Introduction
Follicular thyroid carcinoma (FTC) is a type of differentiated thyroid cancer (DTC) that accounts for about 6–10% of all thyroid cancers and has a good prognosis with a 10-year survival rate of over 90% (1,2). However, FTC can metastasize to distant organs via hematogenous spread, especially to the lungs and bones (3).
Breast metastasis from FTC is extremely rare, but possible, with only a few cases reported in the literature (Table 1) (4-11). The diagnosis of breast metastasis from FTC is challenging because it can mimic primary breast cancer in clinical presentation, imaging features, and histopathology (7,9). Most breast metastases are associated with poor prognosis because of their hematogenous nature (9). Therefore, targeted and more comprehensive diagnosis, treatment, and follow-up should be seriously considered.
Table 1. Review of literature on breast metastasis from follicular thyroid carcinoma including the present study.
| Reference | Age (years) | Sex | Metastases elsewhere | Time interval between primary and metastatic diagnosis | Breast imaging findings | Treatment and outcome |
|---|---|---|---|---|---|---|
| Chisholm et al. [1980] (4) |
75 | Female | Neck (lymph nodes) and lungs | 9 years | Xeromammogram: right breast, upper inner quadrant, mass measuring 40 mm × 50 mm | Resection of the thyroid mass, a modified left neck dissection and a simple mastectomy, radioiodine therapy, replacement therapy with levothyroxine; 6 months later, disappearance of lung metastasis and improvement of symptoms; CR |
| Ascani et al. [1994] (5) | 57 | Female | No | 15 years | Ultrasound and mammography: left breast, upper outer quadrant, nodule measuring 20 mm with ill-defined margins, no microcalcifications | Thyroidectomy, excision of the breast nodule, radioiodine therapy, and postoperative radiotherapy; no evidence of recurrent disease at 10 months follow-up; CR |
| Arslan et al. [2014] (6) | 57 | Female | Lungs and bones | 26 months | Mammography: left breast, mass lesion measuring 25 mm, no calcifications | Total thyroidectomy, radioiodine therapy, adriamycin chemotherapy, surgical excision of breast metastasis, zoledronic acid, supportive care; after 12 months following surgery to the breast she had no recurrent lesions either in the breast or other solid organs, and was on supportive care; SD |
| Candanedo-Gonzalez et al. [2015] (7) | 59 | Female | Bones | 1 year | CT chest (without contrast): left breast, deep plane under sternum, mass measuring 60 mm × 50 mm | The patient received radiotherapy for 3 months without response; PD |
| Tanriverdi et al. [2015] (8) |
68 | Female | Lungs, bones, and mediastinal lymph nodes | 1 year | PET/CT: left breast, superior interior quadrant, mass measuring 25 mm × 18 mm, increased metabolic activity, no micro-calcifications | Total thyroidectomy, palliative radiotherapy to vertebrae and sacrum, referred to nuclear medicine department for radioiodine therapy; no follow-up information |
| Jain et al. [2021] (9) | 55 | Female | Right lung, bones, mediastinal lymph nodes, and spleen | 24 years | Mammography and PET/CT: right breast parenchymal lesion, non-iodine avid on radioiodine scan, FDG-avid | Radioiodine therapy for iodine-avid metastatic disease; breast lesion remained radioiodine refractory; the patient may have a poor prognosis |
| Ertürk et al. [2022] (10) | 33 | Female | Lungs and bones | 17 years | PET/CT: right breast, upper-middle quadrant, nodular lesion measuring 11 mm × 10 mm, increased 18F-FDG uptake | Left neck region operation and right lumpectomy for breast lesion, followed by 200 mCi posttreatment I-131 whole-body scan; 13 months after treatment, thyroglobulin levels decreased; PR |
| Taha et al. [2022] (11) | 90 | Female | Skin and lung (location unspecified) |
12 years | Mammogram and ultrasound: left breast, upper outer quadrant, asymmetric round lesion measuring 8 mm in diameter | Core biopsy confirmed metastatic follicular carcinoma of the thyroid, TSH suppressive therapy, patient declined further intervention; PD |
| This report | 64 | Female | Right lung | 17 years | Ultrasound, mammography and PET/CT: left breast, upper internal quadrant, irregular nodule measuring 31 mm × 23 mm, with calcifications, increased FDG uptake | Total thyroidectomy, left segmental mastectomy, 3 cycles of radioiodine therapy and TSH suppressive therapy; CR of the recurrent FTC lesion and breast metastasis |
CT, computed tomography; PET, positron emission tomography; FDG, fluorodeoxyglucose; FTC, follicular thyroid carcinoma; CR, complete response; SD, stable disease; PD, progressive disease; PR, partial response; TSH, thyroid stimulating hormone.
This case report describes a rare and delayed complication of FTC, which is breast metastasis that occurred 17 years after partial thyroid lobectomy. In addition to discussing the main imaging manifestations, treatment options, and prognosis of this rare complication, we also provide a comprehensive review of the literature in order to gain additional insight into its clinical and pathological features.
Case presentation
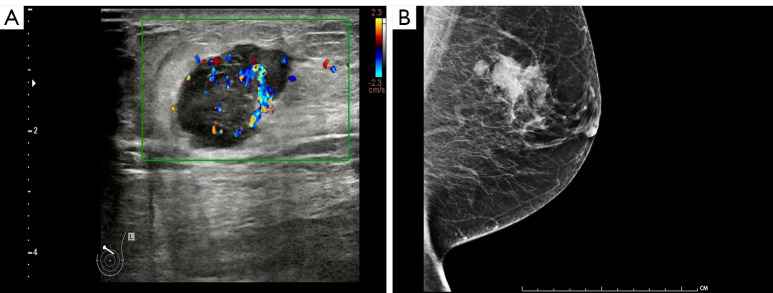
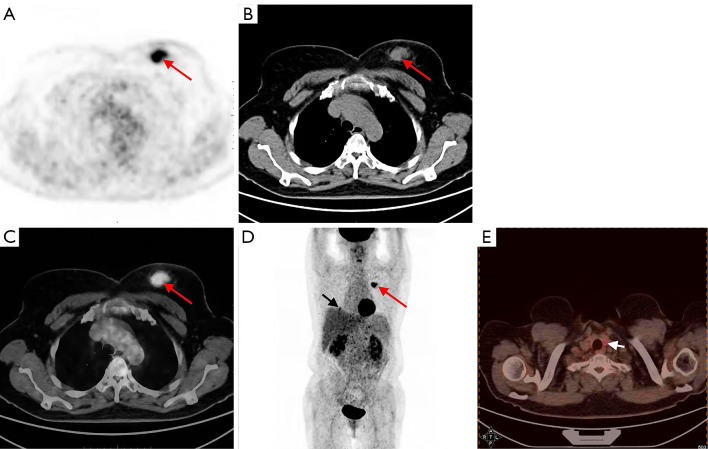
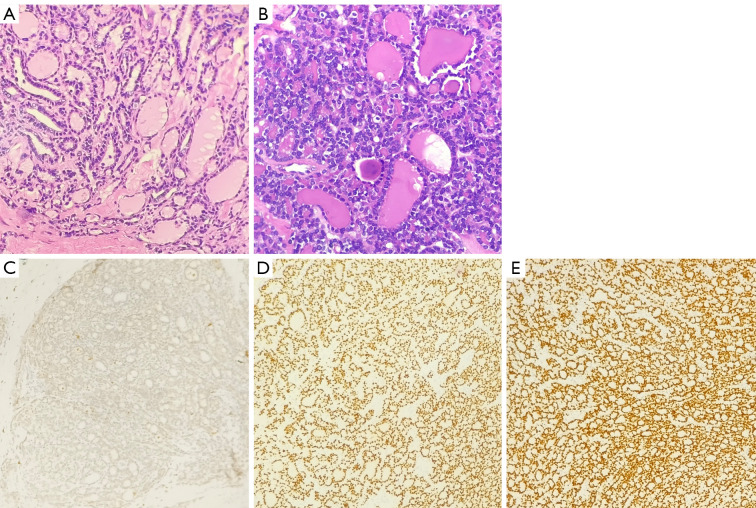
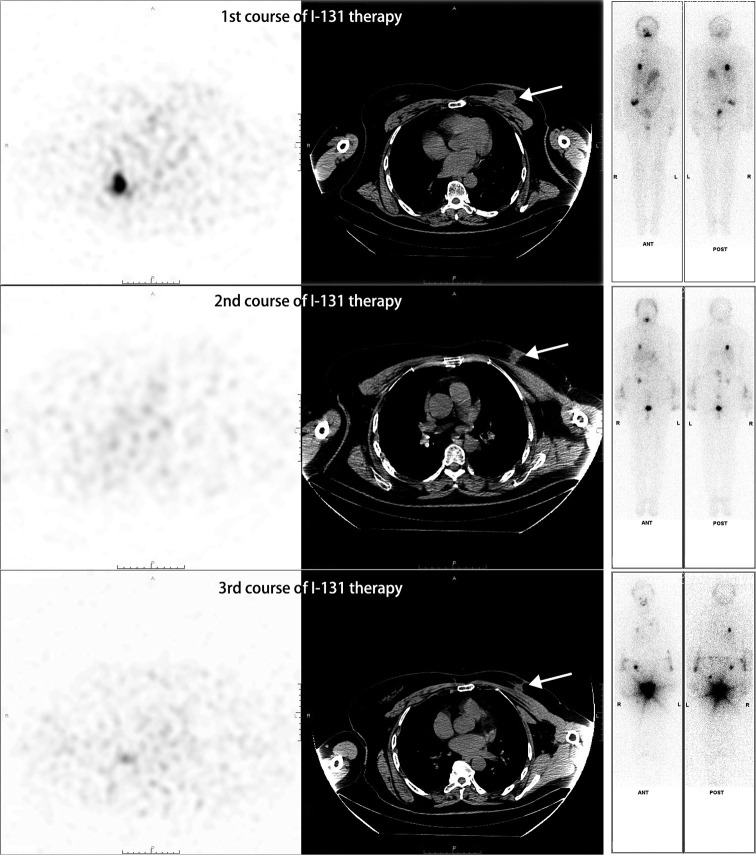
A 64-year-old woman, who had undergone partial thyroid lobectomy for primary FTC in the left lobe of the thyroid 17 years ago, presented with a palpable mass in the upper inner quadrant of the left breast as the main complaint, without any other symptoms. Ultrasound revealed an irregular hypoechoic mass on the left breast with well-defined borders and abundant blood flow signals, which measured about 2.6 cm × 1.9 cm × 2.2 cm (Figure 1A). She then underwent diagnostic mammography. Both craniocaudal and mediolateral oblique revealed an irregular nodule with ill-defined borders, and the surrounding gland structure was distorted; in addition, calcifications were evident in the left breast. No suspicious axillary lymph nodes were found (Figure 1B). The patient then underwent positron emission tomography-computed tomography (PET/CT) with fluorodeoxyglucose (FDG) to obtain baseline data for treatment, which revealed a hypermetabolic nodule in the upper inner quadrant of the left breast with a maximum standardized uptake value (SUVmax) of 7.08 and CT value of approximately 43.2 Hounsfield units (HU) (Figure 2), as well as hypermetabolic nodules in the right lower lung and thyroid gland. Considering her previous medical history of partial thyroid lobectomy, we speculated that thyroid cancer recurrence with breast and lung metastasis was the most likely diagnosis. Subsequently, the patient underwent total thyroidectomy and left segmental mastectomy. Postoperative pathology of the breast mass showed that a large number of abnormal glands were arranged in a follicle-like manner (Figure 3). This finding was not sufficient for the diagnosis of breast metastasis from FTC. Immunohistochemistry (IHC) further confirmed the diagnosis of breast metastasis from FTC, showing thyroid transcription factor-1 (TTF-1) (+), paired box gene 8 (Pax-8) (+), thyroglobulin (TG) (partially weak +), estrogen receptor (ER) (−), and progesterone receptor (PR) (−) (Figure 3). After surgical treatment, the patient received 3 cycles of I-131 radioablation therapy and thyroid stimulating hormone (TSH) suppressive therapy. During this period, the patient underwent I-131 whole-body scan 3 times, all of which showed negative results in the breast area (Figure 4). The patient underwent a follow-up PET/CT at 12 months after partial mastectomy, which showed a 1.0 cm × 1.0 cm nodule with mild metabolic activity in the left breast area, with a SUVmax of 1.32 (Figure 5). After discussion in the nuclear medicine department, the lesion was finally diagnosed as a postoperative change. This indicated that complete response (CR) was achieved for the recurrent FTC lesion and breast metastasis. The patient’s condition will be closely followed up. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was provided by the patient for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Figure 1.
Imaging examination of the left breast. (A) Ultrasound examination revealed an irregular hypoechoic mass in the left breast with well-defined borders and abundant blood flow signals, measuring approximately 2.6 cm × 1.9 cm × 2.2 cm. No suspicious axillary lymph nodes were found. (B) Mammography showed a high-density mass shadow in the left breast, with unclear boundaries and irregular shape, measuring approximately 3.1 cm × 2.3 cm, with distorted surrounding glandular structures. The left breast also showed orbit-like calcification.
Figure 2.
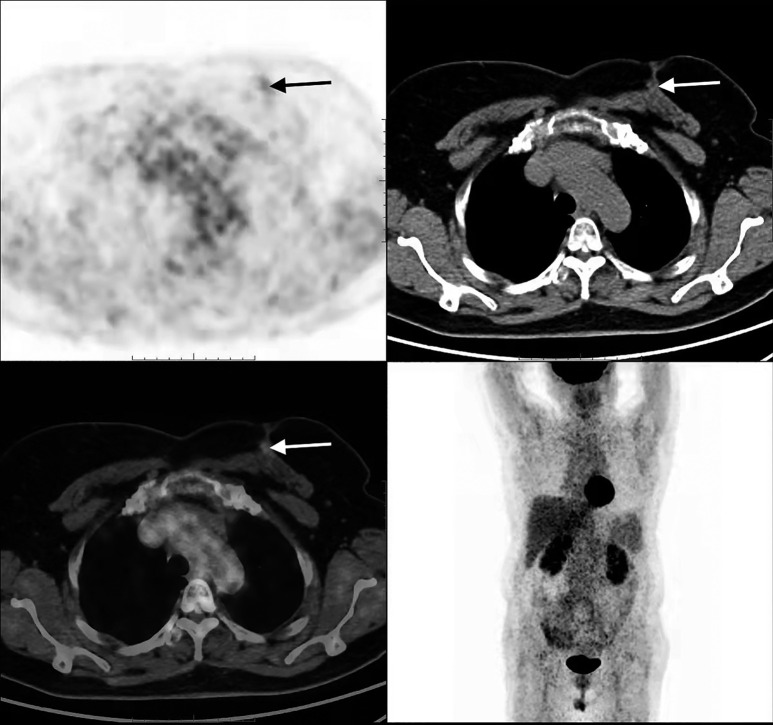
FDG PET/CT scan showing hypermetabolic activity in the breast, lung, and thyroid. (A) PET image of the breast metastasis. (B) CT image of the breast metastasis. (C) Fused PET/CT image of the breast metastasis. (D) Whole-body PET MIP image showing lung metastasis and breast metastasis. (E) Fused PET/CT image of the thyroid recurrence. FDG PET/CT revealed a hypermetabolic nodule (2.8 cm × 2.0 cm) with SUVmax of 7.08 in the upper internal quadrant of the left breast [red arrows in (A), (B), (C), and (D)], along with hypermetabolic nodules in the right lower lung [black arrow in (D)] and the thyroid gland [white arrow in (E)]. FDG, fluorodeoxyglucose; PET, positron emission tomography; CT, computed tomography; SUVmax, maximum standardized uptake value; MIP, maximum intensity projection.
Figure 3.
Histopathological and immunohistochemical features of the primary and metastatic lesions. (A) H&E stain (×200) of the left thyroid lobe showing FTC with colloid-filled follicles. (B) H&E stain (×200) of the left breast mass revealing a tumor composed of thyroid follicles filled with colloid, without a residual breast tissue, indicating metastatic rather than primary breast carcinoma. (C) GATA-3 immunostaining (×100) showing negative nuclear expression in the breast metastasis, supporting non-mammary origin. (D) PAX-8 immunostaining (×100) showing positive nuclear expression in the breast metastasis, confirming thyroidal origin. (E) TTF-1 immunostaining (×100) showing positive nuclear expression in the breast metastasis, further affirming its thyroidal derivation. H&E, hematoxylin and eosin; FTC, follicular thyroid carcinoma; GATA-3, GATA binding protein 3; PAX-8, paired box gene 8; TTF-1, thyroid transcription factor-1.
Figure 4.
The patient underwent left partial mastectomy and total thyroidectomy followed by 3 courses (2, 6, and 10 months after surgery, respectively) of I-131 therapy for the metastatic FTC. The I-131 ablative doses ranged from 100 to 150 mCi. I-131 WBS and SPECT/CT scan were performed five to seven days after each ablative treatment. During all 3 courses of ablation therapy, I-131 WBS showed negative result in the breast site, indicating the absence of significant breast metastasis after FTC. Additional SPECT/CT scans showed that the nodular shadow of the left breast surgical area was gradually absorbed with the 3 courses of I-131 therapy (arrow). FTC, follicular thyroid carcinoma; WBS, whole-body scan; SPECT, single photon emission computed tomography; CT, computed tomography.
Figure 5.
FDG PET/CT showed a nodular shadow (1.0 cm × 1.0 cm) with only mild metabolic activity (SUVmax of 1.32) in the left breast (arrow). FDG PET/CT, positron emission tomography/computed tomography with fluorodeoxyglucose; SUVmax, maximum standardized uptake value.
Discussion
This case report describes a rare and unusual presentation of FTC metastasis to the breast, which mimicked primary breast cancer on imaging and histopathology. The patient had undergone partial thyroid lobectomy for FTC before the breast metastasis was detected. Unlike primary breast carcinoma, architectural distortion, calcification, posterior acoustic shadowing, spiculated margins, and skin or nipple retraction are infrequent in breast metastatic lesions on mammography and ultrasound (12). Breast cancer may spread to regional lymph nodes, such as the axillary, interpectoral, supraclavicular, or internal mammary ones, which affects 25.4% of patients (13,14). Lymph node metastasis from FTC is rare, and it is more common in the central compartment than in the lateral compartment (15,16). The incidence of lymph node metastasis in FTC has been reported to range from 2% to 19%, depending on the degree of invasiveness and the diagnostic methods (16-18). However, in this case, the imaging presentation of breast metastasis was accompanied by distortion and calcification of the surrounding glands, which was not entirely consistent with conventional mammography findings (see Figure 1, Table 1). No abnormal axillary or neck lymph nodes were detected by ultrasonography before and after surgery. Meanwhile, it is noteworthy that neither the imaging tests nor the surgical procedure revealed any signs of local invasion of the primary FTC lesion in the left thyroid lobe. Moreover, the pathology examination of the right thyroid lobe showed no evidence of malignancy. These undoubtedly increased the difficulty of obtaining a correct diagnosis. Therefore, mammography and ultrasound alone do not completely explain the diagnosis of breast metastasis.
The pathology examination (either from the preoperative core biopsy or the postoperative tissue resection) was the only method that confirmed the metastasis of FTC to the breast (4-11). However, FDG PET/CT is a useful imaging modality for the early detection, staging, and response assessment of FTC breast metastasis. We reviewed 9 case reports on this rare complication of FTC (see Table 1) and found that FDG PET/CT was used in 4 of them to evaluate the breast lesions [(8-10), and this case report]. All of them showed significant FDG uptake in the breast metastases, consistent with the diagnosis of other distant metastases (8-10). This indicates that FDG PET/CT can sensitively detect the presence and distribution of FTC breast metastasis and facilitate the diagnosis and staging (8-10). Furthermore, only 1 case report of FTC breast metastasis without prior surgery underwent I-131 whole-body scan, which found the lesion to be non-iodine avid, discordant with the FDG PET/CT results (9) (Table 1). This suggests that FDG PET/CT in combination with I-131 whole-body scan can identify breast metastases from radioiodine-refractory FTC and help to select the optimal treatment strategy. Furthermore, 2 of the 9 case reports, including this case, conducted post-treatment FDG PET/CT follow-up and observed a decrease or disappearance of FDG uptake in the breast metastases, in accordance with the changes in serum TG levels (9). This demonstrates that FDG PET/CT can objectively monitor the treatment response of FTC breast metastasis and assist in adjusting and individualizing the treatment plan. Compared with breast ultrasound and mammography, FDG PET/CT can more accurately assess the size, shape, and metabolic activity of the breast metastases, as well as other potential metastatic sites (8-10). However, FDG PET/CT also has some limitations, such as the inability to differentiate breast metastases from primary breast cancer, and the lack of information on the iodine uptake of the metastatic lesions (8-10). One of the limitations in the case of our patient was that we did not scan with I-131 for the differential diagnosis of the lesion in the breast, which could have confirmed the presence or absence of iodine-avid metastasis and guided the optimal dose of radioiodine therapy (19). Therefore, FDG PET/CT should be combined with other imaging methods to improve the diagnosis and staging of FTC breast metastasis. Additionally, there is no standardized and validated criteria for FDG PET/CT interpretation and quantification in FTC breast metastasis, which may affect the reproducibility and comparability of the results. Further studies are needed to establish the optimal FDG PET/CT protocol and criteria for the diagnosis and staging of FTC breast metastasis.
For the management of DTC, consensus guidelines usually recommend total thyroidectomy, TSH suppression therapy, and I-131 radioablation for patients at high risk of recurrence (20). However, persistence/recurrence still occurs in 20–30% of these patients (20). In this case, the patient did not receive I-131 radioablation and TSH suppression after the initial partial thyroid lobectomy. Breast metastasis is a rare site of metastasis and guidelines are lacking. We performed total thyroidectomy and lumpectomy, followed by I-131 radioablation for this patient, for the following reasons: first, FTC has a worse prognosis than PTC (21); second, unlike FTC, which is confined to the thyroid or cervical lymph nodes, survival for patients with distant metastases drops to 50% (20); third, FDG-positive lesions on PET images reflect dedifferentiation of FTC lesions, indicating poor prognosis. Therefore, we suggest that I-131 radioablation alone is insufficient for treating breast metastases, and local surgical excision of recurrent foci and metastasis should be performed first. Incomplete tumor resection or high-risk patients require ongoing radioiodine therapy and TSH suppression. Here, we achieved CR of the recurrent FTC foci and breast metastasis in a short time with total thyroidectomy, segmental mastectomy, I-131 radioablation, and TSH suppression therapy.
The treatment strategies and outcomes of this condition are not well established due to the limited number of cases reported in the literature (4-11). In this review, we summarize the current evidence and recommendations for the management of breast metastasis from FTC based on the existing literature (Table 1). We used the databases of Medline, PubMed, Ovid, and Web of Science to gather relevant cases. The search covered a time range from 1980 to June 2024. The main treatment modalities for breast metastasis from FTC are radioiodine therapy (7 cases), surgical resection of the primary and metastatic lesions (5 cases), and TSH suppression therapy (2 cases). These 3 modalities, when combined, can achieve CR or partial response (PR) in cases. Some patients also received radiotherapy (3 cases), chemotherapy (1 case), or zoledronic acid (1 case) for palliative or adjuvant purposes. The prognosis of breast metastasis from FTC is poor, and the survival rate is lower than that of primary FTC (7,9). Therefore, patients with breast metastasis from FTC should receive comprehensive and individualized treatment and long-term follow-up.
The common sites of metastases elsewhere in patients with breast metastasis from FTC are lung, bone, lymph node, and spleen (Table 1). Lung metastasis was reported in 7 out of 9 cases, with 4 cases involving both lungs, 2 cases involving only the right lung, and 1 case with an unspecified lung location. Bone metastasis was reported in 5 out of 9 cases, mainly involving the sternum, skull, ribs, and lumbar vertebrae. Lymph node metastasis was reported in 3 out of 9 cases, mainly involving the cervical and mediastinal regions. Spleen metastasis was reported in 1 out of 9 cases. The possible mechanisms underlying these metastatic patterns are related to the rich blood supply and hematogenous dissemination of FTC, the blood circulation and high metabolic activity of bone marrow, the invasiveness and lymphatic pathways of FTC, and the blood filtration function of the spleen.
Conclusions
This case report emphasizes the importance of obtaining a detailed thyroid-related disease history and performing comprehensive imaging and pathological examinations for the differential diagnosis of FTC breast metastasis. We suggest that a combination of total thyroidectomy, segmental mastectomy, I-131 radioablation therapy, and TSH suppressive therapy can be an effective treatment option for this condition. FDG PET/CT can provide valuable information for the diagnosis, staging, and response assessment of FTC breast metastasis, and should be integrated with other imaging modalities and clinical data. Further studies are needed to establish the optimal FDG PET/CT protocol and criteria, and to explore the mechanisms and prognostic factors of FTC breast metastasis.
Supplementary
The article’s supplementary files as
Acknowledgments
We thank our colleague Lili Liu from the Department of Pathology for the diagnosis of this case, and the patient and her family members who agreed to the publication of this case.
Funding: This study was supported by Qingdao 2020 Medical Scientific Research Guidance Plan Project (No. 2020-WJZD078).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was provided by the patient for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Footnotes
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1260/coif). The authors have no conflicts of interest to declare.
References
- 1.Vishveshwaraiah PM, Mukunda A, Laxminarayana KK, Kasim K. Metastatic follicular thyroid carcinoma to the body of the mandible mimicking an odontogenic tumor. J Cancer Res Ther 2013;9:320-3. 10.4103/0973-1482.113412 [DOI] [PubMed] [Google Scholar]
- 2.Badulescu CI, Piciu D, Apostu D, et al. Follicular thyroid carcinoma - clinical and diagnostic findings in a 20-year follow up study. Acta Endocrinol (Buchar) 2020;16:170-7. 10.4183/aeb.2020.170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zunino A, Pitoia F, Faure E, Reyes A, Sala M, Sklate R, Ilera V, Califano I; . Unusual metastases from differentiated thyroid carcinoma: analysis of 36 cases. Endocrine 2019;65:630-6. 10.1007/s12020-019-01991-0 [DOI] [PubMed] [Google Scholar]
- 4.Chisholm RC, Chung EB, Tuckson W, Khan T, White JE. Follicular carcinoma of the thyroid with metastasis to the breast. J Natl Med Assoc 1980;72:1101-4. [PMC free article] [PubMed] [Google Scholar]
- 5.Cristallini EG, Ascani S, Nati S, Liberati F, Farabi R. Breast metastasis of thyroid follicular carcinoma. Acta Oncol 1994;33:71-3. 10.3109/02841869409098381 [DOI] [PubMed] [Google Scholar]
- 6.Arslan Ç, Coşkun HŞ, Göksu SS, et al. Follicular thyroid carcinoma with metastases to the breast: An unusual case. J Breast Health 2014;10:69-71. [Google Scholar]
- 7.Candanedo-Gonzalez F, Romero-Utrilla A, Osuna-Ramos JF, Alvarado-Sanchez C, Camacho-Rebollar L, Mendez-Perez VJ, Cordova-Uscanga C, Mora-Hernandez L. Occult follicular thyroid carcinoma presenting as primary breast tumor with sternal and skull metastasis. American Journal of Cancer Case Reports 2015;3:171-6. [Google Scholar]
- 8.Tanriverdi O, Avci A, Yugunt I, Polat M. A case report of breast and liver metastases of thyroid follicular carcinoma. J Cancer Res Ther 2015;11:652. 10.4103/0973-1482.138003 [DOI] [PubMed] [Google Scholar]
- 9.Jain TK, Krishnaraju VS, Mittal BR, Sood A, Kumar R, Garg R, Kumar S. Follicular Thyroid Carcinoma with Unusual Radioiodine-Refractory Breast Metastasis Mimicking Primary Breast Malignancy. J Nucl Med Technol 2021;49:288-9. 10.2967/jnmt.120.259259 [DOI] [PubMed] [Google Scholar]
- 10.Ertürk SA, Hasbek Z, Duman G, Sariakçali B. Breast metastasis in follicular thyroid cancer patient. J Cancer Res Ther 2022;18:S486-8. 10.4103/jcrt.JCRT_957_20 [DOI] [PubMed] [Google Scholar]
- 11.Taha A, Khalil A, Khan M, Metafa A. Breast metastasis from thyroid follicular carcinoma in a 90-year-old patient 12 years after thyroidectomy and radiotherapy. Radiol Case Rep 2022;17:3911-4. 10.1016/j.radcr.2022.07.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shetty MK. Imaging of the Symptomatic Breast. In Breast & Gynecological Diseases. Springer International Publishing: Cham, Germany; 2021. p. 27-79. [Google Scholar]
- 13.Recht A, Houlihan MJ. Axillary lymph nodes and breast cancer: a review. Cancer 1995;76:1491-512. [DOI] [PubMed] [Google Scholar]
- 14.Nathanson SD, Kwon D, Kapke A, Alford SH, Chitale D. The role of lymph node metastasis in the systemic dissemination of breast cancer. Ann Surg Oncol 2009;16:3396-405. 10.1245/s10434-009-0659-2 [DOI] [PubMed] [Google Scholar]
- 15.Stenman A, Kjellman M, Zedenius J, Juhlin CC. Synchronous lateral lymph node metastases from papillary and follicular thyroid carcinoma: case report and review of the literature. Thyroid Res 2022;15:1. 10.1186/s13044-022-00120-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Witte J, Goretzki PE, Dieken J, Simon D, Röher HD. Importance of lymph node metastases in follicular thyroid cancer. World J Surg 2002;26:1017-22. 10.1007/s00268-002-6668-y [DOI] [PubMed] [Google Scholar]
- 17.Machens A, Holzhausen HJ, Lautenschläger C, Thanh PN, Dralle H. Enhancement of lymph node metastasis and distant metastasis of thyroid carcinoma. Cancer 2003;98:712-9. 10.1002/cncr.11581 [DOI] [PubMed] [Google Scholar]
- 18.Zaydfudim V, Feurer ID, Griffin MR, Phay JE. The impact of lymph node involvement on survival in patients with papillary and follicular thyroid carcinoma. Surgery 2008;144:1070-7; discussion 1077-8. 10.1016/j.surg.2008.08.034 [DOI] [PubMed] [Google Scholar]
- 19.Silberstein EB, Alavi A, Balon HR, Clarke SE, Divgi C, Gelfand MJ, Goldsmith SJ, Jadvar H, Marcus CS, Martin WH, Parker JA, Royal HD, Sarkar SD, Stabin M, Waxman AD. The SNMMI practice guideline for therapy of thyroid disease with 131I 3.0. J Nucl Med 2012;53:1633-51. 10.2967/jnumed.112.105148 [DOI] [PubMed] [Google Scholar]
- 20.Simões-Pereira J, Mourinho N, Ferreira TC, Limbert E, Cavaco BM, Leite V. Avidity and Outcomes of Radioiodine Therapy for Distant Metastasis of Distinct Types of Differentiated Thyroid Cancer. J Clin Endocrinol Metab 2021;106:e3911-22. 10.1210/clinem/dgab436 [DOI] [PubMed] [Google Scholar]
- 21.Sugino K, Kameyama K, Nagahama M, Kitagawa W, Shibuya H, Ohkuwa K, Uruno T, Akaishi J, Suzuki A, Masaki C, Matsuzu K, Kawano M, Ito K. Follicular thyroid carcinoma with distant metastasis: outcome and prognostic factor. Endocr J 2014;61:273-9. 10.1507/endocrj.ej13-0437 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as