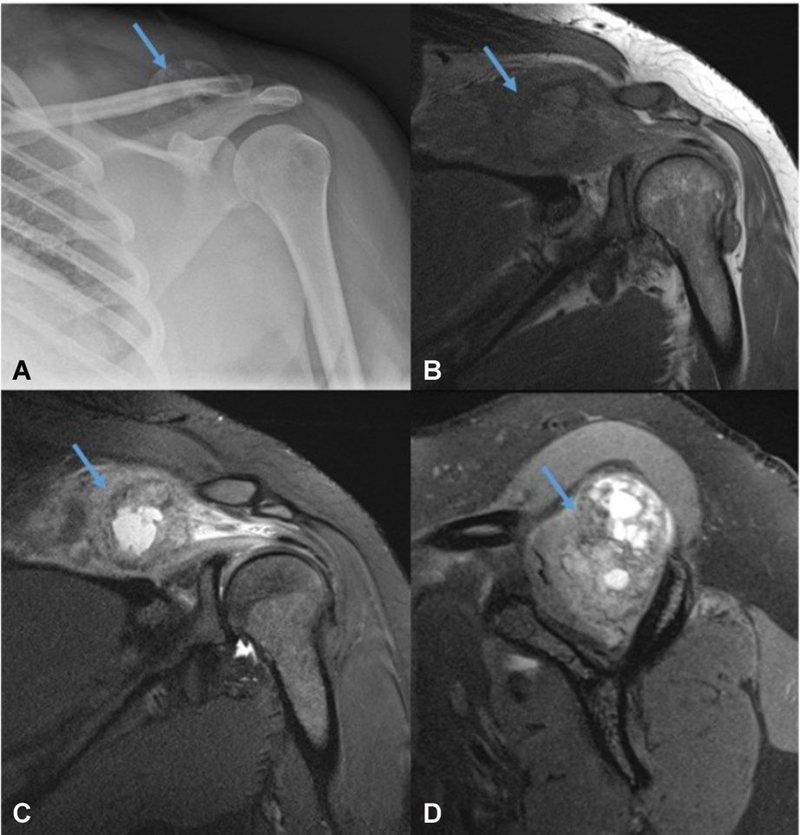
Conventional radiograph ( A ) and magnetic resonance imaging ( B – D ) showing myositis ossificans (arrow) in supraspinous fossa.
Conclusion
The supraspinous fossa is an important location that can be affected by various pathologies. In this pictorial review, we have discussed the detailed anatomy of the supraspinous fossa along with imaging findings of common and uncommon pathological processes affecting it. An awareness of the imaging findings of these entities is essential for a radiologist to avoid misinterpretation and can aid a timely diagnosis.
Funding Statement
Funding None.
Footnotes
Conflict of Interest None declared.
References
- 1.Rengachary S S, Burr D, Lucas S, Brackett C E. Suprascapular entrapment neuropathy: a clinical, anatomical, and comparative study. Part 3: comparative study. Neurosurgery. 1979;5(04):452–455. doi: 10.1227/00006123-197910000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Jeno S H, Munjal A, Schindler G S. Treasure Island (FL): StatPearls Publishing; 2022. Anatomy, shoulder and upper limb, arm supraspinatus muscle. [PubMed] [Google Scholar]
- 3.Alsabieh M, Alzahrani M, Almuhanna A, Bedaiwy N. Spinoglenoid notch ganglion cyst: a case report. Cureus. 2023;15(05):e39279. doi: 10.7759/cureus.39279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wijntjes J, van Alfen N. Muscle ultrasound: present state and future opportunities. Muscle Nerve. 2021;63(04):455–466. doi: 10.1002/mus.27081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flores D V, Mejía Gómez C, Estrada-Castrillón M, Smitaman E, Pathria M N. MR imaging of muscle trauma: anatomy, biomechanics, pathophysiology, and imaging appearance. Radiographics. 2018;38(01):124–148. doi: 10.1148/rg.2018170072. [DOI] [PubMed] [Google Scholar]
- 6.Deshmukh S, Sun K, Komarraju A, Singer A, Wu J S. Peripheral nerve imaging: magnetic resonance and ultrasound correlation. Magn Reson Imaging Clin N Am. 2023;31(02):181–191. doi: 10.1016/j.mric.2023.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Mazal A T, Faramarzalian A, Samet J D, Gill K, Cheng J, Chhabra A. MR neurography of the brachial plexus in adult and pediatric age groups: evolution, recent advances, and future directions. Expert Rev Med Devices. 2020;17(02):111–122. doi: 10.1080/17434440.2020.1719830. [DOI] [PubMed] [Google Scholar]
- 8.Stengaard K, Hejbøl E K, Jensen P T et al. Early-stage inflammation changes in supraspinatus muscle after rotator cuff tear. J Shoulder Elbow Surg. 2022;31(07):1344–1356. doi: 10.1016/j.jse.2021.12.046. [DOI] [PubMed] [Google Scholar]
- 9.Khoury V, Cardinal E, Brassard P. Atrophy and fatty infiltration of the supraspinatus muscle: sonography versus MRI. Am J Roentgenol. 2008;190(04):1105–1111. doi: 10.2214/AJR.07.2835. [DOI] [PubMed] [Google Scholar]
- 10.Thomazeau H, Rolland Y, Lucas C, Duval J M, Langlais F. Atrophy of the supraspinatus belly. Assessment by MRI in 55 patients with rotator cuff pathology. Acta Orthop Scand. 1996;67(03):264–268. doi: 10.3109/17453679608994685. [DOI] [PubMed] [Google Scholar]
- 11.Goutallier D, Postel J M, Gleyze P, Leguilloux P, Van Driessche S. Influence of cuff muscle fatty degeneration on anatomic and functional outcomes after simple suture of full-thickness tears. J Shoulder Elbow Surg. 2003;12(06):550–554. doi: 10.1016/s1058-2746(03)00211-8. [DOI] [PubMed] [Google Scholar]
- 12.Zanetti M, Gerber C, Hodler J. Quantitative assessment of the muscles of the rotator cuff with magnetic resonance imaging. Invest Radiol. 1998;33(03):163–170. doi: 10.1097/00004424-199803000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Choo H J, Lee S J, Kim J H et al. Delaminated tears of the rotator cuff: prevalence, characteristics, and diagnostic accuracy using indirect MR arthrography. Am J Roentgenol. 2015;204(02):360–366. doi: 10.2214/AJR.14.12555. [DOI] [PubMed] [Google Scholar]
- 14.Waheed W, Sneag D. Parsonage-Turner syndrome: fascicular involvement and focal constriction. J Clin Neuromuscul Dis. 2022;23(04):227–228. doi: 10.1097/CND.0000000000000407. [DOI] [PubMed] [Google Scholar]
- 15.Al-Redouan A, Holding K, Kachlik D. “Suprascapular canal”: anatomical and topographical description and its clinical implication in entrapment syndrome. Ann Anat. 2021;233:151593. doi: 10.1016/j.aanat.2020.151593. [DOI] [PubMed] [Google Scholar]
- 16.Joo Y B, Lee W Y, Chung H J. Suprascapular nerve entrapment caused by a large hematoma of the scapula: a case report. BMC Musculoskelet Disord. 2023;24(01):589. doi: 10.1186/s12891-023-06723-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kostretzis L, Theodoroudis I, Boutsiadis A, Papadakis N, Papadopoulos P. Suprascapular nerve pathology: a review of the literature. Open Orthop J. 2017;11:140–153. doi: 10.2174/1874325001711010140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahlawat S, Wadhwa V, Belzberg A J, Batra K, Chhabra A. Spectrum of suprascapular nerve lesions: normal and abnormal neuromuscular imaging appearances on 3-T MR neurography. Am J Roentgenol. 2015;204(03):589–601. doi: 10.2214/AJR.14.12974. [DOI] [PubMed] [Google Scholar]
- 19.Griffin A M, Shaheen M, Bell R S, Wunder J S, Ferguson P C. Oncologic and functional outcome of scapular chondrosarcoma. Ann Surg Oncol. 2008;15(08):2250–2256. doi: 10.1245/s10434-008-9975-1. [DOI] [PubMed] [Google Scholar]
- 20.Waqar S H, Zahid M A. Ewing's sarcoma in scapular region. APSP J Case Rep. 2011;2(03):22. [PMC free article] [PubMed] [Google Scholar]
- 21.Riahi H, Barsaoui M, Zitouna K. Symptomatic scapular osteochondromas: case report. Tunis Med. 2019;97(07):870–873. [PubMed] [Google Scholar]
- 22.Ropp A M, Davis D L. Scapular fractures: what radiologists need to know. Am J Roentgenol. 2015;205(03):491–501. doi: 10.2214/AJR.15.14446. [DOI] [PubMed] [Google Scholar]
- 23.Rhee S M, Bansal V, Jeong H Y, Jeon Y D, Jeong H J, Oh J H. The correlation of the spinoglenoid ganglion cyst size with the electrophysiological alterations of suprascapular nerve and the rotator cuff muscle power. J Orthop Sci. 2024;29(04):969–975. doi: 10.1016/j.jos.2023.06.004. [DOI] [PubMed] [Google Scholar]
- 24.Tham S YY, Ng P HJ, Phua S KA, Ho S WL. Aspiration of a large acromioclavicular joint cyst complicated by recurrence and enlargement: a case report. Cureus. 2023;15(02):e34754. doi: 10.7759/cureus.34754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shyam K, Cicilet S, Philip B. Among the fibers: a multimodality imaging review of intramuscular mass lesions. Indian J Radiol Imaging. 2018;28(02):214–224. doi: 10.4103/ijri.IJRI_299_17. [DOI] [PMC free article] [PubMed] [Google Scholar]


