Introduction
Multilevel disc herniations are commonly occurring diseases that affect the quality of life of patients (1). A portion of disc herniation cases are resolved by spontaneous resorption, but this “self-healing” phenomenon does not occur in all patients, and its underlying mechanism remains poorly understood, particularly in the context of multilevel herniations (2). Therefore, the means to accurately identifying the surgical entry point and reducing unnecessary surgical intervention remains a surgical and clinical challenge. Double inversion recovery (DIR) is a pulse sequence that has proven highly effective at detecting cortical lesions, but its application in the spinal cord has not been extensively studied (3). Herein, we report an additional DIR sequence in the spinal cord that led to unexpected discoveries, which were inconsistent with the clinician’s initial assessment and led to a change in surgical approach.
Case presentation
Our patient was a 56-year-old man with a 3-week history of weakness in the right lower limb. This symptom suddenly progressed after he played badminton and was accompanied by walking with a dragging step and a band-like sensation around the chest. The patient had no pain or numbness of the limbs, no dizziness or blurred vision, no nausea or vomiting, and no chest tightness or palpitation. He was a nonsmoker, and his medical history was characterized by arterial atherosclerosis, arterial hypertension, and fatty liver. Three weeks prior to this admission, he underwent an appendectomy. No allergies were reported, and the results of standard blood tests were within the normal range.
Physical examination revealed weakness of the right lower limb with grade IV muscle strength, normal peripheral pulses, and normal sensibility. Bilateral Achilles tendon and knee tendon reflexes were active (right > left), which suggested that tendon reflexes were pathologically increased in the right lower limbs. The Babinski reflex, straight leg elevation test, and strengthening test of the right lower limb were positive. The muscle strength and tension of both upper limbs and left lower limbs were normal.
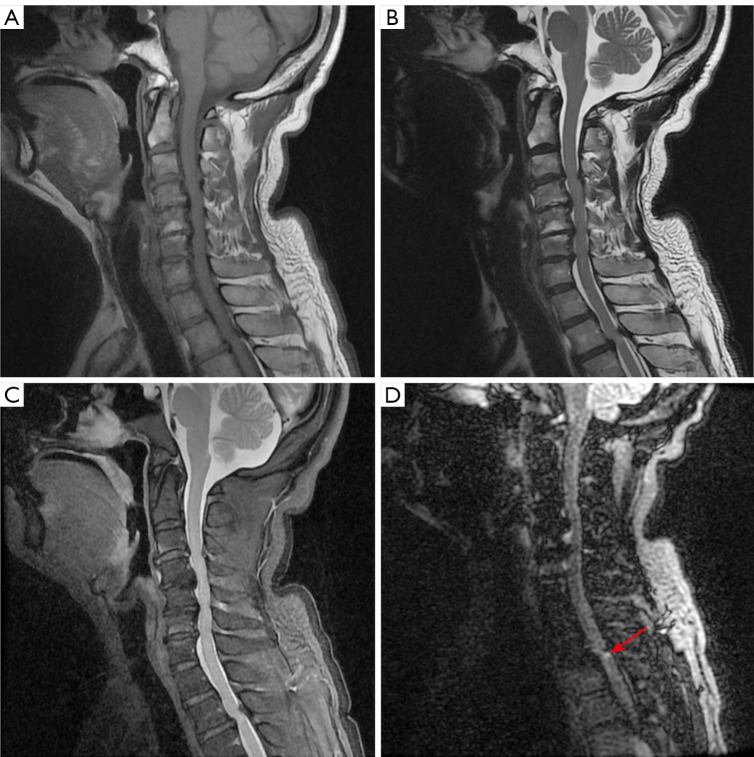
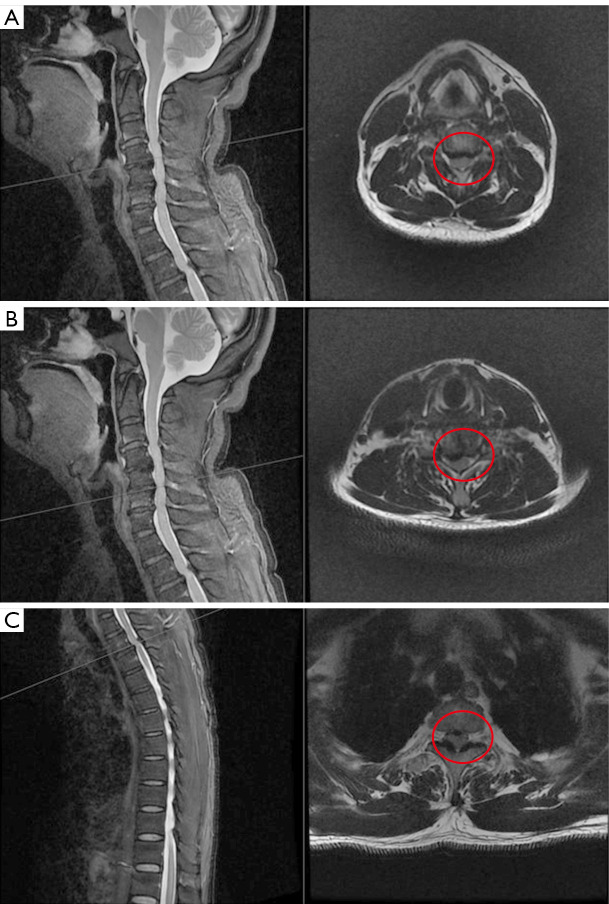
Given the clinical suspicion of spinal cord disorder, the patient was ordered to undergo electromyography and magnetic resonance imaging (MRI) of the spinal cord. Electromyography indicated that the right common peroneal nerve had been damaged, with accompanying chronic neurogenic lesions. According to our clinical experience, damage to the common peroneal nerve is invariably related to foot drop and varus, but our patient did not have these symptoms. The principal physician believed the electromyogram findings in this patient to have no specificity or clinical significance. MRI data were obtained on a 3-T MRI scanner (M750w, GE HealthCare, Chicago, IL, USA) with a 32-channel phased-array head coil. Spinal MRI displayed images compatible with multilevel disc herniation, extending from the third cervical vertebra to the seventh cervical vertebra (C3–C7), the first thoracic vertebra to the third thoracic vertebra (T1–3), and the second lumbar vertebra to the first sacral vertebra (L2–S1), which seriously indented the posterior cord of C3–7 vertebrae and the second thoracic vertebra to the third thoracic vertebra (T2–3) (Figure 1). Sagittal T2-weighted image (T2WI) showed a patchy, slightly high signal within the spinal cord at the T2–3 level (Figure 1B), which was more evident on fat-suppressed T2WI (Figure 1C). To visualize the lesion of the spinal cord clearly, we added an additional DIR sequence with the following parameters: long inversion time =2,894 ms, short inversion time =547 ms, repetition time =7,000 ms, echo time =90 ms, frequency field of view (FOV) =256 mm × 256 mm, phase FOV =95%, matrix of reconstruction =192×192, pixel size =1.3×1.3 mm2, slice thickness =1.3 mm, slice number =512, and time =592 s. The most striking finding of the DIR sequence was the abnormal signal at the posterior cord of the T2–3 vertebrae (Figure 1, red arrow), which was close to the area of the ligamentum flavum thickening, although the spinal canal stenosis was slightly more severe in the cervical vertebrae than in the thoracic vertebrae in the axial images (Figure 2, red circle).
Figure 1.
Sagittal imaging of the spine. (A) Sagittal T1WI. (B) Sagittal T2WI. (C) Sagittal T2WI with fat-saturated series. (D) DIR. Spine magnetic resonance imaging revealed multilevel disc herniation, the DIR sequence showed abnormal signals at the posterior cord of the second thoracic vertebra to the third thoracic vertebra (T2–3) (long red arrow). T1WI, T1-weighted image; T2WI, T2-weighted image; DIR, double inversion recovery.
Figure 2.
Comparison of the degree of spinal stenosis. (A) Sagittal and axial T2WI in the level of the fourth cervical vertebra to the fifth cervical vertebra (C4–5). (B) Sagittal and axial T2WI in the level of the fifth cervical vertebra to the sixth cervical vertebra (C5–6). (C) Sagittal and axial T2WI in the level of the second thoracic vertebra to the third thoracic vertebra (T2–3). Spine magnetic resonance imaging revealed multilevel disc herniation. Axial views at the level of the spinal canal at multiple segments are listed to compare their degree of stenosis (red circles). We found the spinal canal stenosis was more severe in the cervical vertebrae than in the thoracic vertebrae. T2WI, T2-weighted image.
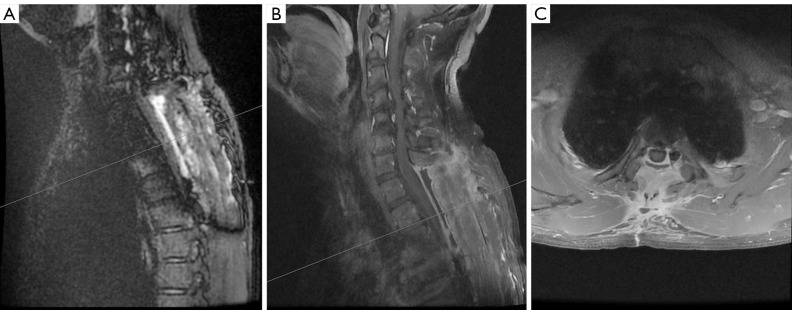
Considering the degree of spinal stenosis, the surgeon surmised that the fourth cervical vertebra to the fifth cervical vertebra (C4–5) and the fifth cervical vertebra to the sixth cervical vertebra (C5–6) were the best entry points for surgery. However, we believed that most of the patient’s clinical symptoms were caused by thoracic pulp compression based on the results of DIR. Thus, we suggested that the clinician perform a surgery of the T2–3 vertebrae and ligamentum flavum. After comprehensive evaluation, the surgeon decided to perform a posterior thoracic laminectomy decompression on the patient. Intraoperatively, the intervertebral disc at the T2–3 level was found to protrude into the spinal canal, the posterior ligamentum flavum was thickened, and the dural sac was compressed. Subsequently, an incision was made in the middle of the lower back, and surgical instruments were used to remove the spinous processes, lamina, and ligamentum flavum to provide extra space for alleviating pressure. Postoperatively, the patient’s muscle strength of the right lower limb gradually improved, with a muscle strength of grade V. MRI performed 3 months postoperatively showed no obvious cord abnormalities (Figure 3).
Figure 3.
Postoperative image of magnetic resonance imaging. (A) Sagittal double inversion recovery. (B) Sagittal T2WI. (C) Sagittal and axial T2WI in the level of the second thoracic vertebra to the third thoracic vertebra (T2–3). Magnetic resonance imaging performed 3 months postoperatively showed no obvious spinal cord abnormalities. T2WI, T2-weighted image.
All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
In this case, an additional DIR sequence in the spinal cord revealed unexpected discoveries, which was inconsistent with the clinician’s initial assessment, and ultimately led to a change in the surgical approach.
DIR, which has gained increasing attention over the past 10 years, is a combination of two inversion pulses with two different inversion times designed to suppress signals from the white matter and cerebrospinal fluid (4). The underlying mechanism of DIR involves producing prominent gray-matter contrast with high spatial resolution and improving the sensitivity and contrast in delineating white-matter signal abnormalities (5). Several studies have shown that this sequence is highly sensitive to inflammatory demyelinating lesions, especially cerebral cortical lesions in multiple sclerosis (MS) (6,7). Therefore, previous clinical application of the DIR sequence has been mostly limited to the detection of MS lesions, particularly intracranial and intracortical lesions.
It is often assumed that the clinical symptoms of disc herniation are caused by compression on the nerve root, which is attributed to simple mechanical involvement. However, the symptoms are typically complex, which is attributed to both chemical and mechanical factors (8). There is some experimental evidence suggesting that the nucleus pulposus in the inner part of a disc has been shown to induce an inflammatory-like reaction in the nerve root after the annulus fibrosus is torn (9). Other significant findings also indicate that disc herniation is highly associated with inflammation in the context of pain generation (10,11). As a chemical effect, the inflammatory process has been confirmed to be critical in the development of degenerative spondylopathy (12).
The diagnosis and treatment of disc herniation are relatively mature in modern medical care, given that accurate evaluation of the spinal cord and spinal canal is crucial for diagnosis and prognosis. The conventional sequences of the spinal cord are the sagittal T1-weighted (T1W), sagittal T2W, axial T2W, fluid-attenuated inversion recovery (FLAIR), and T2W with fat-saturated series. Typically, we evaluate the stenosis of the spinal canal and compression of the spinal cord in patients with disc herniation via sagittal and axial T2W images to assist the surgeon in identifying the optimal location for surgical entry.
To the best of our knowledge, DIR is rarely used in routine spinal imaging. Although DIR is not a new technique, MRI of the spinal cord is still challenging, because of the small size of the spinal cord and its artifacts caused by deglutition, respiration, and cardiac pulsation (13). Recently, some scholars have found that even for spinal cord lesions, the DIR sequence is superior to routinely used sequences, as it can demonstrate a greater number of lesions, particularly in the cervical cord (14). Thus, an analogous DIR sequence for spinal cord imaging should be developed and efficiently used in clinical practice and research.
In our case, the conventional sequences revealed that the spinal canal stenosis was more severe in the cervical vertebrae than in the thoracic vertebrae, but the DIR sequence only showed the abnormal signal at the posterior cord of the T2–3 vertebrae, which was close to the area of ligamentum flavum thickening. Based on our experience with DIR’s high sensitivity in displaying inflammatory demyelinating lesions, we considered that most of the patient’s clinical symptoms were caused by thoracic pulp compression, and choosing the T2–3 level as a surgical entry point could reduce unnecessary surgical intervention for this patient. The final result of the operation also supported our hypothesis.
The limitations of the DIR sequence include a longer scanning time; thus, more effort should be invested to optimize the applicability of DIR as a diagnostic modality in clinical practice. However, we deem that a DIR sequence for spinal cord imaging would be more sensitive than would a conventional sequence, which clinicians should be aware of and apply in a rational manner.
In the case reported here, we refer to the application and advantages of the DIR sequence in spinal cord imaging, which provides a new direction for the diagnosis and precision therapy of multilevel disc herniation. Furthermore, wider use of the DIR sequence is anticipated in clinical settings to assist in selecting the surgical entry point of disc herniation.
Supplementary
The article’s supplementary files as
Acknowledgments
We thank all the participants for their cooperation during this study and Charlesworth Author Services for providing professional language polishing for our manuscript.
Funding: This work was supported by the Pudong New Area Science and Technology Development Fund-Livelihood Research Fund (grant No. PKJ2020-Y24).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Footnotes
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-1008/coif). The authors have no conflicts of interest to declare.
References
- 1.Cunha C, Silva AJ, Pereira P, Vaz R, Gonçalves RM, Barbosa MA. The inflammatory response in the regression of lumbar disc herniation. Arthritis Res Ther 2018;20:251. 10.1186/s13075-018-1743-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hornung AL, Rudisill SS, Barajas JN, Harada G, Fitch AA, Leonard SF, Roberts AC, An HS, Albert HB, Tkachev A, Samartzis D. How Does Resorption Differ Among Single-Level and Multilevel Lumbar Disc Herniations? A Prospective Multi-Imaging and Clinical Phenotype Study. Spine (Phila Pa 1976) 2024;49:763-71. 10.1097/BRS.0000000000004955 [DOI] [PubMed] [Google Scholar]
- 3.Patel S, Dimaandal I, Kuhns T, Creed M, Gershon A, Wolansky I, Imitola J, Wolansky L. Double inversion recovery to detect cervical spinal cord multiple sclerosis lesions. J Neuroimaging 2023;33:521-6. 10.1111/jon.13100 [DOI] [PubMed] [Google Scholar]
- 4.Kulkarni S, Kulkarni MM, Patankar A, Watve A. Role of Double Inversion Recovery Sequence in Neuro-imaging on 3 Tesla MRI. Neurol India 2021;69:394-6. 10.4103/0028-3886.314551 [DOI] [PubMed] [Google Scholar]
- 5.Sharma R, Sekhon S, Lui F, Cascella M. White Matter Lesions. Treasure Island, FL, USA: StatPearls Publishing; 2024. [PubMed] [Google Scholar]
- 6.Umino M, Maeda M, Ii Y, Tomimoto H, Sakuma H. 3D double inversion recovery MR imaging: Clinical applications and usefulness in a wide spectrum of central nervous system diseases. J Neuroradiol 2019;46:107-16. 10.1016/j.neurad.2018.06.002 [DOI] [PubMed] [Google Scholar]
- 7.Dobson R, Giovannoni G. Multiple sclerosis - a review. Eur J Neurol 2019;26:27-40. 10.1111/ene.13819 [DOI] [PubMed] [Google Scholar]
- 8.Cosamalón-Gan I, Cosamalón-Gan T, Mattos-Piaggio G, Villar-Suárez V, García-Cosamalón J, Vega-Álvarez JA. Inflammation in the intervertebral disc herniation. Neurocirugia (Astur : Engl Ed) 2021;32:21-35. 10.1016/j.neucie.2020.04.001 [DOI] [PubMed] [Google Scholar]
- 9.Ethemoğlu KB, Erkoç YS. Is There Any Relationship Between Cervical Disc Herniation and Blood Inflammatory Response? Cureus 2020;12:e10161. 10.7759/cureus.10161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pedersen LM, Schistad E, Jacobsen LM, Røe C, Gjerstad J. Serum levels of the pro-inflammatory interleukins 6 (IL-6) and -8 (IL-8) in patients with lumbar radicular pain due to disc herniation: A 12-month prospective study. Brain Behav Immun 2015;46:132-6. 10.1016/j.bbi.2015.01.008 [DOI] [PubMed] [Google Scholar]
- 11.Ford JJ, Kaddour O, Gonzales M, Page P, Hahne AJ. Clinical features as predictors of histologically confirmed inflammation in patients with lumbar disc herniation with associated radiculopathy. BMC Musculoskelet Disord 2020;21:567. 10.1186/s12891-020-03590-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen B, Liu Y, Zhang Y, Li J, Cheng K, Cheng L. IL-21 Is Positively Associated with Intervertebral Disc Degeneration by Interaction with TNF-α Through the JAK-STAT Signaling Pathway. Inflammation 2017;40:612-22. 10.1007/s10753-017-0508-6 [DOI] [PubMed] [Google Scholar]
- 13.Riederer I, Karampinos DC, Settles M, Preibisch C, Bauer JS, Kleine JF, Mühlau M, Zimmer C. Double inversion recovery sequence of the cervical spinal cord in multiple sclerosis and related inflammatory diseases. AJNR Am J Neuroradiol 2015;36:219-25. 10.3174/ajnr.A4093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gulati P. Double Inversion Recovery: Another Feather in MRI Cap. Neurol India 2021;69:397-8. 10.4103/0028-3886.314550 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as