Abstract
The extent to which the mammalian host is capable of enzymatic degradation and detoxification of bacterial lipopolysaccharides (LPS) is still unknown. Partial deacylation of LPS by the enzyme acyloxyacyl hydrolase (AOAH) provides such a mechanism, but its participation in the disposal of LPS under physiological conditions has not been established. In this study, deacylation of isolated radiolabeled LPS by both cellular and extracellular components of a sterile inflammatory peritoneal exudate elicited in rabbits was examined ex vivo. AOAH-like activity, tested under artificial conditions (pH 5.4, 0.1% Triton X-100), was evident in all components of the exudate (mononuclear cells [MNC] > polymorphonuclear leukocytes [PMN] > inflammatory [ascitic] fluid [AF]). Under more physiological conditions, in a defined medium containing purified LPS-binding protein, the LPS-deacylating activity of MNC greatly exceeded that of PMN. In AF, MNC (but not PMN) also produced rapid and extensive CD14-dependent LPS deacylation. Under these conditions, almost all MNC-associated LPS underwent deacylation within 1 h, a rate greatly exceeding that previously found in any cell type. The remaining extracellular LPS was more slowly subject to CD14-independent deacylation in AF. Quantitative analysis showed a comparable release of laurate and myristate but no release of 3-hydroxymyristate, consistent with an AOAH-like activity. These findings suggest a major role for CD14+ MNC and a secondary role for AF in the deacylation of cell-free LPS at extravascular inflammatory sites.
It is often assumed that successful elimination of invading bacteria by the host animal includes digestion of the bacterial macromolecular constituents. Many of the component molecules of bacteria ingested and killed by polymorphonuclear leukocytes (PMN) in vitro do indeed undergo extensive degradation, but this degradation is quite incomplete, at least within the time frame examined (4, 32). In addition, bacterial cell wall fragments can persist for long periods in vivo (8, 9, 25, 26). Of particular biological importance is the fate of bacterial lipopolysaccharides (LPS; endotoxins), a dominant molecular constituent of the outer leaflet of the outer membrane of the gram-negative bacterial envelope. Despite the extraordinary potency of LPS as a trigger for both protective and destructive host responses, it remains unclear how animal hosts detoxify and dispose of LPS, either while it is still part of the bacterial structure or after its release into the host environment. Indeed, a remarkable feature of the biology of LPS is the fact that enzymes capable of extensive LPS degradation have not been identified either in the bacteria that produce LPS or in animal hosts. The one exception is a host enzyme, the acyloxyacyl hydrolase (AOAH) that selectively removes the secondary acyl chains attached in acyloxyacyl linkage to the hydroxyl groups of glucosamine-linked (primary) 3-hydroxy fatty acyl chains (11). The lipid A moiety of LPS deacylated in this fashion resembles the biosynthetic precursor lipid IVA and the chemically synthesized 406 (LA-14-PP) (16), and it similarly exhibits a markedly reduced toxicity toward rabbits in vivo (15) and a reduced ability to stimulate human endothelial cells (7, 20), PMN (6), and leukocytes in whole blood (24). Deacylated LPS also antagonizes the responses of human endothelial cells (7, 20) and human monocytes/macrophages (21, 23) to intact LPS. During inflammation, AOAH is present in body fluids as well as in phagocytic cells, placing the enzyme strategically at sites where host-LPS interactions take place.
Although much has been learned about the structure of AOAH and its activities under laboratory conditions (10, 28), much less is known about its role in the disposal of LPS in more biological settings (11, 13). Thus, whereas in cell-free assays AOAH activity is maximal at acidic pH values in the presence of detergent, the ability of the enzyme to deacylate LPS in a natural extracellular environment, such as that of an inflammatory fluid, remains uncertain. Moreover, uptake and AOAH-like deacylation of purified LPS by PMN, monocytes, and macrophages accumulating locally in response to an inflammatory stimulus have not been characterized.
In this study, we examined the deacylation of isolated LPS exposed to the cellular and extracellular elements of a sterile inflammatory exudate ex vivo. The results show that under conditions that closely resemble a local inflammatory response, rapid and extensive AOAH-like deacylation depends on CD14+ mononuclear cells (MNC) in the exudate, with PMN playing a much less significant role. In addition, AOAH-like activity in the cell-free inflammatory fluid further contributes to a more gradual deacylation of the remaining extracellular LPS.
MATERIALS AND METHODS
Materials.
Oyster glycogen was from U.S. Biochemical Corp. (Cleveland, Ohio). Human serum albumin (HSA) was from Armour Pharmaceutical Co. (Kanakee, Ill.). Hanks’ balanced salt solutions lacking phenol red, with (HBSS+) and without (HBSS−) divalent cations, were from BioWhittaker Bioproducts (Walkersville, Md.). Silica gel G plates were from Analtech (Newark, Del.); reverse-phase KC18 plates were from Whatman (Clifton, N.J.). Aquasol-2 was from Packard Instrument Co. (Meriden, Conn.). Human recombinant LPS-binding protein (LBP) was provided by Xoma Corp. (Berkeley, Calif.).
Collection of rabbit AF, PMN, and MNC.
Sterile inflammatory exudates were collected from New Zealand White rabbits 16 to 18 h after intraperitoneal injection of 300 ml of 150 mM saline containing 2.5 mg of glycogen/ml. Cells were separated from exudate fluid (ascitic fluid [AF]) by centrifugation for 5 min at 100 to 200 × g, and the AF was centrifuged an additional 10 to 20 min at 20,000 × g to remove particulate matter. Cells were washed once in HBSS−. Cell smears made by using a cytospin apparatus (Shandon, Pittsburgh, Pa.) and stained with HEMA 3 stain (Fisher Scientific) showed that peritoneal exudate preparations contained ≥85% PMN and 8 to 15% MNC. PMN were separated from MNC by centrifugation (320 × g) of exudate cells (resuspended in HBSS− containing 1% HSA) in Histopaque-1077 (Sigma) for 30 min. The MNC layer at the interface and the PMN pellet were collected, washed twice in HBSS−, and resuspended in HBSS+ containing 1% HSA at a cell concentration of 0.5 × 107 to 1 × 107/ml. Purified PMN and MNC suspensions respectively contained >98% PMN and at least 80% MNC as judged by differential counting. Surface expression of CD14 on exudate cells was determined by indirect immunofluorescence staining after incubating fractionated or whole-exudate cells with anti-rabbit CD14 monoclonal antibody (MAb) or the isotype control MAb immunoglobulin G2a (IgG2a; 18 μg/ml) (generous gifts from John C. Mathison, Scripps Research Institute, La Jolla, Calif.) at 4 to 8°C for 30 min. Washed cells were then treated with anti-mouse-IgG fluorescein isothiocyanate-labeled (Fab)2 conjugates (Cappel) before being measured for fluorescence intensity in a FACScan flow cytometer. More than 98% of the exudate cells were CD14 positive, with the MNC fraction showing four- to fivefold more CD14 surface expression than the PMN.
Preparation of cell lysates.
Either stored (−80°C) or fresh exudate cells, PMN or MNC, were resuspended at concentrations of 1 × 107 to 2 × 107/ml in HBSS+ containing 1% HSA, 0.1% Triton X-100, and 1 mM phenylmethylsulfonyl fluoride and were disrupted by sonication (40 W, 1 min, 0 to 4°C [model 550 Sonic Dismembrator; Fisher Scientific]). Cell lysates were stored at −80°C before use.
Preparation and characterization of radiolabeled LPS.
Radiolabeled LPS was prepared from Escherichia coli LCD25 (aceEF gltA) after biosynthetic labeling with [3H]acetate or [14C]acetate as described before (17). The specific activity of the radiolabeled [3H]LPS species was 1.5 × 106 to 2 × 106 dpm/μg, and that of [14C]LPS was 106 dpm/μg. To determine the composition of radiolabeled fatty acids (FA) in purified LPS, LPS was hydrolyzed in 4 N HCl followed by 4 N NaOH (6) and, after reacidification of the sample to pH 4.0 with glacial acetic acid (HAc), the released free FA (FFA) were extracted into the chloroform phase of lipid extracts (1). More than 98% of the LPS-associated radioactivity (acetate labeled) was recovered in the chloroform phase after chemical hydrolysis. LPS-derived nonhydroxylated FA (NFA) and 3-hydroxymyristic acid (3-OH-14:0) were resolved by thin-layer chromatography (TLC) on silica gel G plates, using petroleum ether-diethyl ether-HAc (70:30:1, vol/vol/vol) as the solvent (20). Labeled lipid standards were run in parallel. 3H-labeled lipid species were detected by ENHANCE (Dupont) autoradiography and quantitated by scraping off appropriate spots from TLC plates, resuspending them in 0.1 ml of 1% sodium dodecyl sulfate–10 mM EDTA, and subjecting the suspensions to liquid scintillation counting. 14C-labeled species were quantitated by proportional argon ionization, using an AMBIS-1000 detector (AMBIS, Inc). All acetate-labeled LPS species contained ∼30 to 35% NFA and 60 to 65% 3-OH-14:0, in close agreement with previously reported values (17). To further define the composition of radiolabeled NFA, they were eluted from silica gel G plates with CHCl3-CH3OH-HAc-H2O (55:33:9:4, vol/vol/vol/vol) and resolved by reverse-phase TLC on KC18 plates with HAc-acetonitrile (1:1, vol/vol) as the solvent (20). Resolved individual NFA were visualized and quantitated as described above.
Assay of LPS-deacylating activity in cell lysates and cell-free fluids.
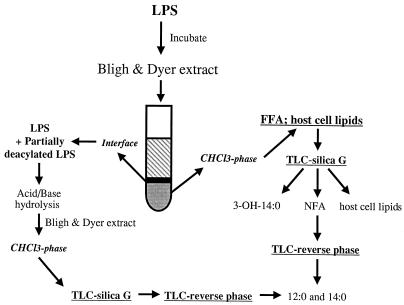
Deacylating activity was assayed in two ways: (i) by determining the accumulation of released labeled FFA in the chloroform phase, and (ii) by quantitating intact and partially deacylated LPS which quantitatively remains in the interface, between the chloroform and the methanol-H2O phase of the extraction mixture (Fig. 1) (19). LPS-deacylating activity of cell lysates, AF, and rabbit serum was measured in the presence of detergent as described previously (18) with minor modifications. Briefly, biological samples were added to a reaction mixture containing 1% HSA in HBSS+, 20 mM sodium acetate (pH 5.4), 5 mM CaCl2, 0.5% (vol/vol) Triton X-100, and radioactive LPS substrate in a total volume of 0.5 ml. Following incubation for 16 h at 37°C, samples were extracted (1), and the released radiolabeled FFA were monitored by measuring radioactivity in aliquots of the chloroform phase. Less than 3% of the radiolabeled purified LPS partitioned into the chloroform phase before incubation. This value was subtracted from levels of radioactive material recovered from experimental samples to calculate the extent of LPS deacylation during the incubation. Less than 1% of radiolabeled material further accumulated in the chloroform phase during incubation of LPS alone in buffered medium for up to 20 h. Identical results were obtained with all radiolabeled LPS species tested. Further characterization of released radiolabeled FFA was accomplished by TLC on silica gel G and KC18 plates as described above (11, 17, 18, 20). FA were also released from the intact and partially deacylated LPS substrate remaining in the interface by acid/base hydrolysis as described above. Samples were then extracted (1), and released radiolabeled FFA were analyzed by TLC as described above. More than 90% of the LPS-associated radioactivity was recovered in the chloroform phase after chemical hydrolysis.
FIG. 1.
Schematic representation of analysis of radiolabeled LPS deacylation.
Assay of LPS uptake and deacylation by AF and/or intact cells.
LPS deacylation was measured under more-physiological conditions by incubation of radioactive LPS at 37°C or at 0 to 4°C either in 0.5 ml of HBSS+ containing 1% HSA, 20 mM HEPES (pH 7.4), and 10 nM recombinant LBP or in 50% AF containing 10 to 20 nM LBP (unpublished measurements) with or without MNC or PMN (0.5 × 107 to 1 × 107/ml). To measure LPS uptake, mixtures were chilled and centrifuged (500 × g, 4 min, 4°C) after 15, 30, 45, and 60 min of incubation. The cell pellets were then washed twice with ice-cold saline, transferred in saline to a fresh tube, spun again, solubilized by boiling (10 min) in 100 μl of 5% sodium dodecyl sulfate–10 mM EDTA, and mixed with Aquasol-2 scintillant to measure cell-associated radioactivity. To correct for the portion of cell-associated radiolabeled LPS that was deacylated and that accumulated as radiolabeled FFA extracellularly, supernatants were extracted and the labeled FFA content in the chloroform phase was measured (1). Thus, the combined total counts recovered from the cell pellets and the chloroform phase counts in the supernatants represent total LPS uptake. Gradual release of the partially deacylated LPS from the cells precluded accurate assessment of LPS binding during longer continuous incubations. This partially deacylated LPS partitions with intact LPS in the extract and hence could not be distinguished from the remaining intact extracellular LPS. Total LPS deacylation in these samples was determined by measuring the accumulation of radiolabeled material in the chloroform phases of separately extracted cell pellets and supernatants or of whole suspensions. Nearly all radiolabeled material in the chloroform phase accumulating during incubations for up to 60 min was FFA (NFA), and most of the FFA was recovered from the extracellular medium. To measure LPS deacylation during longer incubations, intact MNC or PMN (0.5 × 107 to 1 × 107/ml) purified from the same exudate were incubated at 37°C for up to 20 h with radiolabeled LPS (100 ng/ml) in a total volume of 0.5 ml in defined medium (HBSS+ containing 1% HSA, 20 mM HEPES [pH 7.4], and 10 nM recombinant LBP) or 50% AF (AF diluted 1:1 with HBSS+ containing 20 mM HEPES [pH 7.4]). Radiolabeled LPS was also added to samples containing 50% AF alone. To measure the effect of anti-CD14 antibodies on LPS deacylation, samples were preincubated with anti-rabbit CD14 MAb or the isotype control IgG2a (18 μg/ml) for 30 min at room temperature before the addition of LPS. After incubation for various time periods, whole suspensions were extracted (1), and the radioactivity in an aliquot of the chloroform phase was counted to measure conversion of labeled LPS to products in a chloroform-soluble form (e.g., FFA). Further characterization of the labeled lipid species in the chloroform phase was accomplished by TLC on silica gel G and KC18 plates as described above. Radiolabeled species (see Table 1) that comigrated at the solvent front with exudate cell triglyceride during TLC on silica gel G were observed. To identify the labeled FA compositions of these species, they were eluted, chemically hydrolyzed, reextracted (1), and analyzed by silica gel G and reverse-phase TLC.
TABLE 1.
TLC analysis of LPS deacylation by AF with and without intact MNCa
| Type of cells or fluid used, or treatment | Incubation time (h) | CHCl3-soluble cpm
|
||||
|---|---|---|---|---|---|---|
| Total | Originb | NFA | 3-OH-14:0 | SF(TG)c | ||
| MNC | 0 | 3.0 | 2.4 | 0.3 | 0 | 0.4 |
| 2 | 6.1 ± 1.1 | 1.1 | 5.3 | 0 | 0.7 | |
| 4 | 11.3 ± 2.0 | 0.8 | 8.7 | 0 | 2.9 | |
| 8 | 13.3 ± 0.6 | 1.1 | 7.0 | 0.3 | 4.7 | |
| 20 | 17.2 ± 1.3 | 1.5 | 6.6 | 0.4 | 8.2 | |
| AF | 0 | 1.5 ± 0.7 | 0.7 | 0.7 | 0.3 | 0.4 |
| 4 | 3.7 ± 0.9 | 1.2 | 3.1 | 0.9 | 0.3 | |
| 8 | 4.8 ± 1.3 | 0.6 | 3.6 | 0.5 | 0.8 | |
| 20 | 8.3 ± 1.8 | 0.6 | 5.7 | 1.1 | 0.8 | |
| Direct chemical hydrolysis of purified LPS | NAd | NA | 6 ± 1.0 | 32 ± 2.8 | 62 ± 2.4 | NA |
Radiolabeled LPS (100 ng/ml) was incubated in 50% AF with and without MNC (0.5 × 107 to 1 × 107/ml) as indicated, as described in the legend to Fig. 5. At each time point, samples were extracted (20), and labeled lipids recovered in the chloroform phase were analyzed by TLC on silica gel G as described in Materials and Methods. The data shown represent percentages of the total LPS added and are the means of three to five determinations (± standard errors of the means where indicated).
Origin, site of application of extracts to TLC plates.
SF(TG), solvent front host cell triglycerides radiolabeled by incorporation of FA released from LPS. To confirm this, the labeled species were eluted, chemically hydrolyzed, and analyzed as described in Materials and Methods. After chemical hydrolysis, essentially all radiolabeled material was recovered as FFA (12:0, 14:0, or 16:0) in approximately the same ratio (30:45:10, respectively) as FA in NFA from LPS (14.5:20:2.3).
NA, not applicable.
RESULTS
Assessment of LPS deacylation by the cellular and extracellular components of a sterile inflammatory exudate.
Previous studies have demonstrated that both intact and lysed human peripheral blood PMN and murine peritoneal mononuclear phagocytes deacylate LPS under assay conditions that reveal selective removal of NFA (AOAH activity) (11, 13, 19). To initiate the exploration of the fate of LPS in an inflammatory setting provided by a sterile exudate elicited in the peritoneal cavity of the rabbit, the same assay conditions (see Materials and Methods) were used to measure the deacylating activities of various elements in the exudate (Fig. 2).
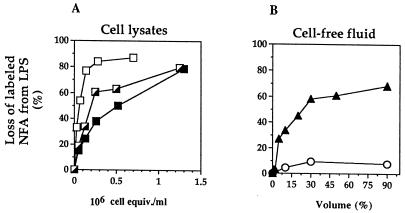
FIG. 2.
Comparison of LPS deacylation by lysates of rabbit inflammatory peritoneal exudate cells (A) and by AF and serum (B). Purified radiolabeled LPS (40 ng/ml) was incubated with increasing concentrations of cell lysate (in cell equivalents per milliliter) (A) or cell-free fluids (AF [▴] or serum [○]) (in volume percent) (B) for 20 h at 37°C, and LPS deacylation was measured as described in Materials and Methods. Whole exudates (┌) contained >85% PMN and <15% mononuclear leukocytes (monocytes/macrophages). Purified PMN (■) contained 98% PMN, and enriched MNC (□) contained >80% mononuclear leukocytes. In all cases, radiolabeled products of LPS deacylation recovered in the chloroform phase were essentially all NFA. Results are therefore expressed as the percentage of labeled NFA lost from the LPS (recovered in the interface). The data shown represent the means of at least three independent determinations ± the standard errors of the means. (Error bars are not visible because they are smaller than the plot symbols.)
Lysates of the exudate cells deacylated LPS in a dose-dependent fashion during incubation for 20 h at pH 5.4 in the presence of detergent (Fig. 2A). At the time of the exudate was collected (16 to 18 h), approximately 85 to 90% of the cells were PMN and the remainder were MNC (i.e., monocytes/macrophages). Again in line with previous studies of rabbit cells, per cell equivalent, the deacylating activity of MNC lysates was about five times higher than that of the PMN lysates (7).
The cell-free AF also contained substantial LPS-deacylating activity. In contrast, serum collected either from unchallenged animals or at the same time as the exudate was nearly devoid of activity (Fig. 2B).
Analysis of the reaction products of LPS deacylation.
The FA composition of the LPS of E. coli LCD25 matches that of other strains of E. coli reported in the literature, consisting of three principal fatty acid species: 3-OH-14:0 (∼65 mass%), lauric acid (12:0; ∼15 mass%), and myristic acid (14:0; ∼18 mass%). The last two NFA occupy the acyloxyacyl positions in lipid A and represent two of the six acyl groups of the LPS of the E. coli species examined (5, 17, 27). The composition of the radiolabeled FA in LPS purified from E. coli LCD25 after growth of this bacterium in medium supplemented with [3H]- or [14C]acetate (see Materials and Methods) is in close accord with the chemical composition of E. coli LCD25 LPS (17) (Table 1).
The products of deacylation of radiolabeled LPS incubated with AF or lysates of exudate cells were analyzed by measuring the released radiolabeled FA accumulating in the chloroform phase (Fig. 3A) and by monitoring the loss of these FA from the interface between the chloroform and methanol-water phases (Fig. 3B), where intact and partially deacylated LPS are quantitatively recovered. After chemical hydrolysis of this fraction, the FA released from the LPS were also subjected to TLC analysis and quantification. Both AF and lysates of exudate cells caused a dose-dependent accumulation in the chloroform phase of up to 90% of 12:0 and 14:0 from the LPS, as well as a corresponding loss of these FA from the interface. In contrast, essentially no 3-OH-14:0 appeared in the chloroform phase or was lost from the interface. Thus, under these conditions, in both the AF and the cell lysates, the LPS-deacylating activity is entirely attributable to AOAH-like activity.
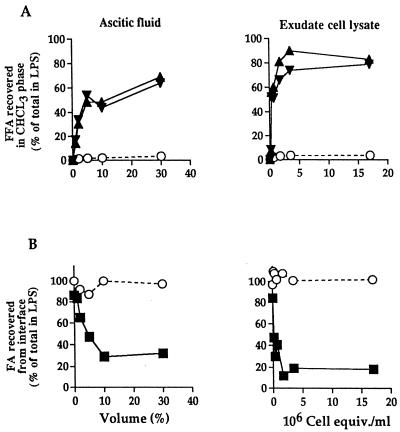
FIG. 3.
AOAH-like deacylation of LPS by AF and lysates of whole-exudate cells. The incubation conditions were as described in the legend to Fig. 2. (A) Identification and quantitation of individual FA recovered as FFA in the chloroform phase were determined by TLC analysis as described in Materials and Methods. NFA, 12:0 (▴) and 14:0 (▾); HFA, 3-OH-14:0 [○]). (B) FA remaining linked to LPS and recovered in the interface were first released by chemical hydrolysis before TLC analysis as described in Materials and Methods. NFA, ■; HFA (3-OH-14:0), ○. The amount of each radiolabeled FA species present in LPS before incubation with AF or cell lysates is defined as 100%. Results shown represent the means of two independent determinations. Note that the loss of NFA from the interface closely matches their accumulation in the chloroform phase. equiv., equivalents.
Uptake and deacylation of LPS by intact exudate cells.
The demonstration that both the cells and the extracellular fluid of the inflammatory exudate contain LPS-deacylating activity, as assayed in the presence of detergent at pH 5.4, set the stage for assessing the ability of each component to deacylate LPS under more-physiological conditions. Uptake and deacylation of purified LPS by the two main cell types in the exudate were measured in two settings: (i) in a buffered salt solution supplemented with purified LBP to stimulate LPS delivery to the cells, and (ii) in a medium that more closely resembles the in vivo inflammatory conditions of the peritoneal exudate, i.e., in AF. Because fibrin formation tends to occur when cells are incubated in nearly undiluted (90%) AF, incubations in this medium were carried out in a 50% dilution.
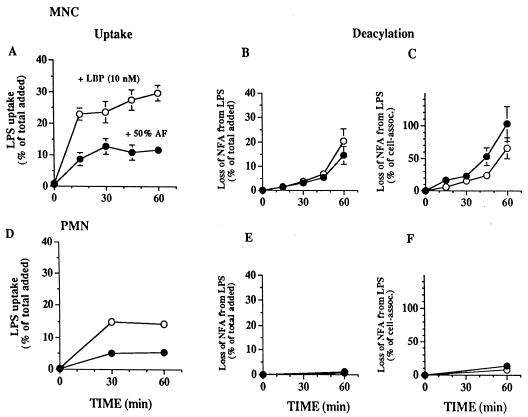
Radioactivity from the radiolabeled LPS promptly became cell associated in both the simpler medium and in AF (Fig. 4A and D). During the first hour of incubation, the rate of LPS uptake by MNC was approximately two times higher than that by PMN and the rate in medium containing purified LBP was two to three times higher than that in 50% AF. However, accumulations of radiolabeled, chloroform-soluble degradation products (i.e., released NFA) during incubation of LPS with MNC for 1 h were similar in the two media (Fig. 4B). Under both sets of conditions, the release of FA was limited to the NFA (12:0 and 14:0). There was no detectable release of 3-OH-14:0 (Table 1). Uptake preceded LPS deacylation (compare Fig. 4A and B), but after 60 min of incubation with MNC plus AF, the loss of NFA from LPS was essentially the same as the amount of LPS taken up by the cells (again compare Fig. 4A and B). This suggested a nearly quantitative release of NFA from LPS taken up by MNC in the presence of AF and was confirmed by analysis of the FA composition of cell-associated LPS recovered after 60 min of incubation (data not shown). In contrast, approximately one-third of the LPS delivered to MNC in buffered salt medium containing purified LBP remained intact after 1 h of incubation.
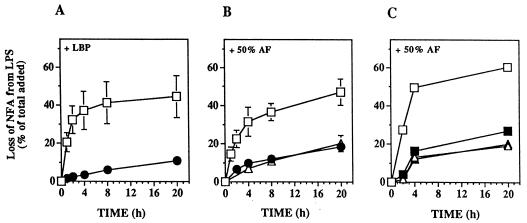
FIG. 4.
Uptake and deacylation of radiolabeled LPS by rabbit inflammatory exudate mononuclear and PMN leukocytes in the presence of 50% AF or LBP. MNC or PMN (0.5 × 107 to 1 × 107 cells/ml) were incubated for the indicated times with radiolabeled LPS (100 ng/ml) in HBSS+ containing 1% HSA, 20 mM HEPES (pH 7.4), and 10 nM LBP or in AF diluted 1:1 with HBSS+ containing 20 mM HEPES (pH 7.4), in a total volume of 0.5 ml. At the indicated time points, LPS uptake and deacylation were measured as described in Materials and Methods. Washed cells from parallel, duplicate samples and the supernatants were extracted (1) to measure the release of radiolabeled FFA. (A and D) Uptake is shown as a percentage of the total radioactivity in the LPS added. Deacylation of LPS was determined at each time point by measuring the chloroform-soluble radioactivity in combined extracts of the supernatant and the washed cells and is expressed as the percentage of labeled NFA lost from the total LPS added (B and E) or from cell-associated (assoc.) LPS (C and F). Less than 1% of the added LPS was deacylated during incubations with purified LBP or 50% AF in the absence of MNC. The data represent the means ± standard errors of the means of values from at least three independent experiments. When no error bars are shown, they are hidden by the symbols, except for the results shown in panels E and F, which represent the means of values from two independent experiments.
Since AF also contains AOAH-like activity (Fig. 2 and 3), at least some of the deacylation that was observed under the conditions mimicking the whole exudate might have taken place extracellularly. LPS deacylation did occur in AF alone, but its rate was much lower than that observed in the presence of MNC. Further, anti-CD14 MAbs blocked nearly all deacylation during the first 2 h of incubation of MNC with LPS in AF (as well as in the medium supplemented with LBP [data not shown]) but did not inhibit deacylation by AF alone (Fig. 5C).
FIG. 5.
Time course of deacylation of purified LPS by AF alone or by exudate cells in the presence of purified LBP or 50% AF: effect of anti-CD14 antibody. MNC (□) or PMN (●) (0.5 × 107 to 1 × 107 cells/ml) were incubated with purified LPS (100 ng/ml) in HBSS+ containing 1% HSA, 20 mM HEPES (pH 7.4), and 10 nM LBP (A) or in AF, diluted 1:1 with HBSS+ containing 20 mM HEPES (pH 7.4) or undiluted (▵) (B), in a total volume of 0.5 ml for up to 20 h at 37°C. (C) To measure the effect of anti-CD14 antibodies on LPS deacylation, samples were preincubated in the presence of the CD14 antibody (closed symbols) or an isotype-matched control MAb (IgG2a) (open symbols) for 30 min at room temperature before the addition of LPS. At the indicated time points, samples were extracted (1), and the radiolabeled FFA recovered in the chloroform phase of the extracts of the whole suspension were measured by TLC analysis as described in Materials and Methods. Since deacylation involves almost exclusively NFA (see Table 1), deacylation is expressed as the percentage of labeled NFA lost from LPS. The data shown in panels A and B represent the mean of values from three to five independent experiments ± the standard errors of the means; the data shown in panel C represent the means of values from two independent experiments.
In contrast to the nearly quantitative LPS deacylation by MNC, less than 10% of PMN-associated LPS was deacylated during incubation of LPS with PMN for 1 h in either medium (Fig. 4F). During longer incubations (up to 20 h), both PMN in medium containing purified LBP and AF alone also produced (limited) deacylation (Fig. 5A and 4B). Addition of PMN to AF did not increase LPS deacylation (Fig. 5B). This contrasts with the effect of added MNC, which resulted in deacylation of up to 40% of the added LPS within 4 h in medium plus LBP or in AF (Fig. 5A and B). During incubation of LPS with MNC and AF, anti-CD14 MAbs nearly completely blocked deacylation within the first 2 h, but not at later times (Fig. 5C). These findings suggest that rapid CD14-dependent intracellular LPS deacylation by MNC may be followed by CD14-independent deacylation of some of the remaining extracellular LPS in AF. Whether the incomplete effects of the anti-CD14 MAb at the later time points also are attributable to internalization of antibody and recycling of CD14 to the cell surface or to a slower CD14-independent pathway has not been determined.
Under all conditions and at all time points, radiolabeled FA released from LPS were nearly exclusively NFA (Table 1), indicating that under these more-physiological conditions the deacylation was also AOAH like. Chloroform-soluble radiolabeled products accumulating during incubation of LPS with AF alone were entirely free NFA. When MNC were also present during incubation, the radioactive material accumulating in the chloroform phase of the cell extracts included material near the solvent front on the TLC plate. With time, the proportion of free NFA radioactivity decreased as that at the solvent front increased. This front-running material comigrated with triglycerides. Analysis of the radiolabeled FA composition of this material by chemical hydrolysis and TLC showed nearly the same radiolabeled FA profile of 12:0, 14:0 and palmitic acid (16:0) as the NFA in substrate LPS. Thus, this material represents incorporation of the released FA into host cell lipids (11, 22).
DISCUSSION
We have previously employed a sterile inflammatory exudate elicited in the peritoneal cavity of the rabbit to provide an ex vivo experimental model for identifying mammalian cellular and extracellular contributors to antibacterial host defense at a local inflammatory site (30, 31). We have used this same model for the study of the ability of the cells and fluid in this inflammatory setting to deacylate LPS. By using the assay conditions (i.e., acid pH in the presence of detergent) that had served before to detect AOAH activity in cells and body fluids (18), we confirmed that lysates of PMN and MNC in the exudate contained AOAH-like deacylating activities (8). The cell-free AF (in contrast to serum collected at the same time) also contained readily detectable AOAH-like activity (Fig. 2 and 3), but at levels per milliliter of whole exudate that were about 10-fold less than those found in the cells.
Exploration of deacylation of isolated LPS under more-physiological conditions revealed that among the various elements of the inflammatory exudate that contain AOAH-like activity, MNC stood out in their ability to deacylate LPS. Both in a buffered medium with added LBP (to promote uptake of LPS) and in a reconstituted whole exudate, these cells rapidly incorporated LPS and deacylated up to 25% of the added LPS in 1 h (Fig. 4B). Deacylation continued to progress rapidly in both media, reaching up to 40% during the first 4 h of incubation and then leveling off at a maximum of approximately one-half of the total added LPS (Fig. 5A and B). The hydrolyzed FA were exclusively NFA, accumulating either as FFA or in host lipids (Table 1). No 3-OH-14:0 was detected in the chloroform phase of the extracts, and none was lost from the interface, which contained all the undegraded and partially deacylated LPS (data not shown). Thus, under these more-physiological incubation conditions, the LPS-deacylating activity is also almost exclusively AOAH like. However, a very small amount of 3-OH-14:0 does accumulate during incubation of LPS with cell-free AF (Table 1). It is possible that accumulation of this product of deacylation would be more prominent if less LPS could be added as the substrate. Therefore, further exploration of this potentially interesting finding will require an LPS substrate of a higher specific radioactivity than is currently available.
Under our experimental conditions, particularly when AF is used as the extracellular medium, deacylation of LPS appears to be limited mainly by CD14-dependent LPS uptake by MNC. Within 1 h, essentially all LPS associated with MNC was partially deacylated, a rate and extent of LPS degradation that should contribute substantially to down regulation of LPS signaling of these cells (2). The much smaller contribution of the exudate PMN to LPS deacylation may reflect much lower levels of both surface expression of CD14 (14) and AOAH-like activity in PMN than are found in MNC (Fig. 2), accounting for limited LPS uptake and also less subsequent degradation.
The LBP concentrations in 50% AF (10 to 20 nM, as measured by enzyme-linked immunosorbent assay, are well within the range needed for optimal delivery of LPS to host myeloid cells (1 to 100 nM) (11a). Therefore, the finding that both MNC and PMN bind less than half the amount of LPS in AF than they bind in the medium with added purified LBP (10 nM) is probably best explained by competition for LPS between LBP and other LPS-binding (lipo)proteins in the AF that may inhibit delivery of LPS to the cells (12, 29, 31). Nevertheless, the rates of deacylation by MNC in the two media are nearly the same, suggesting that the capacity of these cells to deacylate LPS was exceeded by the LBP-mediated capacity of MNC to bind LPS. The extent to which LPS-interactive proteins in AF, such as BPI, p15, other LPS-binding proteins (e.g., the phospholipid transfer protein) (12, 29, 31), and lipoproteins, influence the distribution and the processing of LPS in the complete inflammatory setting remains to be studied. Data not shown indicate that some of the LPS, after incorporation and partial deacylation by MNC in AF, may be extruded from the cells and transferred to (lipo)proteins in the inflammatory fluid.
It should be noted that our focus on the quantitative assessment of LPS deacylation dictated the use of relatively large amounts of radiolabeled LPS for reliable detection of the products of hydrolysis. However, very similar results were generally obtained in experiments with 10-fold-lower LPS concentrations (data not shown). This study demonstrates for the first time, therefore, that among the cellular elements of a local inflammatory exudate elicited in a mammalian host, the MNC are the most capable of prompt and substantial deacylation and hence detoxification of LPS. To a lesser extent, and at a lower rate, the cell-free AF can also contribute to this process. As the inflammatory process continues and resolves, the proportion of MNC increases. These long-lived cells, with their greater capacity for LPS deacylation, may play a progressively larger role in the elimination of the signaling and toxic actions of LPS. Further, the mononuclear phagocytes have been shown to ingest apoptotic PMN (3). Microscopic inspection of exudates collected after 16 h has regularly shown MNC that had phagocytosed PMN (unpublished observations). We envision, therefore, that undegraded LPS associated with PMN, destined for removal by scavenging macrophages, may ultimately be subject to degradation. Such an extension of an incomplete digestive process, initiated by the PMN (representing the first-line response), to the mononuclear phagocyte may also be invoked during host defense against live gram-negative bacteria (11b).
So long as relatively few MNC are present in the whole exudate, deacylation of LPS is a slow process that leaves most of the LPS intact for many hours (Fig. 5) (8). This raises questions about the short-term biological effectiveness of this process. However, since extracellular partially deacylated LPS can block signaling of host cells by extracellular intact LPS (23), even limited deacylation might play a greater role than would be reflected by the extent of degradation. Thus, cellular LPS responses may be dampened not only by intracellular deacylation but also when partially deacylated LPS accumulate extracellularly due to extrusion from the MNC and because of the AOAH-like activity in the AF. In addition, deacylation may represent only part of a complex array of disposal mechanisms that includes other, as-yet-undefined degradative pathways and the formation of bioinactive LPS complexes (8). The latter is likely to play a dominant role in the inactivation of LPS in the circulation, where AOAH-like activity is barely detectable, even when this activity is high in AF (Fig. 2). In contrast, in the more stagnant environment of a localized infection or inflammation, where LPS associated with cells or complexed to host (lipo)proteins is not readily delivered to sites involved in final LPS removal (liver/bile) (8), deacylation may well contribute importantly to anti-LPS defense.
In summary, this study shows for the first time that both the extracellular and the cellular elements of an inflammatory exudate can participate in the deacylation of isolated LPS under close to physiological conditions. The results suggest the following sequence of events during evolution of the inflammatory response to the LPS of invading gram-negative bacteria. Initially, PMN are mobilized to fulfill their primary role in bacterial clearance, aided by the proinflammatory effects of LPS. The limited capacity of PMN (and AF) to deacylate and detoxify LPS may therefore optimize early host defense. With the later influx of AOAH-rich MNC, continued and potentially excessive LPS responses are blunted as these cells increasingly take up and deacylate the remaining extracellular LPS. The AF may contribute further to this process by virtue of its content of AOAH-like activity and LPS-complexing (lipo)proteins that also downgrade LPS toxicity. How these findings extend to LPS as a constituent of live bacteria, the form in which the host generally encounters the molecule, will be addressed in the future.
ACKNOWLEDGMENTS
We thank Jan Vilcek, John Gerecitano, and the members of our group for advice and assistance.
This work was supported in part by United States Public Health Service grants R37 DK 05472 and AI 18188 and by a grant from the Xoma Corporation (Berkeley, Calif.). Seth Katz was supported by National Institutes of Health training grant 5T32GM07308 from the National Institute of General Medical Sciences.
REFERENCES
- 1.Bligh E G, Dyer W J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 2.Dedrick R L, Conlon P J. Prolonged expression of lipopolysaccharide (LPS)-induced inflammatory genes in whole blood requires continual exposure to LPS. Infect Immun. 1995;63:1362–1368. doi: 10.1128/iai.63.4.1362-1368.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Devitt A, Moffatt O D, Raykundalia C, Capra J D, Simmons D L, Gregory C D. Human CD14 mediates recognition and phagocytosis of apoptotic cells. Nature. 1998;392:505–509. doi: 10.1038/33169. [DOI] [PubMed] [Google Scholar]
- 4.Elsbach P. Degradation of microorganisms by phagocytic cells. Rev Infect Dis. 1980;2:106–128. doi: 10.1093/clinids/2.1.106. [DOI] [PubMed] [Google Scholar]
- 5.Erwin A L, Mandrell R E, Munford R S. Enzymatically deacylated Neisseria lipopolysaccharide (LPS) inhibits murine splenocyte mitogenesis induced by LPS. Infect Immun. 1991;59:1881–1887. doi: 10.1128/iai.59.6.1881-1887.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erwin A L, Munford R S. Deacylation of structurally diverse lipopolysaccharides by human acyloxyacyl hydrolase. J Biol Chem. 1990;265:16444–16449. [PubMed] [Google Scholar]
- 7.Erwin A L, Munford R S. Plasma lipopolysaccharide-deacylating activity (acyloxyacyl hydrolase) increases after lipopolysaccharide administration to rabbits. Lab Investig. 1991;65:138–144. [PubMed] [Google Scholar]
- 8.Erwin A L, Munford R S. Processing of LPS by phagocytes. In: Morrison D C, Ryan J L, editors. Bacterial endotoxic lipopolysaccharides. Vol. 1. Boca Raton, Fla: CRC Press; 1992. pp. 405–434. [Google Scholar]
- 9.Ginsburg I, Sela M N. The role of leukocytes and their hydrolases in the persistence, degradation, and transport of bacterial constituents in tissues: relation to chronic inflammatory processes in staphylococcal, streptococcal, and mycobacterial infections and in chronic periodontal disease. Crit Rev Microbiol. 1976;4:249–322. doi: 10.3109/10408417609106944. [DOI] [PubMed] [Google Scholar]
- 10.Hagen F S, Grant F J, Kuijper J L, Slaughter C A, Moomaw C R, Orth K, O’Hara P J, Munford R S. Expression and characterization of recombinant human acyloxyacyl hydrolase, a leukocyte enzyme that deacylates bacterial lipopolysaccharides. Biochemistry. 1991;30:8415–8423. doi: 10.1021/bi00098a020. [DOI] [PubMed] [Google Scholar]
- 11.Hall C L, Munford R S. Enzymatic deacylation of the lipid A moiety of Salmonella typhimurium lipopolysaccharides by human neutrophils. Proc Natl Acad Sci USA. 1983;80:6671–6675. doi: 10.1073/pnas.80.21.6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11a.Iovine, N. Unpublished data.
- 11b.Katz, S. S., Y. Weinrauch, R. S. Munford, P. Elsbach, and J. Weiss. Submitted for publication.
- 12.Lagrost L, Desrumaux C, Masson D, Deckert V, Gambert P. Structure and function of the plasma phospholipid transfer protein. Curr Opin Lipidol. 1998;9:203–209. doi: 10.1097/00041433-199806000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Luchi M, Munford R S. Binding, internalization, and deacylation of bacterial lipopolysaccharide by human neutrophils. J Immunol. 1993;151:959–969. [PubMed] [Google Scholar]
- 14.Mathison J, Wolfson E, Steinemann S, Tobias P, Ulevitch R. Lipopolysaccharide (LPS) recognition in macrophages. Participation of LPS-binding protein and CD14 in LPS-induced adaptation in rabbit peritoneal exudate macrophages. J Clin Investig. 1993;92:2053–2059. doi: 10.1172/JCI116801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDermott C, Fenwick B. Neutrophil activation associated with increased neutrophil acyloxyacyl hydrolase activity during inflammation in cattle. Am J Vet Res. 1992;53:803–807. [PubMed] [Google Scholar]
- 16.McDermott C M, Morrill J L, Fenwick B W. Deacylation of endotoxin during natural cases of bovine mastitis. J Dairy Sci. 1991;74:1227–1234. doi: 10.3168/jds.S0022-0302(91)78278-7. [DOI] [PubMed] [Google Scholar]
- 17.Munford R S, DeVeaux L C, Cronan J E, Jr, Rick P D. Biosynthetic radiolabeling of bacterial lipopolysaccharide to high specific activity. J Immunol Methods. 1992;148:115–120. doi: 10.1016/0022-1759(92)90164-o. [DOI] [PubMed] [Google Scholar]
- 18.Munford R S, Erwin A L. Eukaryotic lipopolysaccharide deacylating enzyme. Methods Enzymol. 1992;209:485–492. doi: 10.1016/0076-6879(92)09059-c. [DOI] [PubMed] [Google Scholar]
- 19.Munford R S, Hall C L. Uptake and deacylation of bacterial lipopolysaccharides by macrophages from normal and endotoxin-hyporesponsive mice. Infect Immun. 1985;48:464–473. doi: 10.1128/iai.48.2.464-473.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Munford R S, Hunter J P. Acyloxyacyl hydrolase, a leukocyte enzyme that deacylates bacterial lipopolysaccharides, has phospholipase, lysophospholipase, diacylglycerollipase, and acyltransferase activities in vitro. J Biol Chem. 1992;267:10116–10121. [PubMed] [Google Scholar]
- 21.Nigam V N, Malchow D, Rietschel E T, Lüderitz O, Westphal O. Enzymatic liberation of long-chain fatty acids from bacterial lipopolysaccharides with the aid of extracts from amoebae of Dictyostelium discoideum. Hoppe-Seyler’s Z Physiol Chem. 1970;351:1123–1132. [PubMed] [Google Scholar]
- 22.Patriarca P, Beckerdite S, Pettis P, Elsbach P. Phospholipid metabolism by phagocytic cells. VII. The degradation and utilization of phospholipids of various microbial species by rabbit granulocytes. Biochim Biophys Acta. 1972;280:45–56. [PubMed] [Google Scholar]
- 23.Pohlman T H, Munford R S, Harlan J M. Deacylated lipopolysaccharide inhibits neutrophil adherence to endothelium induced by lipopolysaccharide in vitro. J Exp Med. 1987;165:1393–1402. doi: 10.1084/jem.165.5.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riedo F X, Munford R S, Campbell W B, Reisch J S, Chien K R, Gerard R D. Deacylated lipopolysaccharide inhibits plasminogen activator inhibitor-1, prostacyclin, and prostaglandin E2 induction by lipopolysaccharide but not by tumor necrosis factor-alpha. J Immunol. 1990;144:3506–3512. [PubMed] [Google Scholar]
- 25.Schwab J H. Phlogistic properties of peptidoglycan-polysaccharide polymers from cell walls of pathogenic and normal-flora bacteria which colonize humans. Infect Immun. 1993;61:4535–4539. doi: 10.1128/iai.61.11.4535-4539.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smialowicz R J, Schwab J H. Processing of streptococcal cell walls by rat macrophages and human monocytes in vitro. Infect Immun. 1977;17:591–598. doi: 10.1128/iai.17.3.591-598.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Somerville J, Jr, Cassiano L, Bainbridge B, Cunningham M D, Darveau R P. A novel Escherichia coli lipid A mutant that produces an antiinflammatory lipopolysaccharide. J Clin Investig. 1996;97:359–365. doi: 10.1172/JCI118423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Staab J F, Ginkel D L, Rosenberg G B, Munford R S. A saposin-like domain influences the intracellular localization, stability, and catalytic activity of human acyloxyacyl hydrolase. J Biol Chem. 1994;269:23736–23742. [PubMed] [Google Scholar]
- 29.Tobias P S, Soldau K, Iovine N M, Elsbach P, Weiss J. Lipopolysaccharide (LPS)-binding proteins BPI and LBP form different types of complexes with LPS. J Biol Chem. 1997;272:18682–18685. doi: 10.1074/jbc.272.30.18682. [DOI] [PubMed] [Google Scholar]
- 30.Weinrauch Y, Elsbach P, Madsen L M, Foreman A, Weiss J. The potent anti-Staphylococcus aureus activity of a sterile rabbit inflammatory fluid is due to a 14-kD phospholipase A2. J Clin Investig. 1996;97:250–257. doi: 10.1172/JCI118399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weinrauch Y, Foreman A, Shu C, Zarember K, Levy O, Elsbach P, Weiss J. Extracellular accumulation of potently microbicidal bactericidal/permeability-increasing protein and p15s in an evolving sterile rabbit peritoneal inflammatory exudate. J Clin Investig. 1995;95:1916–1924. doi: 10.1172/JCI117873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wright G C, Weiss J, Kim K S, Verheij H, Elsbach P. Bacterial phospholipid hydrolysis enhances the destruction of Escherichia coli ingested by rabbit neutrophils. Role of cellular and extracellular phospholipases. J Clin Investig. 1990;85:1925–1935. doi: 10.1172/JCI114655. [DOI] [PMC free article] [PubMed] [Google Scholar]