Abstract
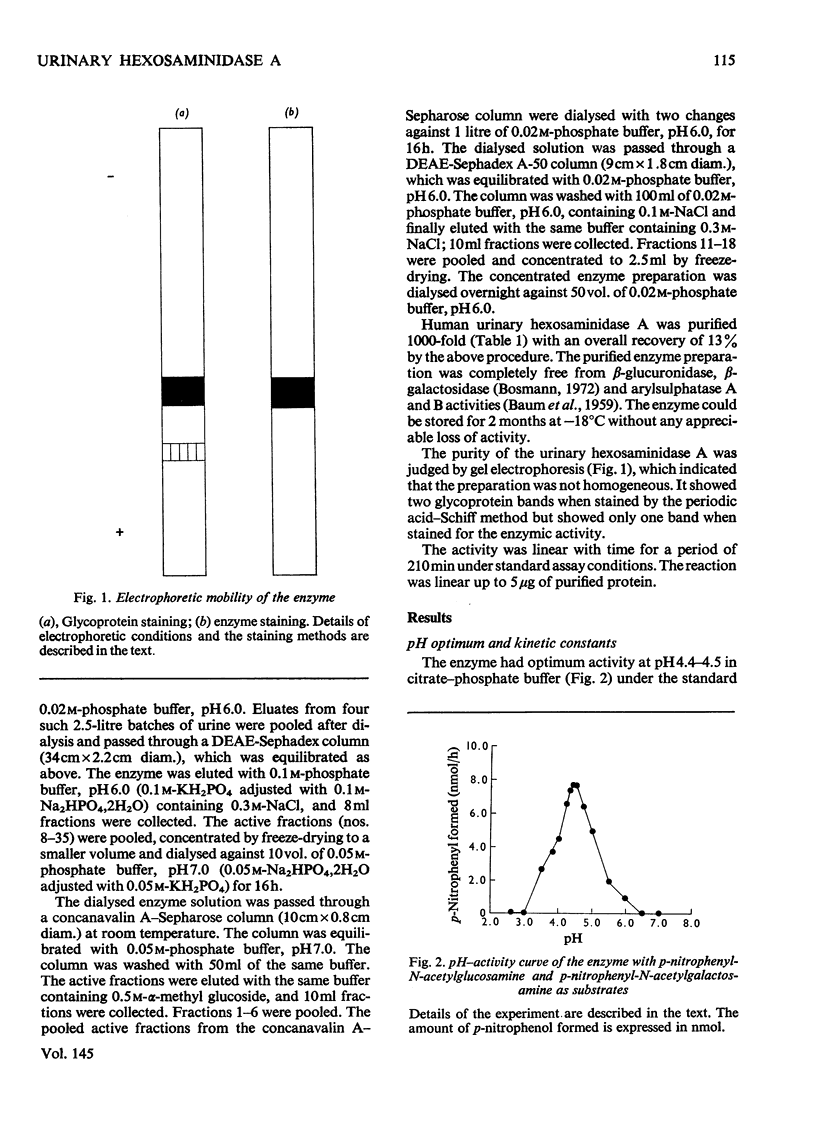
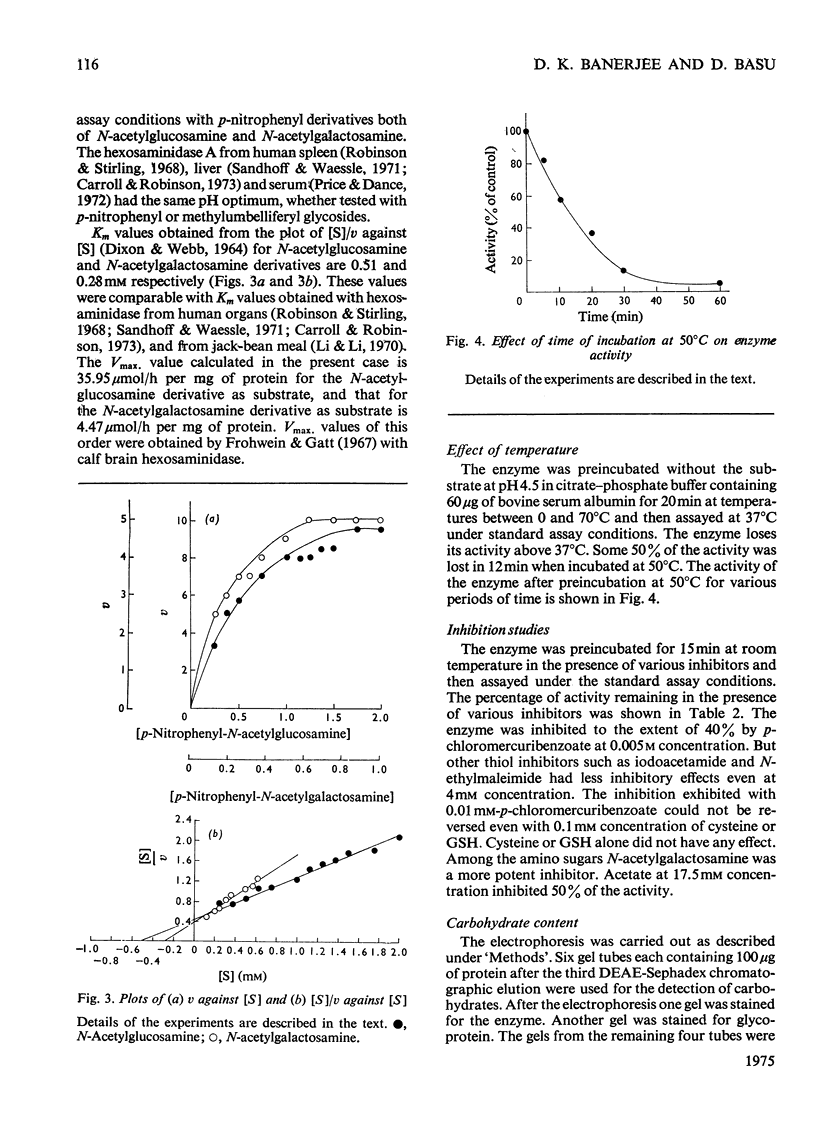
N-Acetyl-beta-hexosaminidase A was purified 1000-fold from human urine by chromatography on DEAE-Sephadex followed by concanavalin A--Sepharose affinity chromatography. The optimal pH range was 4.4--4.5 for both the N-acetylglucosamine and N-acetylgalactosamine derivatives. The Km values were 0.51 mM and 0.28 mM respectively for the N-acetylglucosamine and N-acetylgalactosamine derivatives. The glycoprotein nature of the urinary enzyme was established by its affinity towards concanavalin A as well as by the presence of sialic acid, galactose, glucose, mannose and hexosamines in the molecule.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAUM H., DODGSON K. S., SPENCER B. The assay of arylsulphatases A and B in human urine. Clin Chim Acta. 1959 May;4(3):453–455. doi: 10.1016/0009-8981(59)90119-6. [DOI] [PubMed] [Google Scholar]
- Bahl O. P., Agrawal K. M. Glycosidases of Aspergillus niger. I. Purification and characterization of alpha- and beta-galactosidases and beta-N-acetylglucosaminidase. J Biol Chem. 1969 Jun 10;244(11):2970–2978. [PubMed] [Google Scholar]
- Bishayee S., Bachhawat B. K. ConA-sepharose affinity chromatography: a study on the glycoprotein nature of brain acid hydrolases. Neurobiology. 1974;4(1):48–56. [PubMed] [Google Scholar]
- Bosmann H. B. Identification, purification and characteristics of glycosidases of human blood platelets. Biochim Biophys Acta. 1972 Jan 20;258(1):265–273. doi: 10.1016/0005-2744(72)90984-9. [DOI] [PubMed] [Google Scholar]
- Brady R. O. Lipidoses. Biochimie. 1972;54(5):723–733. doi: 10.1016/s0300-9084(72)80173-1. [DOI] [PubMed] [Google Scholar]
- Carroll M., Robinson D. A low-molecular-weight protein cross-reacting with human liver N-acetyl-beta-D-glucosaminidase. Biochem J. 1974 Feb;137(2):217–221. doi: 10.1042/bj1370217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll M., Robinson D. Immunological properties of N-acetyl-beta-D-glucosaminidase of normal human liver and of GM2-gangliosidosis liver. Biochem J. 1973 Jan;131(1):91–96. doi: 10.1042/bj1310091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dance N., Price R. G., Cattell W. R., Lansdell J., Richards B. The excretion of N-acetyl-beta-glucosaminidase and beta-galactosidase by patients with renal disease. Clin Chim Acta. 1970 Jan;27(1):87–92. doi: 10.1016/0009-8981(70)90378-5. [DOI] [PubMed] [Google Scholar]
- Dance N., Price R. G., Robinson D., Stirling J. L. Beta-galactosidase, beta-glucosidase and N-acetyl-beta-glucosaminidase in human kidney. Clin Chim Acta. 1969 May;24(2):189–197. doi: 10.1016/0009-8981(69)90311-8. [DOI] [PubMed] [Google Scholar]
- FINDLAY J., LEVVY G. A. Purification of beta-N-acetylglucosaminidase from the pig epididymis. Biochem J. 1960 Oct;77:170–175. doi: 10.1042/bj0770170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohwein Y. Z., Gatt S. Isolation of beta-N-acetylhexosaminidase, beta-N-acetylglucosaminidase, and beta-N-acetylgalactosaminidase from calf brain. Biochemistry. 1967 Sep;6(9):2775–2782. doi: 10.1021/bi00861a018. [DOI] [PubMed] [Google Scholar]
- GOLDSTEIN I. J., HOLLERMAN C. E., SMITH E. E. PROTEIN-CARBOHYDRATE INTERACTION. II. INHIBITION STUDIES ON THE INTERACTION OF CONCANAVALIN A WITH POLYSACCHARIDES. Biochemistry. 1965 May;4:876–883. doi: 10.1021/bi00881a013. [DOI] [PubMed] [Google Scholar]
- Goldstone A., Koenig H. Lysosomal hydrolases as glycoproteins. Life Sci II. 1970 Dec 8;9(23):1341–1350. doi: 10.1016/0024-3205(70)90115-3. [DOI] [PubMed] [Google Scholar]
- Goldstone A., Konecny P., Koenig H. Lysosomal hydrolases: Conversion of acidic to basic forms by neuraminidase. FEBS Lett. 1971 Feb 12;13(1):68–72. doi: 10.1016/0014-5793(71)80667-1. [DOI] [PubMed] [Google Scholar]
- Grebner E. E., Tucker J. Human urinary N-acetyl-beta-hexosaminidases. Biochim Biophys Acta. 1973 Sep 15;321(1):228–233. doi: 10.1016/0005-2744(73)90077-6. [DOI] [PubMed] [Google Scholar]
- Hultberg B. N-acetylhexosaminidase activities in Tay-Sachs disease. Lancet. 1969 Nov 29;2(7631):1195–1195. doi: 10.1016/s0140-6736(69)92520-3. [DOI] [PubMed] [Google Scholar]
- Ikonne J. U., Ellis R. B. N-acetyl-beta-D-hexosaminidase component A. Different forms in human tissues and fluids. Biochem J. 1973 Nov;135(3):457–462. doi: 10.1042/bj1350457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mega T., Ikenaka T., Matsushima Y. Studies on N-acetyl-beta-D-glucosaminidase of Aspergillus oryzae. I. Purification and characterization of N-acetyl-beta-D-glucosaminidase obtained from Takadiastase. J Biochem. 1970 Jul;68(1):109–117. [PubMed] [Google Scholar]
- Okada S., O'Brien J. S. Tay-Sachs disease: generalized absence of a beta-D-N-acetylhexosaminidase component. Science. 1969 Aug 15;165(3894):698–700. doi: 10.1126/science.165.3894.698. [DOI] [PubMed] [Google Scholar]
- Poenaru L., Dreyfus J. C. Electrophoretic study of hexosaminidases. Hexosaminidase C. Clin Chim Acta. 1973 Feb 12;43(3):439–442. doi: 10.1016/0009-8981(73)90486-5. [DOI] [PubMed] [Google Scholar]
- Price R. G., Dance N., Richards B., Cattell W. R. The excretion of N-acetyl-beta-glucosaminidase and beta-galactosidase following surgery to the kidney. Clin Chim Acta. 1970 Jan;27(1):65–72. doi: 10.1016/0009-8981(70)90375-x. [DOI] [PubMed] [Google Scholar]
- Price R. G., Dance N. The demonstration of multiple heat stable forms of N-acetyl- -glucosaminidase in normal human serum. Biochim Biophys Acta. 1972 Jun 22;271(1):145–153. doi: 10.1016/0005-2795(72)90142-0. [DOI] [PubMed] [Google Scholar]
- Robinson D., Carrol M., Stirling J. L. Identification of a possible subunit of hexosaminidase A and B. Nature. 1973 Jun 15;243(5407):415–416. doi: 10.1038/243415a0. [DOI] [PubMed] [Google Scholar]
- Robinson D., Stirling J. L. N-Acetyl-beta-glucosaminidases in human spleen. Biochem J. 1968 Apr;107(3):321–327. doi: 10.1042/bj1070321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAIFER A., GERSTENFELD S. Photometric determination of sialic acids in serum and cerebrospinal fluid with the thiobarbituric acid method. Clin Chim Acta. 1962 Jul;7:467–475. doi: 10.1016/0009-8981(62)90086-4. [DOI] [PubMed] [Google Scholar]
- Sandhoff K. Auftrennung der Säuger-N-Acetyl-beta-D-hexosaminidase in multiple Formen durch Elektrofokusserung. Hoppe Seylers Z Physiol Chem. 1968 Sep;349(9):1095–1098. [PubMed] [Google Scholar]
- Sandhoff K. Variation of beta-N-acetylhexosaminidase-pattern in Tay-Sachs disease. FEBS Lett. 1969 Aug;4(4):351–354. doi: 10.1016/0014-5793(69)80274-7. [DOI] [PubMed] [Google Scholar]
- Sandhoff K., Wässle W. Anreicherung und Charakterisierung zweier Formen der menschlichen N-acetyl- -D-hexosaminidase. Hoppe Seylers Z Physiol Chem. 1971 Aug;352(8):1119–1133. [PubMed] [Google Scholar]
- Srivastava S. K., Beutler E. Antibody against purified human hexosaminidase B cross-reacting with human hexosaminidase A. Biochem Biophys Res Commun. 1972 May 26;47(4):753–759. doi: 10.1016/0006-291x(72)90556-6. [DOI] [PubMed] [Google Scholar]
- Tarentino A. L., Maley F. Multiple forms of a highly purified -N-acetylhexosaminidase from hen oviduct. Arch Biochem Biophys. 1971 Dec;147(2):446–456. doi: 10.1016/0003-9861(71)90400-0. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Yoshikawa J., Koide H., Yamanaka M. Demonstration of the multiplicity of N-acetyl-beta-glucosaminidase by electrophoresis. Biochem Biophys Res Commun. 1972 Jan 14;46(1):11–15. doi: 10.1016/0006-291x(72)90622-5. [DOI] [PubMed] [Google Scholar]
- Young E. P., Ellis R. B., Lake B. D., Patrick A. D. Tay-sachs disease and related disorders: Fractionation of brain N-acetyl-beta-hexosaminidase on DEAE-cellulose. FEBS Lett. 1970 Jul 15;9(1):1–4. doi: 10.1016/0014-5793(70)80295-2. [DOI] [PubMed] [Google Scholar]
- Zacharius R. M., Zell T. E., Morrison J. H., Woodlock J. J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem. 1969 Jul;30(1):148–152. doi: 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]


