Abstract
Purpose Meniscal injuries are a common occurrence in sports-related activities, often leading to pain, reduced joint function, and impaired athletic performance. This study aimed to evaluate the role of ultrasound-guided intra-articular platelet-rich plasma (PRP)-rich fluid injection which was obtained through serial centrifugation in the treatment of meniscal injuries resulting from sports activities.
Materials and Methods A prospective study was conducted involving 54 cases with grade I, II, and III meniscal injuries, aged 18 and 43 years. PRP-rich fluid was prepared by subjecting autologous blood samples to a two-step centrifugation process. Patients were assessed pretreatment and at regular intervals posttreatment.
Results Patients reported reduced pain and improved joint functionality following treatment. Average age of the patients was 34.4 years, and average follow-up period was 275.1 days. It is noteworthy that no cases of bilateral meniscal injuries were identified; indicating that the focus was primarily on single knee injuries. Predominance of grade II injuries suggests that the PRP intervention might be particularly effective in addressing more severe meniscal tears.
Conclusion The results of our study provide compelling evidence for the positive impact of PRP augmentation in meniscus repair. Our findings indicate that PRP therapy has the potential to bring about substantial benefits for individuals with meniscus tears of the knee, particularly in terms of pain relief and enhanced functional capabilities.
Keywords: meniscal injuries, sports activity, joint function, athletic performance, platelet-rich plasma, autologous, centrifugation, knee injuries
Introduction
Meniscal injuries are indeed among the most commonly reported sports-related injuries affecting the knee joint. Due to its crucial role in knee function, the meniscus is susceptible to injuries, especially during activities that involve rapid twisting or pivoting motions, sudden stops and starts, and direct impact to the knee. The choice of therapeutic approaches is contingent upon the extent and location of the injury. 1 Our study aimed to evaluate the efficacy of ultrasound (USG)-guided intra-articular injection therapy using platelet-rich plasma (PRP) in patients who sustained meniscal injuries due to sports. 2 3 USG is a very useful tool for interventional, diagnostic, and therapeutic assessment in orthopaedics.
PRP, a blood constituent abundant in platelet-derived growth factors and fibrinogen, exhibits significantly elevated platelet concentration levels—up to fourfold greater than standard blood platelet levels. 4 5 The regenerative properties of these platelet growth factors facilitate a robust response to damaged or compromised cells, promoting effective healing and tissue restoration. This is crucial in zones with limited blood supply, promoting better integration of repaired tissue. Typically, human blood samples comprise around 94% red blood cells (RBCs), 6% platelets, and 1% white blood cells. 6 The fundamental objective of PRP enrichment is to shift the RBC-to-platelet ratio, aiming for a composition of 95% platelets and 5% RBCs.
Over the recent years, there has been a notable upsurge in the utilization of PRP, driven by advancements in regenerative medicine, particularly in wound healing and skin rejuvenation, dental procedures, cosmetic and plastic surgery, fat grafting, bone regeneration, tendinopathies, ophthalmology, hepatocyte recovery, aesthetic surgery, orthopaedics, soft-tissue ulcers, skeletal muscle injuries, and various other applications. 7 8 9 Despite the occasional occurrence of transient pain and localized swelling following injections, the overall incidence of adverse reactions remains quite low. 10 11 However, further investigations are imperative to evaluate the therapeutic efficacy of PRP and its potential long-term adverse effects. 12 13 14
Materials and Methods
This is a prospective study of USG-guided PRP injections in 54 cases of isolated meniscal, sports-related injuries that were reported and followed up at the Department of Arthroscopy and Sports Medicine, Government Multi-Super-Specialty Hospital, Chennai, Tamil Nadu, India, from July 1, 2020 to May 31, 2023.All standard protocols and norms were followed. Our study comprised of patients aged between 18 and 43 years, who exhibited grade I, II, and III meniscal injuries (as per the Reicher classification) confirmed via magnetic resonance imaging (MRI), resulting from participation in contact sports. Reicher defined four grades on MRI 15 : Grade I (no tear) are defined as homogenously black meniscus; grade II (unlikely tear), with a region of minimally increased signal intensity within the meniscus, usually not present on two adjacent scans; grade III (probable tear), with a small, linear region of increased signal intensity/a small-to-moderate nonlinear area of increased signal intensity within the meniscus; and grade IV (definite tear), with gross distortion of the normal shape, truncation of the meniscus/a large focus or line of increased signal intensity within the meniscus. We included cases with grade I to III meniscal injuries with horizontal and vertical tear. Additionally, patients with meniscal injuries who did not show improvement following conservative treatment for a minimum of 6 weeks were also incorporated. Individuals with a prior history of PRP treatment, degenerative meniscal injuries, radial tear, concurrent fractures, or other ligament injuries were excluded from the study.
Method of PRP Preparation
To prepare the PRP, we collected 27 mL of peripheral venous blood in a sterile vacutainer, to which 3 mL of acid citrate dextrose phosphate buffer was added as an anticoagulant. Following this, the blood was subjected to two sequence of centrifugation. Initially, the 30 mL vacutainer was centrifuged at 2,800 revolutions per minute (rpm; first spin) for 10 minutes, leading to the separation of the supernatant fluid and the buffy coat, which were then transferred into another sterile vacutainer. In the subsequent step (second spin), the supernatant plasma underwent centrifugation at 3,200 rpm for 10 minutes. Ultimately, this process yielded 4 mL of PRP ( Fig. 1 ).
Fig. 1.
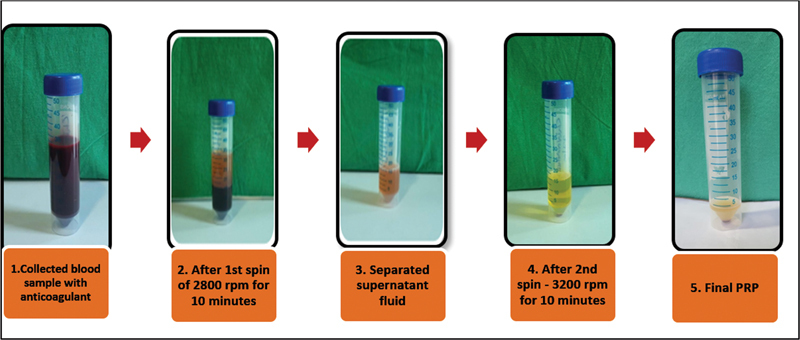
Sequential steps in platelet-rich plasma (PRP) preparation.
Procedure Performed
Under USG guidance (5–15 MHz frequency probe used) in sterile condition, with knee flexed at 90 degrees, meniscus was identified as hypoechoic shadow ( Figs. 2 and 3 ). Intra-articular perimeniscal administration of PRP into the knee joint was performed using a 25-gauge needle, employing either a lateral or medial approach depending on the meniscal involvement. When the needle touches a specific wall or point, it is retracted by 1 mm. This precise needle manipulation may be used to ensure accurate placement of the PRP injection. These changes in resistance are indicative of the presence of meniscal tissue. Specifically, when the needle encounters increased resistance, it suggests the presence of meniscal tissue. Conversely, when there is a loss of resistance, it indicates a change in tissue properties, which corresponds to the meniscus “red zone.” The reason for omission of local anesthesia is the concern that it could negatively influence the effectiveness of the PRP. This concern is tied to potential pH modifications caused by the local anesthetic. Subsequently, knee cycling was performed to facilitate even distribution of PRP throughout the joint. This procedure involved two PRP injections, spaced 2 weeks apart, for each patient.
Fig. 2.
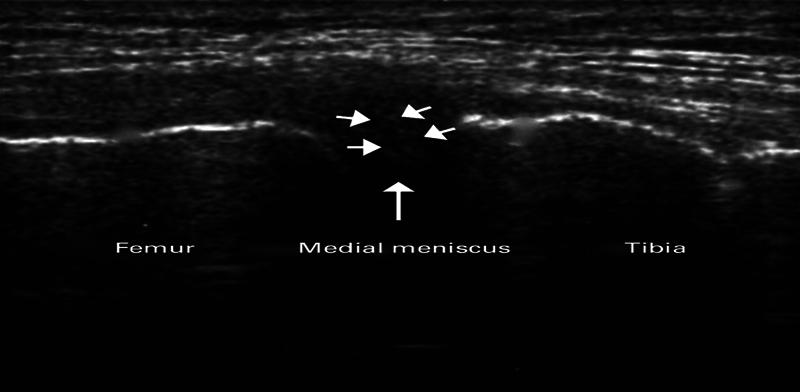
Identification of medial meniscus in USG.
Fig. 3.
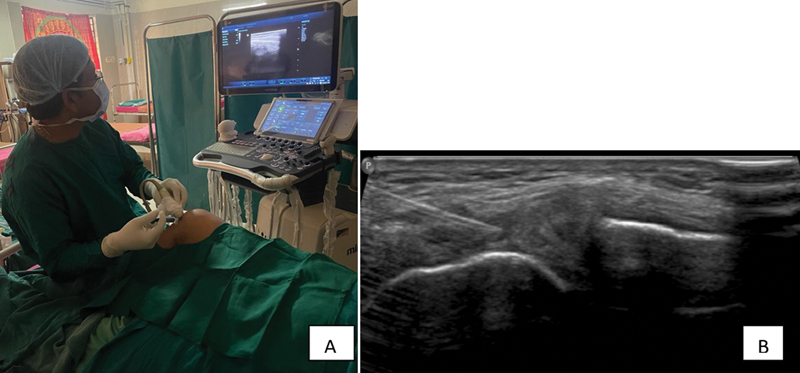
Injection of PRP under USG guidance in sterile condition (A) and intra-articular needle reaching perimeniscal region (B).
Postprocedure Protocol
Following the PRP injection, patients were instructed to avoid nonsteroidal anti-inflammatory drugs. Instead, they were advised to take oral 650 mg paracetamol three times daily as analgesic for the initial 2 days. Standard ice application was recommended for the subsequent 48 to 72 hours.
Results
Demography Data
In our study, male was predominant (85%) and the mean age was 34.4 years. Since our institution is a sports injury center, we received cases with sports injuries of both the sexes.
Causes of Meniscal Injury
We noticed that football and kabaddi players were predominant ( Table 1 ).
Table 1. Various sports activities causing meniscal injury.
| Sports injuries | Number of cases ( n = 54) |
|---|---|
| Football | 20 |
| Kabaddi | 19 |
| Cricket | 7 |
| Judo | 3 |
| Athlete | 3 |
| Gymnastic | 2 |
Side of Involvement
Right side was predominant in this series (79%).
Type and Site of Meniscus Involved
We observed that lateral meniscal injuries (35 cases) were very common in our study ( Table 2 ). We also had combined involvement of body and posterior horn of lateral meniscus in 3 cases and in medial meniscus in 2 cases.
Table 2. Clinical data about type of meniscal injuries.
| Lateral meniscus | Medial meniscus | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Grade | Anterior horn (no. of cases) | Body (no. of cases) | Posterior horn (no. of cases) | Combined | Anterior horn (no. of cases) | Body (no. of cases) |
Posterior horn (no. of cases) | Combined | |
| I | 1 | 1 | 2 | 0 | 0 | 1 | 1 | 0 | |
| II | 2 | 5 | 16 | 2 | 1 | 3 | 6 | 1 | |
| III | 0 | 1 | 4 | 1 | 0 | 0 | 5 | 1 | |
MRI Follow-up
All cases were followed up with repeat MRI knee done 6 to 7 months posttreatment, which showed a well-healed meniscal tears ( Fig. 4 ).
Fig. 4.
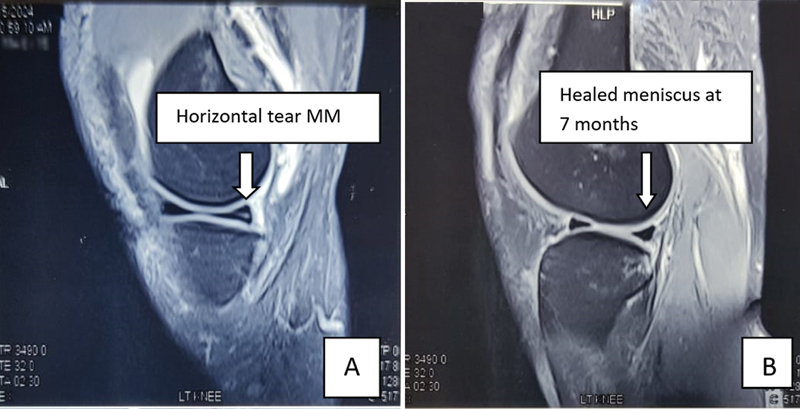
Magnetic resonance imaging (MRI) pictures showing horizontal medial meniscus (MM) tear ( A ) and healed lesion at 7 months ( B ).
Observation
The effectiveness of PRP hinges on the concentration of platelets achieved in the final centrifugation step. In our institution, we conducted a preinjection complete hemogram for all patients to assess the platelet count, which fell within the normal range of 1.5 to 4.5 lakhs/cu mm, and the white blood cell count ranged from 8,300 to 11,500 cells/cu mm. Following the final centrifugation, a substantial concentration of platelet-rich cells (up to three- to fourfold increase) was observed within the vacutainer, which was subsequently confirmed at our blood bank. The white blood cell count in the PRP was notably lower ( Table 3 ).
Table 3. Platelet and WBC levels at both spins.
| Patient values before PRP preparation (average) | After first spin (average) | After second spin (average) | |
|---|---|---|---|
| Platelets | 150,000–450,000/cu mm | 31,000–63,000/cu mm | 491,000–1,540,000/cu mm |
| White blood cells | 8,300–11,500 cells/cu mm | 1,800–3,300 cells/cu mm | Nil to 440 cells/cu mm |
Abbreviations: PRP, platelet-rich plasma; WBC, white blood cell.
Rehabilitation Protocol
The strategy is aimed at rebuilding strength, stability, and function in the injured knee. Strengthening regimen begins with static isometric exercises, gradual progressive weight-bearing, and day to day activities, a common approach we performed in the rehabilitation process after PRP injections. This approach is patient-centered and emphasizes gradual progression to ensure that the injured area is not overstressed during the recovery process. It is designed to prevent exacerbation of the injury and to promote healing while avoiding discomfort.
The phased approach may include the following components:
-
Initial recovery and healing (weeks 1–2):
During the initial weeks, the aim is on allowing the injured knee to heal. Activities are limited to those that do not stress the meniscus or jeopardize the healing process.
Weight-bearing activities and movements are controlled, and individuals use assistive devices like crutches/walkers if necessary.
Physical therapy exercises focus on gentle range of motion, strengthening of supporting muscles, and minimizing joint stiffness.
-
Controlled rehabilitation (weeks 3–4):
As healing progresses, physical therapy is intensified. Range of motion exercises are expanded, and resistance exercises for muscle strengthening are introduced under supervision.
Low-impact activities like swimming or stationary cycling permitted, depending on individual progress.
-
Progressive activity resumption (weeks 5–6):
With proper guidance, patients gradually resume more activities of daily living.
Weight-bearing exercises are increased, and the emphasis remains on controlled movements and gradual load increase.
-
Gradual return to sports (weeks 6–8):
Depending on the individual's progress and responsiveness to treatment, a gradual return to sports activities is initiated.
Sports-specific training, drills, and simulations are introduced, keeping in mind the need to maintain proper form and minimize the risk of reinjury.
Individualized training and rehabilitation programs address specific sport-related movements and demands.
The patients were back to their regular sports over 3 to 4 months' period. They were also followed up for MRI.
Outcome Analysis
The efficacy of intra-articular PRP injection was evaluated using both clinical and functional assessments. Clinical outcomes were gauged using the Numeric Pain Rating Scale (NPRS) as referenced in literature. 16 17 Functional outcomes, on the other hand, were evaluated using the Knee Injury and Osteoarthritis Outcome Score (KOOS) 18 19 and the Lysholm scale 20 21 ( Table 4 ).
Table 4. Comparison of various scoring systems and their intervention.
| Outcome | Preinjection scale | 3 months postinjection scale | 6 months postinjection | 1 year postinjection |
|---|---|---|---|---|
| Numeric Pain Rating Scale (NPRS) | 9 | 6 | 2 | 1 |
| Knee Injury and Osteoarthritis Outcome Score (KOOS) | 64 ± 8.4 | 83 ± 2.5 | 87 ± 3.7 | 89 ± 5.6 |
| Lysholm knee score | 62 | 75 | 96 | 98 |
The NPRS is a patient-centric numerical scale used to quantify the severity of pain. The KOOS, on the other hand, is a subjective measure utilized to assess both symptoms and functional capabilities in individuals experiencing knee issues. Additionally, the Lysholm knee score consists of patient-reported subscales designed to evaluate symptoms and functional aspects of knee injuries.
We did not observe any complications after PRP injection in our series.
Discussion
Numerous studies have investigated the efficacy of PRP therapy in meniscal tears with USG guidance, while findings are generally positive, variations in PRP preparation, patient characteristics, tear severity, and outcome measures make direct comparisons challenging.
USG-guided intra-articular perimeniscal PRP injections have demonstrated promising and effective pain relief for patients with meniscal injuries. The healing progress of the meniscus was assessed using MRI scans conducted 6 to 7 months post-PRP injection, revealing significant and favorable advancement in healing. 22 23 Remarkably, our study indicated that 85 to 92% of patients were able to resume their regular activities without experiencing any symptoms and noteworthy the value of USG in intra-articular injection.
Nonoperative choices include rest, immobilization, weight-bearing limitation, physical therapy, therapeutic exercise, and intra-articular injections, steroid or hyaluronic acid. No drugs or therapies have proven to result in clinically relevant benefits at 12 months of follow-up in comparison with arthroscopic partial meniscectomy.
Existing literature showcases the positive contributions of fibroblast growth factor, transforming growth factor B1, bone morphogenic proteins, and platelet-derived growth factor to meniscal regeneration. 24 25 It serves as an autologous source of these growth factors, making it a valuable therapeutic option for meniscal injuries. In contrast, earlier studies have shown favorable outcomes for procedures such as arthroscopic needling or partial meniscectomy—as it is reserved as a final resort for patients with unstable meniscus lesions. 26
Notably, in our current study, we utilized leukocyte-poor PRP, which mitigates the potential for leukocyte-induced pain. 27 Clinical and functional benefits of percutaneous PRP injections for meniscal lesions of our study is consistent with the findings described by Blanke et al for intrasubstance meniscus injuries as it provided the insights into the positive effects of PRP. The standardization of PRP parameters remains a challenge due to the inherent variability in an individual's circulating blood products.
Despite these challenges, ongoing research is dedicated to better understanding PRP's mechanisms of action, refining preparation techniques, and identifying optimal treatment protocols. 28 Researchers are working to establish guidelines for PRP application based on specific injury types, patient profiles, and desired outcomes.
However, due to the personalized nature of PRP therapy, tailoring treatments to individual patients remains an essential aspect of achieving successful outcomes. The methodologies for PRP preparation have been a subject of ongoing debate and research since the introduction of this therapy. This debate arises from the need to establish standardized protocols that can consistently produce PRP with predictable and optimized therapeutic properties. Different preparation methods can result in PRP products with varying concentrations of platelets, growth factors, and other bioactive substances, 29 and we confirmed the effectiveness through MRI follow-up adds to the growing body of evidence supporting the use of it in meniscal injuries.
The use of USG enhances the targeted delivery of PRP, potentially leading to better radiological results compared with using fluoroscopy, but the main limitations of this study is absence of a control group.
Conclusion
This study ensures that USG guidance can help in precise and accurate delivery of the PRP into the affected site, enhancing the effectiveness of the treatment. This single-center prospective study conclusively demonstrates that PRP presents an intriguing orthobiologic approach for managing meniscal injuries. This therapy has shown positive outcomes in terms of healing and clinical improvement with substantial enhancements in patient-reported pain levels and functional outcomes have been observed, supported by pertinent data: MRI. Additionally, long-term follow-up studies are needed to assess the sustainability of PRP's effects and potential complications. Intra-articular USG-guided PRP injections offer a viable alternative to surgical intervention, as it has the potential to play a significant role for managing stable isolated meniscal injuries in sports persons.
Funding Statement
Funding None.
Footnotes
Conflict of Interest None declared.
References
- 1.Makris E A, Hadidi P, Athanasiou K A. The knee meniscus: structure-function, pathophysiology, current repair techniques, and prospects for regeneration. Biomaterials. 2011;32(30):7411–7431. doi: 10.1016/j.biomaterials.2011.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Medina-Porqueres I, Martin-Garcia P, Sanz-De-Diego S et al. Clinical and functional outcome of meniscal injuries treated with platelet-rich plasma: a single-center case series. Int J Environ Res Public Health. 2022;19(12):7118. doi: 10.3390/ijerph19127118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Z, Weng X. Platelet-rich plasma use in meniscus repair treatment: a systematic review and meta-analysis of clinical studies. J Orthop Surg Res. 2022;17(01):446. doi: 10.1186/s13018-022-03293-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burnouf T, Goubran H A, Chen T M, Ou K L, El-Ekiaby M, Radosevic M. Blood-derived biomaterials and platelet growth factors in regenerative medicine. Blood Rev. 2013;27(02):77–89. doi: 10.1016/j.blre.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Dohan Ehrenfest D M, Rasmusson L, Albrektsson T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF) Trends Biotechnol. 2009;27(03):158–167. doi: 10.1016/j.tibtech.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 6.Premasiri W R, Lee J C, Ziegler L D. Surface-enhanced Raman scattering of whole human blood, blood plasma, and red blood cells: cellular processes and bioanalytical sensing. J Phys Chem B. 2012;116(31):9376–9386. doi: 10.1021/jp304932g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeChellis D M, Cortazzo M H. Regenerative medicine in the field of pain medicine: prolotherapy, platelet-rich plasma therapy, and stem cell therapy—theory and evidence. Tech Reg Anesth Pain Manage. 2011;15(02):74–80. [Google Scholar]
- 8.Chen F M, Zhao Y M, Jin Y, Shi S. Prospects for translational regenerative medicine. Biotechnol Adv. 2012;30(03):658–672. doi: 10.1016/j.biotechadv.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Ntege E H, Sunami H, Shimizu Y. Advances in regenerative therapy: a review of the literature and future directions. Regen Ther. 2020;14:136–153. doi: 10.1016/j.reth.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Martino A, Boffa A, Andriolo L et al. Leukocyte-rich versus leukocyte-poor platelet-rich plasma for the treatment of knee osteoarthritis: a double-blind randomized trial. Am J Sports Med. 2022;50(03):609–617. doi: 10.1177/03635465211064303. [DOI] [PubMed] [Google Scholar]
- 11.Filippiadis D, Charalampopoulos G, Mazioti Aet al. Interventional radiology techniques for pain reduction and mobility improvement in patients with knee osteoarthritis Diagn Interv Imaging 2019100(7-8):391–400. [DOI] [PubMed] [Google Scholar]
- 12.Grossen A A, Lee B J, Shi H H, Shakir H J, Cornett E M, Kaye A D. Platelet-rich plasma injections: pharmacological and clinical considerations in pain management. Curr Pain Headache Rep. 2022;26(10):741–749. doi: 10.1007/s11916-022-01082-2. [DOI] [PubMed] [Google Scholar]
- 13.Middleton K K, Barro V, Muller B, Terada S, Fu F H. Evaluation of the effects of platelet-rich plasma (PRP) therapy involved in the healing of sports-related soft tissue injuries. Iowa Orthop J. 2012;32:150–163. [PMC free article] [PubMed] [Google Scholar]
- 14.Chirichella P S, Jow S, Iacono S, Wey H E, Malanga G A. Treatment of knee meniscus pathology: rehabilitation, surgery, and orthobiologics. PM R. 2019;11(03):292–308. doi: 10.1016/j.pmrj.2018.08.384. [DOI] [PubMed] [Google Scholar]
- 15.Reicher M A, Hartzman S, Duckwiler G R, Bassett L W, Anderson L J, Gold R H. Meniscal injuries: detection using MR imaging. Radiology. 1986;159(03):753–757. doi: 10.1148/radiology.159.3.3754645. [DOI] [PubMed] [Google Scholar]
- 16.Abbott J H, Schmitt J. Minimum important differences for the patient-specific functional scale, 4 region-specific outcome measures, and the numeric pain rating scale. J Orthop Sports Phys Ther. 2014;44(08):560–564. doi: 10.2519/jospt.2014.5248. [DOI] [PubMed] [Google Scholar]
- 17.Swart N M, van Oudenaarde K, Reijnierse M et al. Effectiveness of exercise therapy for meniscal lesions in adults: a systematic review and meta-analysis. J Sci Med Sport. 2016;19(12):990–998. doi: 10.1016/j.jsams.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 18.Roos E M, Roos H P, Lohmander L S, Ekdahl C, Beynnon B D. Knee Injury and Osteoarthritis Outcome Score (KOOS)–development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998;28(02):88–96. doi: 10.2519/jospt.1998.28.2.88. [DOI] [PubMed] [Google Scholar]
- 19.Salavati M, Mazaheri M, Negahban H et al. Validation of a Persian-version of Knee injury and Osteoarthritis Outcome Score (KOOS) in Iranians with knee injuries. Osteoarthritis Cartilage. 2008;16(10):1178–1182. doi: 10.1016/j.joca.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Bengtsson J, Möllborg J, Werner S. A study for testing the sensitivity and reliability of the Lysholm knee scoring scale. Knee Surg Sports Traumatol Arthrosc. 1996;4(01):27–31. doi: 10.1007/BF01565994. [DOI] [PubMed] [Google Scholar]
- 21.Kocher M S, Steadman J R, Briggs K K, Sterett W I, Hawkins R J. Reliability, validity, and responsiveness of the Lysholm knee scale for various chondral disorders of the knee. J Bone Joint Surg Am. 2004;86(06):1139–1145. doi: 10.2106/00004623-200406000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Elnemr R A, Abdelnaby H M, Elshafei M M.Does intra-articular platelet rich plasma injection improve meniscal repair outcomes?. Asian J Sports Med 2019;10(03)
- 23.Lopez-Vidriero E, Goulding K A, Simon D A, Sanchez M, Johnson D H. The use of platelet-rich plasma in arthroscopy and sports medicine: optimizing the healing environment. Arthroscopy. 2010;26(02):269–278. doi: 10.1016/j.arthro.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 24.Tumia N S, Johnstone A J. Platelet derived growth factor-AB enhances knee meniscal cell activity in vitro. Knee. 2009;16(01):73–76. doi: 10.1016/j.knee.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 25.Fortier L A, Barker J U, Strauss E J, McCarrel T M, Cole B J. The role of growth factors in cartilage repair. Clin Orthop Relat Res. 2011;469(10):2706–2715. doi: 10.1007/s11999-011-1857-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blanke F, Vavken P, Haenle M, von Wehren L, Pagenstert G, Majewski M. Percutaneous injections of platelet rich plasma for treatment of intrasubstance meniscal lesions. Muscles Ligaments Tendons J. 2015;5(03):162–166. doi: 10.11138/mltj/2015.5.3.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaminski R, Kulinski K, Kozar-Kaminska K et al. A prospective, randomized, double-blind, parallel-group, placebo-controlled study evaluating meniscal healing, clinical outcomes, and safety in patients undergoing meniscal repair of unstable, complete vertical meniscal tears (bucket handle) augmented with platelet-rich plasma. BioMed Res Int. 2018;2018:9.315815E6. doi: 10.1155/2018/9315815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Genechten W, Verdonk P, Krych A J, Saris D B. Biologic adjuvants in meniscus repair: a review of current translational and clinical evidence. Oper Tech Sports Med. 2020;28(03):150758. [Google Scholar]
- 29.Chen F M, Zhang J, Zhang M, An Y, Chen F, Wu Z F. A review on endogenous regenerative technology in periodontal regenerative medicine. Biomaterials. 2010;31(31):7892–7927. doi: 10.1016/j.biomaterials.2010.07.019. [DOI] [PubMed] [Google Scholar]


