Abstract
Background
Abnormalities of neostriatum have been reported to be implicated in Crohn’s disease (CD). However, there are few systematic explorations. We aim to explore the changes that occur in the structure and function of the neostriatum and whether these changes are related to the clinical characteristics of CD.
Methods
In this cross-sectional and prospective study, we enrolled 34 CD patients and 31 healthy controls (HCs) for analysis. We performed voxel-based morphometry (VBM) and seed-based functional connectivity (FC) to evaluate the structural and functional changes in the neostriatum. Correlation analysis was used to evaluate the possible relationships between clinical characteristics and neuroimaging findings.
Results
CD patients had significantly increased gray matter volume (GMV) in the bilateral putamen compared with HCs. The results showed that CD patients had significantly decreased FC related to the putamen-calcarine cortex, putamen-fusiform gyrus, putamen-inferior temporal cortex (ITC), putamen-parahippocampus, and increased FC associated with the putamen-cuneus/precuneus. Moreover, CD patients showed a positive correlation between the GMV in the left putamen and illness duration (r=0.42, P=0.013).
Conclusions
Our study indicated that CD patients had increased GMV and abnormal FC related to the putamen. The structural and functional differences could reflect that neostriatum may be linked with alterations of aberrant patterns of the default mode network (DMN) and visual processing area.
Keywords: Crohn’s disease (CD), neostriatum, voxel-based morphometry (VBM), functional connectivity (FC), magnetic resonance imaging (MRI)
Introduction
Several lines of evidence have demonstrated that the neostriatum is composed of the caudate nucleus and putamen (1-4). As the primary input part of the basal ganglia, the neostriatum receives information from the cerebral cortex and thalamus. The neostriatum can be roughly divided into sensorimotor regions processing sensorimotor information, social regions processing cognitive information, and regions for reward and motivation information (5,6). Specifically, its subregions are enrolled in goal-oriented (action result) and habitual (stimulus response) learning (7). Previous studies have shown that the neostriatum is associated with certain disorders, such as Parkinson’s disease, drug addiction, Huntington’s disease, depression, and schizophrenia (8,9). On the base of brain-gut axis theory, the neostriatum has also been found to be involved in gastrointestinal diseases including inflammatory bowel diseases (IBDs) as well as irritable bowel syndrome (IBS) (10,11).
As one IBD subtype, Crohn’s disease (CD) is characterized by repeated attacks of intestinal inflammation, abdominal pain, chronic diarrhea, weight loss, etc. (12-14). CD causes widespread high-level stress, anxiety and a decline in sleep quality (15,16). Worldwide prevalence of CD in adults and children is increasing year by year (14,17). The etiology of CD is complex and is currently considered to be implicated in the interaction of genetic factors, environmental factors and changes in intestinal flora (12,17). However, the inflammatory response of the gastrointestinal tract can not sufficiently explain processes related to psychological stress and anxiety, as well as depression (18). In fact, the pathophysiology of CD has transcended the gastrointestinal tract. There is a two-way exchange of information between various visceral signals from the gastrointestinal tract and nerve signals in the brain (19). This mechanism may suggest that neural abnormalities in brain regions are associated with brain-gut axis disorders (18,20).
Recent neuroimaging studies have indicated the complex two-way interaction between brain and gut in CD, specially related to neostriatum (21-23). Bao et al. found significantly changed gray matter volume (GMV) of the putamen, globus pallidus, insula and other regions in CD patients compared to healthy controls (HCs), and the left insula and orbitofrontal cortex were negatively correlated with disease duration (24). Li et al. found that the putamen in CD patients had higher regional homogeneity (ReHo) values compared to those of the HCs (21). Zhang et al. found altered functional connectivity (FC) of insula with the caudate nucleus, parahippocampus/hippocampus and other brain areas in CD patients, and the FC of the parahippocampus/hippocampus with insula were negatively correlated with Crohn’s Disease Activity Index (CDAI) (22). Goodyear et al. observed significantly increased activity and FC in cognitive and emotional processing brain regions, including parts of the limbic system, basal ganglia, and hypothalamus of IBD patients compared to HCs, especially the volume of the thalamus was positively correlated with C-reactive protein (CRP) concentration and was increased in females experiencing pain (23). It was also found that the amygdala, putamen and other brain regions of CD patients were implicated in pro-inflammatory reactions and autonomic stress reactions, and regulated changes in stress habits (25). Although abnormalities of neostriatum have been reported, there is still no detailed research and systematic exposition specifically targeting the structure and function of the neostriatum in CD patients, which could provide valuable evidence to improve our understanding of CD.
The hypothesis of this study is that the changed structure in the neostriatum and related intrinsic connectivity could be found between CD patients and HCs using voxel-based morphometry (VBM) and seed-based FC analyses. Furthermore, based on structural and functional alterations associated with the neostriatum, the relationships could be identified between clinical data and these neuroimaging findings in CD patients. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1603/rc).
Methods
Participants
The study consisted of 65 subjects, including 34 CD patients and 31 HCs. CD patients were recruited from the Outpatient Clinic of Gastroenterology at the Second Affiliated Hospital of Xi’an Jiaotong University. Patients with a previous CD diagnosis were contacted by registered telephone to determine whether they were willing to participate in the experiment for a certain amount of monetary compensation, while HCs were recruited through advertisement.
CD patients were assessed by illness duration, Inflammatory Bowel Disease Questionnaire (IBDQ) and CDAI (26,27). Visual analog scale (VAS) was used to evaluate the pain index. Pain was divided into 11 grades ranging from 0 (no pain) to 10 points (maximum pain). All subjects were assessed with the Hospital Anxiety and Depression Scale (HADS) (28). HADS includes two parts: HADS-anxiety (HADS-A) and HADS-depression (HADS-D). Both HADS-A and HADS-D are composed of 7 items, and the score of each item ranges from 0 to 21 points (score ranges: normal, 0 to 7; mild, 8 to 10; moderate, 11 to14; and severe, 15 to 21).
CD has active phase and remission phase, which are usually studied in groups in existing studies. The CD patients in this study were in remission, so patients who had an episode of CD in the past 12 months were excluded to avoid confounding the findings. The inclusion criteria were based on gastroenterologists at the Second Affiliated Hospital of Xi’an Jiaotong University and reference to existing literature. CD patients with mild disease, especially those with anxiety and depression scores of no more than 7, were included in the study to prevent the influence of comorbid factors and to reduce the potential impact of disease severity on study results. CD patients with mild disease may be more representative of the pathophysiological processes in the early stages of the disease, which would help us better understand the mechanisms of disease occurrence and progression.
The inclusion criteria of CD patients were as follows: (I) right handedness; (II) between 20 and 45 years of age; (III) education years >6 years; (IV) disease remission for more than 12 months; (V) CDAI <150; and (VI) HADS-A and HADS-D scores ≤7. The exclusion criteria for CD patients were as follows: (I) patients with metal implants; (II) claustrophobic patients; (III) women in menstruation, pregnancy or lactation; (IV) patients who underwent CD-related abdominal surgery; (V) those who have a history of 5-aminosalicylic acid (5-ASA), azathioprine (AZA), or psychotropic drug use in the past 3 months; and (VI) participants with a history of neurological or psychiatric illness.
The basic inclusion criteria for HCs were as follows: (I) right handedness; (II) between 20 and 45 years of age; (III) education years >6 years; (IV) HADS-A and HADS-D scores ≤7; and (V) no abdominal symptoms, intestinal transport disorders or abdominal surgery history in the past 12 months. HCs performed a basic evaluation to ensure they did not have familial psychosis or neurological disorders and did not drink alcohol or take drugs 1 week prior. And the exclusion criteria for HCs were as follows: (I) controls with metal implants; (II) claustrophobic controls; (III) women in menstruation, pregnancy, or lactation; and (IV) those who have a history of corticosteroids, antitumor necrosis factor alpha agent, or psychotropic drug use in the past 3 months.
This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All examinations were performed by specialist gastroenterologists and experienced psychiatrists in the Second Affiliated Hospital of Xi’an Jiaotong University. The Ethics Committee of the Second Affiliated Hospital of Xi’an Jiaotong University in China approved all of the procedures of this study (No. 2020081). Participants were informed of all experimental procedures and signed an informed consent form.
Image acquisition
Participants were scanned on a 3.0-T magnetic resonance scanner (Signa HDXT, GE Healthcare, Chicago, IL, USA) to obtain magnetic resonance imaging (MRI) data. All participants were instructed to lie on their backs along a pair of foam pads to reduce scanner noise and minimize head movement. High-resolution brain structural images were obtained with a T1-weighted three-dimensional spoiled gradient recall sequence. Sequence parameters were as follows: repetition time (TR) =8.78 ms, echo time (TE) =2.83 ms, field of view (FOV) =240 mm × 240 mm, matrix size =256×256, in-plane resolution =0.94 mm × 0.94 mm, slice thickness =1.0 mm (no gaps), flip angle (FA) =12°, and slices =150. The resting-state functional MRI (rs-fMRI) data were acquired using a gradient-echo echo-planar imaging (EPI) sequence with the following parameters: TR =2,000 ms, TE =30 ms, FOV =240 mm × 240 mm, matrix size =64×64, in-plane resolution =3.75 mm × 3.75 mm, slice thickness =3.5 mm (no gaps), FA =90°, and slices =43. All subjects were instructed to remain awake and relax with their eyes closed throughout the scan. For this purpose, the participants received the necessary explanation and training before the scan and were questioned after the data collection.
Structural MRI data preprocessing
All the structural images were processed using computational anatomy toolbox (CAT12) to perform VBM analyses. First, structural MRI images were reoriented to the anterior commissure by the Montreal Neurological Institute (MNI) 152 coordinate system. Next, the images were segmented into gray matter, white matter, and cerebrospinal fluid (CSF). All gray matter images were normalized to the MNI space using the DARTEL method and modulated by the Jacobian determinant of the deformation derived from the spatial normalization, avoiding bias in their intensity due to the expansion of the region during the distortion process. Last, the gray matter images were smoothed with a full width at half maximum (FWHM) isotropic Gaussian kernel of 6 mm (22,29,30).
FMRI data preprocessing
Data reprocessing of all the original functional images was performed with the Data Processing & Analysis for Brain Imaging (DPABI) toolkit (version v5.2, http://rfmri.org/dpabi) based on the MATLAB platform. The data underwent processing through the following steps. The fMRI data were collected at 180 volumes. To achieve magnetization equilibrium, the initial 5 functional volumes were discarded. A slice-timing correction was performed to adjust for different acquisition time of slices. Head motion correction was performed to eliminate movement artifacts in the time series. Any fMRI data with excessive head movement (translation >2 mm in any plane or rotation >2° in any direction) was discarded. All CD patients and HCs meet the above requirements. The spatial normalization of the realigned functional images into standard MNI152 space was performed using an EPI template. And the normalized functional images were resampled to 3 mm × 3 mm × 3 mm. After this, the images were then smoothed with a Gaussian kernel with a FWHM of 6 mm. Meanwhile, a regression model was utilized to eliminate the effects of nuisance variables (including head motion parameters from the Friston 24-parameter model, white matter signals, and CSF signals) from the time series of each voxel. Global signal regression alters the distribution of signal correlations in regions of the brain and can induce artificial inverse correlations, potentially removing real neural signals. Therefore, global signal regression is not performed in this step. After detrending the time series, the filter with a bandwidth of 0.01–0.1 Hz was used to remove the interference of low-frequency drift and high-frequency physiological noise.
Statistical analysis
Demographic and clinical data
The Chi-squared test and two-sample t-test were applied to estimate differences in the demographic and clinical data, as well as the differences of the mean GMV of the caudate nucleus and putamen bilaterally between the two groups. All analyses were executed with SPSS 19.0 (IBM, Armonk, NY, USA). We set significance level at P<0.05.
VBM analysis
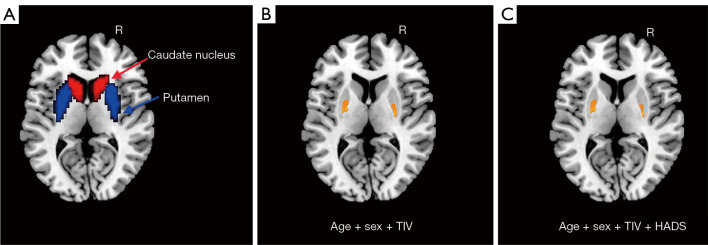
We used Statistical Parametric Mapping (SPM12) (http://www.fil.ion.ucl.ac.uk/spm) to perform a two-sample t-test to test for group differences of GMV within the caudate nucleus and putamen bilaterally. The regions of the caudate nucleus and putamen bilaterally in the automated anatomical labeling (AAL) template were shown (Figure 1A). Covariates including age, sex, and total intracranial volume (TIV) were controlled [P<0.05; cluster-level false discovery rate (FDR)]. Statistical analysis was performed again after HADS was added to the covariate.
Figure 1.
The GMV differences between CD patients and HCs in the bilateral neostriatum: (A) the region of the caudate nucleus and putamen bilaterally; (B) significant differences in the bilateral putamen between CD patients and HCs (covariates were age, sex and TIV); and (C) significant differences in the bilateral putamen between CD patients and HCs (covariates were age, sex, TIV and HADS). R, right; TIV, total intracranial volume; HADS, Hospital Anxiety and Depression Scale; GMV, gray matter volume; CD, Crohn’s disease; HCs, healthy controls.
Seed-based FC analysis
Different peak values of clusters within the neostriatum as assessed by VBM were evaluated as regions of interest (ROIs) for seed-based FC analysis. Each ROI was selected to create a sphere with a 3 mm radius. The mean blood-oxygen-level-dependent (BOLD) time course was extracted from each ROI, and its Pearson correlation with the time course of every voxel in the entire brain was computed to generate the resting-state FC map for each subject. Finally, we applied a Fisher r-to-z transformation to the resting-state FC map to improve normality, resulting in the generation of a z-FC map for each subject. Group differences of FC between CD patients and HCs were tested using two-sample t-tests by SPM12. Age and sex were entered as covariates. The FC analysis was reperformed with HADS-A and HADS-D scores as additional covariates (P<0.05; cluster-level FDR).
Correlation analysis
Different peak values of clusters within the neostriatum as assessed by VBM were evaluated as ROIs. Each ROI was selected to create a sphere with a 3 mm radius. The mean GMV was extracted from each ROI. Pearson correlation was used to detect the relationship between the average GMV and clinical variables, including VAS, illness duration, HADS-A, and HADS-D. Age and sex were included as covariates.
Results
Demographics and clinical characteristics result
As shown in Table 1, there were no significantly statistical differences by age, sex and weight (P>0.05). However, HADS-A and HADS-D scores of CD patients were significantly higher (P<0.05).
Table 1. Clinical and demographic information of CD patients and HCs.
| Variables | CD (n=34) | HCs (n=31) | P value |
|---|---|---|---|
| Age (years) | 30.32±4.89 | 30.13±3.82 | 0.860† |
| Sex (male/female) | 26/8 | 21/10 | 0.432‡ |
| Weight (kg) | 57.12±8.10 | 60.01±6.89 | 0.127† |
| Illness duration (months) | 76.06±48.76 | – | – |
| CDAI | 69.91±39.26 | – | – |
| IBDQ | 175.90±23.39 | – | – |
| VAS | 1.23±1.79 | – | – |
| HADS-A | 6.06±2.71 | 3.32±2.06 | <0.001†*** |
| HADS-D | 4.44±2.93 | 2.87±1.93 | 0.014†* |
Data were expressed as the mean ± SD or number. †, the P value was obtained by two-sample t-test; ‡, the P value was obtained by Chi-squared; *, P<0.05; ***, P<0.001. CD, Crohn’s disease; HCs, healthy controls; CDAI, Crohn’s Disease Activity Index; IBDQ, Inflammatory Bowel Disease Questionnaire; VAS, visual analog scale; HADS-A, Hospital Anxiety and Depression Scale-anxiety; HADS-D, Hospital Anxiety and Depression Scale-depression; SD, standard deviation.
VBM result
Within the identified neostriatum, group differences were found to be located in the bilateral putamen between the CD patients and the HCs {left putamen MNI coordinate: [(−24, −6, 9), P<0.001]; right putamen MNI coordinate: [(27, −9, 9), P<0.001]} (Figure 1B,1C). Peak coordinates of VBM results before and after regression HADS were not affected, and GMV results are shown in Figure 2.
Figure 2.
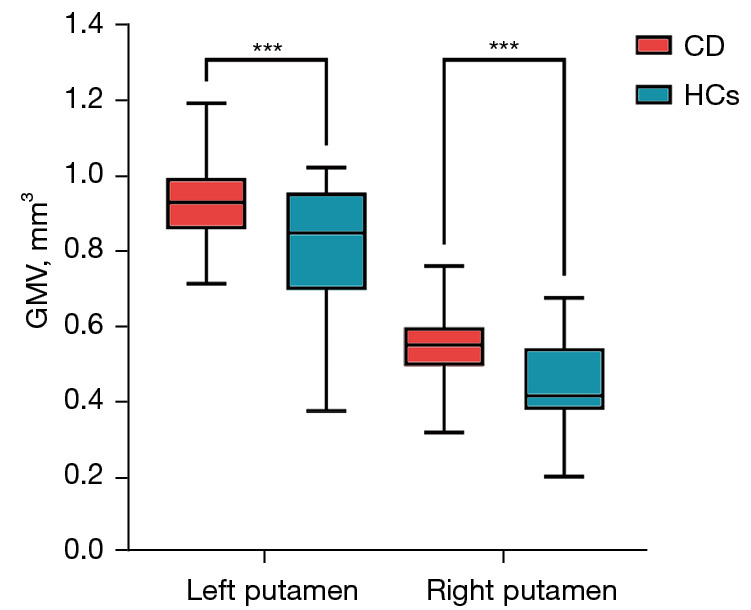
Comparison of GMV in CD patients and HCs at peak coordinates. Box represents the quartile range of data. The upper boundary of the box is Q3, the lower boundary is Q1, and the median inside the box is represented by a line. Whisker: a straight line extending from the upper and lower ends of the box to represent the maximum and minimum values of the data. ***, P value <0.001. CD, Crohn’s disease; HCs, healthy controls; GMV, gray matter volume.
FC result
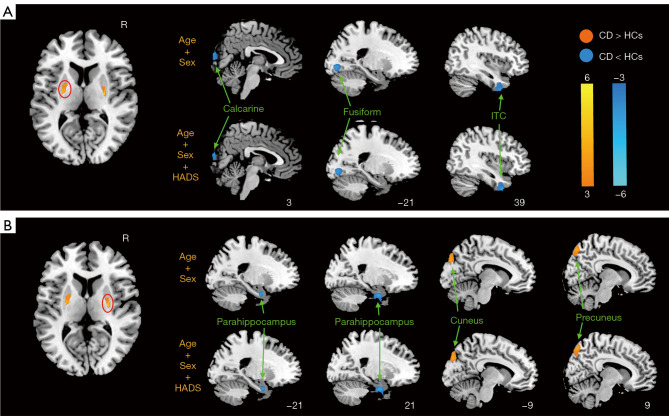
The putamen-related FC results are shown in Figure 3 and Table 2. Compared with HCs, CD patients had significantly decreased FC related to the left putamen-right calcarine cortex, left putamen-left fusiform gyrus, left putamen-inferior temporal cortex (ITC) as well as right putamen-bilateral parahippocampus, and increased FC associated with the right putamen-right precuneus as well as right putamen-left cuneus.
Figure 3.
Brain regions with significant differences in FC between CD patients and HCs of (A) the left putamen and (B) the right putamen. Circles: the activation region where the ROIs are located. R, right; HADS, Hospital Anxiety and Depression Scale; ITC, inferior temporal cortex; CD, Crohn’s disease; HCs, healthy controls; FC, functional connectivity; ROIs, regions of interest.
Table 2. Brain regions showing significantly altered FC between CD patients and HCs.
| Brain region | BA | Hem | Peak MNI | Cluster size | T value | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Seed: L putamen | |||||||
| CD < HCs | |||||||
| Calcarine | 18 | R | 3 | −93 | 12 | 104 | −3.53 |
| Fusiform | 18 | L | −21 | −84 | −6 | 63 | −3.73 |
| ITC | 20 | R | 39 | 9 | −45 | 93 | −3.92 |
| Seed: R putamen | |||||||
| CD < HCs | |||||||
| Parahippocampus | 28 | L | −21 | −3 | −27 | 67 | −3.83 |
| 35 | R | 21 | −3 | −30 | 79 | −4.16 | |
| CD > HCs | |||||||
| Cuneus | 19 | L | −9 | −84 | 36 | 62 | 3.82 |
| Precuneus | 7 | R | 9 | −75 | 54 | 82 | 3.95 |
FC, functional connectivity; CD, Crohn’s disease; HCs, healthy controls; BA, Brodmann’s area; Hem, hemisphere; MNI, Montreal Neurological Institute; X, Y, Z, peak coordinate in the MNI space; ITC, inferior temporal cortex; L, left; R, right.
Correlation result
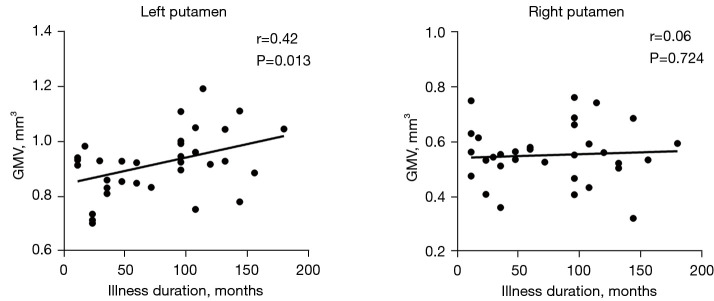
As shown in Figure 4, CD patients had a significant positive correlation between the GMV in the left putamen and illness duration (r=0.42, P=0.013). However, there was no significant correlation between the right putamen and the illness duration.
Figure 4.
Correlation between the illness duration and GMV in the left and right putamen. GMV, gray matter volume.
Discussion
In this study, we investigated changes of GMV and FC in the neostriatum in CD patients compared with HCs. The main findings are as follows: Compared with HCs, (I) CD patients had increased GMV in the bilateral putamen; (II) CD patients had significantly decreased FC related to the putamen-calcarine cortex, putamen-fusiform gyrus, putamen-ITC, putamen-parahippocampus, and increased FC associated with the putamen-cuneus/precuneus; and (III) CD patients showed a positive correlation between the GMV in the left putamen and illness duration.
Group differences in GMV between CD and HCs
In our study, the increased GMV in the putamen was found in CD patients compared with HCs. As the receiving part of the neostriatum, the putamen contains various neuronal populations of relatively uniform density and plays a significant role in motor planning, emotional regulation as well as cognition activity. As part of the brain-gut axis, gut motor and visceral discomfort information in CD patients could activate visceral afferent neurons and transmit to the central nervous system, thereby affecting the putamen. And putamen also contains a somatotopic representation of bodily pain (31). Changed GMV in the putamen may reflect increased pain sensitivity in CD patients. Further, previous studies have revealed that the putamen-related GMV is affected by cognition activity and is associated with the illness duration (32,33). Our results complemented the relationship between GMV in the putamen and illness duration. Combined with the evidence, it is possible that self-reflection, interoceptive stimuli, and visceral discomfort in CD patients may be affected by increased GMV in the putamen and illness duration. Notably, there were different patterns of correlation between GMV and illness duration in the left and right putamen. It may be caused by the division of labor between left and right putamen in CD patients and the connection with different brain networks. It could also involve differences in hemispheric specialization, structural connectivity, or functional roles of the putamen. And GMV in the putamen decreased significantly in patients with IBS (34). Studies indicated that ulcerative colitis (UC) patients also showed increased GMV in the putamen. This may be the characteristic that distinguishes CD and UC from other gastroenteric diseases (35).
Group differences in FC between CD and HCs
Compared with HCs, CD patients showed increased putamen-cuneus/precuneus FC in our study. The cuneus/precuneus partakes in the default mode network (DMN) (36,37). As one of the functional cores of DMN, the cuneus/precuneus is involved in higher-level cortical structures and broader functions such as information integration related to environmental perception and emotional response to pain (38,39). It also plays a role in the fields of attention and self-awareness (40,41). Meanwhile, abnormalities of the DMN have been reported several times in CD (19,42). The processing of visceral sensation information caused by CD is related to the DMN, especially to the cuneus/precuneus. The cuneus/precuneus may integrate visceral information including somatic sensory disturbance and expected pain of the gastrointestinal disease. The cuneus/precuneus responses induced by destructive stimuli may enhance processing of self-awareness. Thereby, we speculate that the increased FC putamen-cuneus/precuneus may represent abnormal perception or abnormal regulation of visceral discomfort in CD patients.
Compared with HCs, CD patients had significantly decreased FC related to the putamen-calcarine cortex, putamen-fusiform gyrus, putamen-ITC, and putamen-parahippocampus in our study. The calcarine cortex and the fusiform gyrus are inner central nodes in the DMN and visual network (43,44). The ITC is a central part of the tertiary visual association cortex and language expression area, associated with visual perception, language, and memory functions (45,46). The parahippocampus, located in the medial temporal lobe, is the key structure for processing declarative memory (47). Damage to the orbital and optic nerves has been reported in CD patients (48). And there is evidence that CD is associated with verbal abnormalities, possibly due to pro-inflammatory cytokines (49). Gut inflammation and stress can produce damaging messages to the central nervous system, thereby prolonging or amplifying the sensitization of visceral afferents. It may cause high neuronal activity in endogenous nociceptive regulatory areas and central sensory amplification areas and could lead to occasional interruption within the temporal lobe network (50). We suppose that long-term chronic inflammation and abdominal stimulation might cause increased pressure on the optic nerve, stimulating the visual area and affecting the calcarine cortex and fusiform gyrus. In addition, existing literature shows that FC increased in the precuneus and FC decreased in the parahippocampal region are common in CD patients in the periaqueductal gray, insula and whole brain network, which is consistent with our results (22,42,51,52). This suggests the widespread alteration of DMN in CD patients and its close association with subcortical nuclei.
In our study, to a certain extent, emotional factors affected the putamen-related FC results but the impact was not significant. According to the previous studies, the structural differences among several regions including the putamen and caudate nucleus were not significant after controlling for emotional factors (anxiety and depression) (24,35). In addition, the effects of emotional factors on CD-related brain structures and functions were inconsistent (22-24,35). The relationship between the neostriatum and emotional factors in CD patients remains to be studied.
Limitations
There are several limitations in this study. First, there were no significant GMV differences emerged in the caudate nucleus. It might be due to the effects of CD being in remission. Moreover, the factors of anxiety and depression had no significant effect on the abnormality of putamen. Further studies should be applied to assess this in the future. The laterality of the putamen also needs further investigation. This will require the inclusion of different CD populations and more detailed grouping of CD patients based on emotional factors. VBM and seed-based FC analyses have inherent limitations. Whole-brain connectivity analyses or machine learning approaches, could provide a more comprehensive understanding of brain alterations.
Conclusions
In summary, we found that CD patients had increased GMV in the bilateral putamen and changed patterns of FC based on the bilateral putamen as ROI. And CD patients had a positive correlation between the GMV in left putamen and illness duration. These imaging abnormalities might be related to aberrant patterns of DMN and visual processing area. It is indicated that the putamen might be a valuable marker to distinguish CD patients from HCs.
Supplementary
The article’s supplementary files as
Acknowledgments
We would like to thank all participants and the Second Affiliated Hospital of Xi’an Jiaotong University for their cooperation.
Funding: This work was supported by the National Natural Science Foundation of China (No. 82270696), the Shaanxi Province TCM “Double Chain Integration” Young and Middle-Aged Scientific Research Innovation Team Construction Project (No. 2022-SLRH-LJ-012), the Shaanxi Key Research and Development Program (No. 2023-YBSF-673), the Young Outstanding Scientific and Technological Talent of Guizhou Province (No. Qiankehepingtairencai[2021]5620), the Lingang Laboratory (No. LGLG-TKN-202205-01), and the Key Laboratory of Acupuncture and Drug Combination in Shaanxi Province (No. KF2219).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Ethics Committee of the Second Affiliated Hospital of Xi’an Jiaotong University in China approved all of the procedures of this study (No. 2020081). All participants were informed of all experimental procedures and signed informed consent form prior to the experiment.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-23-1603/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1603/coif). The authors have no conflicts of interest to declare.
References
- 1.Zheng Q, Ba X, Wang Q, Cheng J, Nan J, He T. Functional differentiation of the dorsal striatum: a coordinate-based neuroimaging meta-analysis. Quant Imaging Med Surg 2023;13:471-88. 10.21037/qims-22-133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garma LD, Harder L, Barba-Reyes JM, Marco Salas S, Díez-Salguero M, Nilsson M, Serrano-Pozo A, Hyman BT, Muñoz-Manchado AB. Interneuron diversity in the human dorsal striatum. Nat Commun 2024;15:6164. 10.1038/s41467-024-50414-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu P, Wang H, Zheng S, Zhang F, Zhang X. Parkinson's Disease Diagnosis Using Neostriatum Radiomic Features Based on T2-Weighted Magnetic Resonance Imaging. Front Neurol 2020;11:248. 10.3389/fneur.2020.00248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Báez-Mendoza R, Schultz W. The role of the striatum in social behavior. Front Neurosci 2013;7:233. 10.3389/fnins.2013.00233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bostan AC, Dum RP, Strick PL. Functional Anatomy of Basal Ganglia Circuits with the Cerebral Cortex and the Cerebellum. Prog Neurol Surg 2018;33:50-61. 10.1159/000480748 [DOI] [PubMed] [Google Scholar]
- 6.Chen SY, Lu KM, Ko HA, Huang TH, Hao JH, Yan YT, Chang SL, Evans SM, Liu FC. Parcellation of the striatal complex into dorsal and ventral districts. Proc Natl Acad Sci U S A 2020;117:7418-29. 10.1073/pnas.1921007117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lanciego JL, Luquin N, Obeso JA. Functional neuroanatomy of the basal ganglia. Cold Spring Harb Perspect Med 2012;2:a009621. 10.1101/cshperspect.a009621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Tella S, Anzuino I, Biassoni F, Ciceri MR, Gnerre M, Nemni R, Cabinio M, Baglio F, Silveri MC. The role of the dorsal striatum in the recognition of emotions expressed by voice in Parkinson's disease. Neurol Sci 2021;42:2085-9. 10.1007/s10072-020-04959-5 [DOI] [PubMed] [Google Scholar]
- 9.Miyanishi H, Nitta A. A Role of BDNF in the Depression Pathogenesis and a Potential Target as Antidepressant: The Modulator of Stress Sensitivity "Shati/Nat8l-BDNF System" in the Dorsal Striatum. Pharmaceuticals (Basel) 2021;14:889. 10.3390/ph14090889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirayama M, Ohno K. Parkinson's Disease and Gut Microbiota. Ann Nutr Metab 2021;77 Suppl 2:28-35. 10.1159/000518147 [DOI] [PubMed] [Google Scholar]
- 11.Chen XF, Guo Y, Lu XQ, Qi L, Xu KH, Chen Y, Li GX, Ding JP, Li J. Aberrant Intraregional Brain Activity and Functional Connectivity in Patients With Diarrhea-Predominant Irritable Bowel Syndrome. Front Neurosci 2021;15:721822. 10.3389/fnins.2021.721822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gajendran M, Loganathan P, Catinella AP, Hashash JG. A comprehensive review and update on Crohn's disease. Dis Mon 2018;64:20-57. 10.1016/j.disamonth.2017.07.001 [DOI] [PubMed] [Google Scholar]
- 13.Sinopoulou V, Gordon M, Akobeng AK, Gasparetto M, Sammaan M, Vasiliou J, Dovey TM. Interventions for the management of abdominal pain in Crohn's disease and inflammatory bowel disease. Cochrane Database Syst Rev 2021;11:CD013531. 10.1002/14651858.CD013531.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dolinger M, Torres J, Vermeire S. Crohn's disease. Lancet 2024;403:1177-91. 10.1016/S0140-6736(23)02586-2 [DOI] [PubMed] [Google Scholar]
- 15.Zanoli L, Tuttolomondo A, Inserra G, Cappello M, Granata A, Malatino L, Castellino P; . Anxiety, depression, chronic inflammation and aortic stiffness in Crohn's disease: the brain--gut--vascular axis. J Hypertens 2020;38:2008-17. 10.1097/HJH.0000000000002517 [DOI] [PubMed] [Google Scholar]
- 16.Qazi T, Farraye FA. Sleep and Inflammatory Bowel Disease: An Important Bi-Directional Relationship. Inflamm Bowel Dis 2019;25:843-52. 10.1093/ibd/izy334 [DOI] [PubMed] [Google Scholar]
- 17.Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L. Crohn's disease. Lancet 2017;389:1741-55. 10.1016/S0140-6736(16)31711-1 [DOI] [PubMed] [Google Scholar]
- 18.Thomann AK, Reindl W, Wüstenberg T, Kmuche D, Ebert MP, Szabo K, Wolf RC, Hirjak D, Niesler B, Griebe M, Thomann PA. Aberrant brain structural large-scale connectome in Crohn's disease. Neurogastroenterol Motil 2019;31:e13593. 10.1111/nmo.13593 [DOI] [PubMed] [Google Scholar]
- 19.Kong N, Gao C, Xu M, Gao X. Changes in the anterior cingulate cortex in Crohn's disease: A neuroimaging perspective. Brain Behav 2021;11:e02003. 10.1002/brb3.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lv K, Fan YH, Xu L, Xu MS. Brain changes detected by functional magnetic resonance imaging and spectroscopy in patients with Crohn's disease. World J Gastroenterol 2017;23:3607-14. 10.3748/wjg.v23.i20.3607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li L, Ma J, Xu JG, Zheng YL, Xie Q, Rong L, Liang ZH. Brain functional changes in patients with Crohn's disease: A resting-state fMRI study. Brain Behav 2021;11:e2243. 10.1002/brb3.2243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang S, Chen F, Wu J, Liu C, Yang G, Piao R, Geng B, Xu K, Liu P. Altered structural covariance and functional connectivity of the insula in patients with Crohn's disease. Quant Imaging Med Surg 2022;12:1020-36. 10.21037/qims-21-509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goodyear BG, Heidari F, Ingram RJM, Cortese F, Sharifi N, Kaplan GG, Ma C, Panaccione R, Sharkey KA, Swain MG. Multimodal Brain MRI of Deep Gray Matter Changes Associated With Inflammatory Bowel Disease. Inflamm Bowel Dis 2023;29:405-16. 10.1093/ibd/izac089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bao CH, Liu P, Liu HR, Wu LY, Shi Y, Chen WF, Qin W, Lu Y, Zhang JY, Jin XM, Wang XM, Zhao JM, Liu XM, Tian J, Wu HG. Alterations in brain grey matter structures in patients with crohn's disease and their correlation with psychological distress. J Crohns Colitis 2015;9:532-40. 10.1093/ecco-jcc/jjv057 [DOI] [PubMed] [Google Scholar]
- 25.Agostini A, Filippini N, Benuzzi F, Bertani A, Scarcelli A, Leoni C, Farinelli V, Riso D, Tambasco R, Calabrese C, Rizzello F, Gionchetti P, Ercolani M, Nichelli P, Campieri M. Functional magnetic resonance imaging study reveals differences in the habituation to psychological stress in patients with Crohn's disease versus healthy controls. J Behav Med 2013;36:477-87. 10.1007/s10865-012-9441-1 [DOI] [PubMed] [Google Scholar]
- 26.Best WR, Becktel JM, Singleton JW. Rederived values of the eight coefficients of the Crohn's Disease Activity Index (CDAI). Gastroenterology 1979;77:843-6. [PubMed] [Google Scholar]
- 27.Guyatt G, Mitchell A, Irvine EJ, Singer J, Williams N, Goodacre R, Tompkins C. A new measure of health status for clinical trials in inflammatory bowel disease. Gastroenterology 1989;96:804-10. [PubMed] [Google Scholar]
- 28.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361-70. 10.1111/j.1600-0447.1983.tb09716.x [DOI] [PubMed] [Google Scholar]
- 29.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 2002;17:825-41. 10.1016/s1053-8119(02)91132-8 [DOI] [PubMed] [Google Scholar]
- 30.Geng B, Gao M, Wu J, Yang G, Liu C, Piao R, Zhang S, Xu K, Yuan J, Liu P. Smaller volume and altered functional connectivity of the amygdala in patients with lifelong premature ejaculation. Eur Radiol 2021;31:8429-37. 10.1007/s00330-021-08002-9 [DOI] [PubMed] [Google Scholar]
- 31.Viñas-Guasch N, Wu YJ. The role of the putamen in language: a meta-analytic connectivity modeling study. Brain Struct Funct 2017;222:3991-4004. 10.1007/s00429-017-1450-y [DOI] [PubMed] [Google Scholar]
- 32.MacEachern SJ, Santoro JD, Hahn KJ, Medress ZA, Stecher X, Li MD, Hahn JS, Yeom KW, Forkert ND. Children with epilepsy demonstrate macro- and microstructural changes in the thalamus, putamen, and amygdala. Neuroradiology 2020;62:389-97. 10.1007/s00234-019-02332-8 [DOI] [PubMed] [Google Scholar]
- 33.Kubota Y, Sato W, Kochiyama T, Uono S, Yoshimura S, Sawada R, Sakihama M, Toichi M. Putamen volume correlates with obsessive compulsive characteristics in healthy population. Psychiatry Res Neuroimaging 2016;249:97-104. 10.1016/j.pscychresns.2016.01.014 [DOI] [PubMed] [Google Scholar]
- 34.Su C, Liu W, Wang Q, Qiu S, Li M, Lv Y, Yu Y, Jia X, Li H. Abnormal resting-state local spontaneous functional activity in irritable bowel syndrome patients: A meta-analysis. J Affect Disord 2022;302:177-84. 10.1016/j.jad.2022.01.075 [DOI] [PubMed] [Google Scholar]
- 35.Zhang S, Chen F, Wu J, Liu C, Yang G, Piao R, Geng B, Xu K, Liu P. Regional Gray Matter Volume Changes in Brains of Patients With Ulcerative Colitis. Inflamm Bowel Dis 2022;28:599-610. 10.1093/ibd/izab252 [DOI] [PubMed] [Google Scholar]
- 36.Deng Z, Wu J, Gao J, Hu Y, Zhang Y, Wang Y, Dong H, Yang Z, Zuo X. Segregated precuneus network and default mode network in naturalistic imaging. Brain Struct Funct 2019;224:3133-44. 10.1007/s00429-019-01953-2 [DOI] [PubMed] [Google Scholar]
- 37.Aryutova K, Paunova R, Kandilarova S, Stoyanova K, Maes MH, Stoyanov D. Differential aberrant connectivity of precuneus and anterior insula may underpin the diagnosis of schizophrenia and mood disorders. World J Psychiatry 2021;11:1274-87. 10.5498/wjp.v11.i12.1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gallace A, Bellan V. The parietal cortex and pain perception: a body protection system. Handb Clin Neurol 2018;151:103-17. 10.1016/B978-0-444-63622-5.00005-X [DOI] [PubMed] [Google Scholar]
- 39.Mattioli P, Pardini M, Famà F, Girtler N, Brugnolo A, Orso B, Meli R, Filippi L, Grisanti S, Massa F, Bauckneht M, Miceli A, Terzaghi M, Morbelli S, Nobili F, Arnaldi D. Cuneus/precuneus as a central hub for brain functional connectivity of mild cognitive impairment in idiopathic REM sleep behavior patients. Eur J Nucl Med Mol Imaging 2021;48:2834-45. 10.1007/s00259-021-05205-6 [DOI] [PubMed] [Google Scholar]
- 40.Palejwala AH, Dadario NB, Young IM, O'Connor K, Briggs RG, Conner AK, O'Donoghue DL, Sughrue ME. Anatomy and White Matter Connections of the Lingual Gyrus and Cuneus. World Neurosurg 2021;151:e426-37. 10.1016/j.wneu.2021.04.050 [DOI] [PubMed] [Google Scholar]
- 41.He X, Li X, Fu J, Xu J, Liu H, Zhang P, Li W, Yu C, Ye Z, Qin W. The morphometry of left cuneus mediating the genetic regulation on working memory. Hum Brain Mapp 2021;42:3470-80. 10.1002/hbm.25446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen F, Zhang S, Li P, Xu K, Liu C, Geng B, Piao R, Liu P. Disruption of Periaqueductal Gray-default Mode Network Functional Connectivity in Patients with Crohn's Disease with Abdominal Pain. Neuroscience 2023;517:96-104. 10.1016/j.neuroscience.2023.03.002 [DOI] [PubMed] [Google Scholar]
- 43.Chen N, Liu G, Guo M, Li Y, Yao Z, Hu B. Calcarine as a bridge between brain function and structure in irritable bowel syndrome: A multiplex network analysis. J Gastroenterol Hepatol 2021;36:2408-15. 10.1111/jgh.15382 [DOI] [PubMed] [Google Scholar]
- 44.Bartolomeo P. Visual objects and their colors. Handb Clin Neurol 2022;187:179-89. 10.1016/B978-0-12-823493-8.00022-5 [DOI] [PubMed] [Google Scholar]
- 45.Vincent MB. Vision and migraine. Headache 2015;55:595-9. 10.1111/head.12531 [DOI] [PubMed] [Google Scholar]
- 46.Marzoli SB, Criscuoli A. The role of visual system in migraine. Neurol Sci 2017;38:99-102. 10.1007/s10072-017-2890-0 [DOI] [PubMed] [Google Scholar]
- 47.Ward AM, Schultz AP, Huijbers W, Van Dijk KR, Hedden T, Sperling RA. The parahippocampal gyrus links the default-mode cortical network with the medial temporal lobe memory system. Hum Brain Mapp 2014;35:1061-73. 10.1002/hbm.22234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jordan DR, Park JSY, Al-Breiki D. Acute orbital inflammation with loss of vision: a paradoxical adverse event associated with infliximab therapy for Crohn's disease. Orbit 2022;41:791-6. 10.1080/01676830.2021.1939726 [DOI] [PubMed] [Google Scholar]
- 49.Nair VA, Dodd K, Rajan S, Santhanubosu A, Beniwal-Patel P, Saha S, Prabhakaran V. A Verbal Fluency Task-Based Brain Activation fMRI Study in Patients with Crohn's Disease in Remission. J Neuroimaging 2019;29:630-9. 10.1111/jon.12634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen HJ, Qi R, Ke J, Qiu J, Xu Q, Zhang Z, Zhong Y, Lu GM, Chen F. Altered dynamic parahippocampus functional connectivity in patients with post-traumatic stress disorder. World J Biol Psychiatry 2021;22:236-45. 10.1080/15622975.2020.1785006 [DOI] [PubMed] [Google Scholar]
- 51.Hou J, Mohanty R, Nair VA, Dodd K, Beniwal-Patel P, Saha S, Prabhakaran V. Alterations in resting-state functional connectivity in patients with Crohn's disease in remission. Sci Rep 2019;9:7412. 10.1038/s41598-019-43878-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thapaliya G, Eldeghaidy S, Radford SJ, Francis ST, Moran GW. An examination of resting-state functional connectivity in patients with active Crohn's disease. Front Neurosci 2023;17:1265815. 10.3389/fnins.2023.1265815 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as