Cystatin C is a kidney filtration marker that, when used in combination with serum creatinine, provides a more precise estimate of glomerular filtration rate (eGFR) than using serum creatinine alone.1 Major kidney organizations, including the National Kidney Foundation, the American Society of Nephrology, and the Kidney Disease: Improving Global Outcomes have urged incorporation of cystatin C testing in routine clinical care.2,3 Despite these recommendations, prevalence of cystatin C testing is low and many clinicians are unfamiliar with the assay.4 In contrast, cystatin C testing has been widely performed in Sweden for over a decade.5 Using the health system in Stockholm, Sweden as a model, we report rates of cystatin C testing and clinical characteristics associated with cystatin C testing.
Methods
Study Sample
We used data from the Stockholm Creatinine Measurements project, a healthcare utilization cohort from the region of Stockholm, Sweden with data collected between 2006 to 2019, the details of which have been described previously.6 In brief, 20% to 25% of the population of Sweden resides in the Stockholm region and are covered by universal health care. Data from these individuals on demographics, laboratory testing, vital measurements, medications, and diagnoses was extracted as part of the Stockholm Creatinine Measurements project. The Regional Ethical Review Board in Stockholm approved the study (reference 2017/793–31); informed consent was not deemed necessary since all data were de-identified at the Swedish Board of Health and Welfare.
We included all adult patients (age ≥18 years) with a plasma creatinine tested between January 1, 2010, and December 31, 2018 (N = 1,369,183).
Supplemental methods detail the measurements for eGFRcr and eGFRcys as well as the covariates and outcomes.
Analyses
Detailed analyses description may be found in the Supplement. In brief, we described the number of participants with cystatin C testing by year and then, using 2014 as a cross-sectional sample, compared people who received additional cystatin C testing with those who received creatinine testing alone. We used logistic regression to examine associations of all covariates with cystatin C testing status in a multivariate model.
We evaluated the frequency of re-testing of cystatin C within 5 years and evaluated characteristics associated with retesting using multivariate logistic regression. Within those individuals who had cystatin C testing prior to 2014 and re-testing 1 to 5 years later, we compared the percent change in eGFRcr with the percent change in eGFRcys, estimating the sensitivity and specificity of 30% decline in eGFRcr for detecting a 30% decline in eGFRcys.
Results
From 2010 through 2018 in the Stockholm region, 1,369,183 adults received creatinine tests, and of those, 11.2% (N = 152,669) ever had cystatin C tested. On an annual basis, between 4% to 7% of individuals with creatinine testing had cystatin C testing each year (Supplementary Figure S1). The highest proportion of cystatin C testing was among individuals with lower eGFRcr and higher albuminuria (Supplementary Table S1, Supplementary Figure S2).
Those who had both creatinine and cystatin C tested were more likely be male, have a lower eGFRcr, and have more comorbidities, compared to those with only creatinine tested (Table 1). Those with albuminuria testing were much more likely to receive cystatin C testing than those without (12% vs. 4.8%; adjusted odds ratio [95% CI]: 2.27 [2.22, 2.33]; Supplementary Figure S3, Supplementary Table S2–S3). Among people without albuminuria testing, older age was a strong predictor for receiving cystatin C testing. The odds of cystatin C testing per 10 years older was 0.80 (0.79, 0.81) in those with albuminuria testing and 1.13 (1.12, 1.15) in those without albuminuria testing. Women were much less likely to receive cystatin C testing than men in both groups. This was consistent in all years of data from 2010–2018 (Supplementary Table S4A–S4I).
Table 1.
Characteristics of individuals tested for creatinine and/or cystatin C in 2014
| Characteristics | Overall | Cystatin C and creatinine tested | Only creatinine tested |
|---|---|---|---|
| N | 552909 | 37100 | 515809 |
| eGFRcr (SD), ml/min per 1.73m2 | 90 (22) | 75 (27) | 91 (21) |
| eGFRcys (SD), ml/min per 1.73m2 | 69 (32) | 69 (32) | |
| KDIGO G-stage by eGFRcr, % | |||
| eGFR 90 + ml/min per 1.73m2 | 55 | 33 | 57 |
| eGFR 60–89 ml/min per 1.73m2 | 36 | 38 | 36 |
| eGFR 45–59 ml/min per 1.73m2 | 5.6 | 14 | 5.1 |
| eGFR 30–44 ml/min per 1.73m2 | 2.3 | 9.5 | 1.8 |
| eGFR <30 ml/min per 1.73m2 | 1.0 | 6.3 | 0.64 |
| Any urine protein measured, % | 26 | 47 | 24 |
| ACR/PCR measured, % | 13 | 34 | 11 |
| Dipstick measured, % | 13 | 13 | 13 |
| ACR/PCRa (IQI), mg/g | 14 (4–69) | 16.8 (4.4–110.6) | 8.0 (2.7–23.9) |
| Dipstick + and above, % | 6.1 | 8.2 | 5.9 |
| Age (SD), yr (OR per 10 yr) | 58 (19) | 63 (18) | 57 (19) |
| Female, % | 55 | 46 | 55 |
| Hypertension, % | 47 | 68 | 45 |
| Hypertension medication use, % | 44 | 64 | 42 |
| RAAS inhibitor use, % | 30 | 45 | 28 |
| Diuretics, % | 19 | 34 | 18 |
| Diabetes, % | 13 | 23 | 13 |
| Statin, % | 19 | 29 | 18 |
| History of coronary heart disease, % | 6.7 | 12 | 6.3 |
| History of cerebrovascular disease, % | 5.7 | 9.8 | 5.4 |
| History of heart failure, % | 5.5 | 13 | 5.0 |
| History of peripheral artery disease, % | 1.2 | 2.7 | 1.04 |
| History of atrial fibrillation, % | 8.0 | 15.8 | 7.4 |
| Liver disease, % | 2.4 | 3.7 | 2.3 |
| Recent cancer, % | 12 | 17 | 11 |
| Chronic obstructive pulmonary disease, % | 4.1 | 6.8 | 3.9 |
| Potassium >5 mmol/l, % | 0.28 | 1.1 | 0.22 |
| Anemia by hemoglobin,b % | 4.3 | 9.1 | 4.0 |
Measurement from 2014 used as a representative cross-sectional sample. Percents are of the column sample.
ACR/PCR, albumin-to-creatinine ratio or protein-to-creatinine ratio ;eGFRcr, estimated glomerular filtration rate based on creatinine; eGFRcys, estimated glomerular filtration rate based on cystatin C; RAAS, renin-angiotensin-aldosterone system.
ACR measured in 2014, imputed by PCR if ACR was not available.
Anemia is defined as hemoglobin<12 g/dL for female and <13 g/dl for male.
Of the 80,560 individuals with an initial cystatin C test before 2014, 41,109 (51%) were retested within the subsequent 1 to 5 years. The clinical characteristics related to retesting cystatin C in the subsequent 5 years were like those related to initial testing: patients with retesting were more likely to have a lower eGFR and higher albuminuria, and more likely to have had albuminuria measured (Supplementary Table S5). Patients who underwent retesting were no longer likely to have large discrepancies (>30%) between eGFRcys and eGFRcr than people who were not retested.
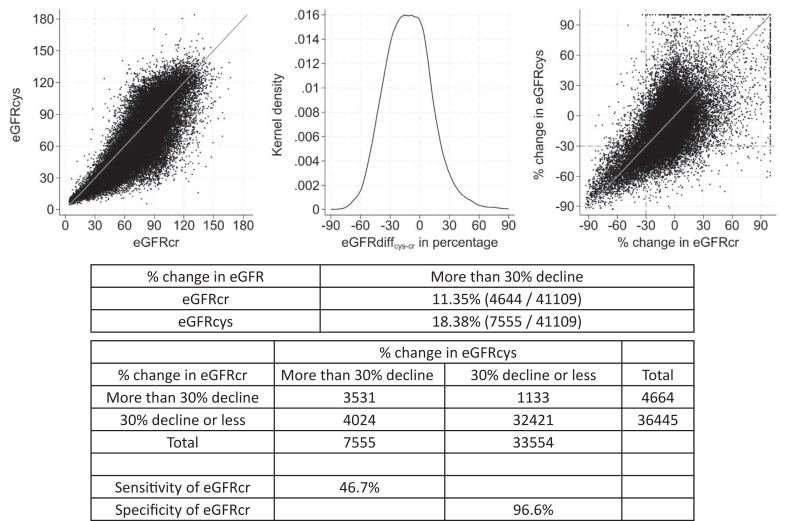
To assess whether increased cystatin C testing could identify people with unrecognized, meaningful kidney function decline, we assessed the incidence of >30% decline in eGFRcr and eGFRcys as well as the sensitivity and specificity of the former to detect the latter. Among people with cystatin C retesting, more individuals had a decline of more than 30% in eGFRcys than in eGFRcr (18.4% with decline of more than 30% in eGFRcys and 11.4% with decline of more than 30% in eGFRcr; Figure 1). For a decline of more than 30% in eGFRcys, a more than 30% decline in eGFRcr had a sensitivity of 47% and a specificity of 97%.
Figure 1.
Plot of eGFRcys vs. eGFRcr values at first measurement, kernel density of difference of eGFRcys-eGFRcr at first measurement, percent change in eGFRcys vs. eGFRcr over up to 5 calendar years, and the sensitivity and specificity of a decline of 30% or more in eGFRcr. Included the population with any cystatin C measurement by 2014 to allow for up to 5 years of follow-up for retesting. eGFRcr, estimated glomerular filtration rate based on creatinine; eGFRcys, estimated glomerular filtration rate based on cystatin C.
Discussion
Using practice patterns in Stockholm, Sweden, as an example of how cystatin C is used in real-world clinical care, we highlighted which patients were tested, how often they were tested, and what extra insight cystatin C might bring to clinical care. Among 1,369,183 adult individuals who received creatinine testing, 11% were also tested for cystatin C, at a rate of approximately 5% annually. While much higher than estimates of current use in the US, this is still a modest amount of testing given the long-standing availability and low cost of cystatin C in Sweden. Cystatin C measurement is recommended in Kidney Disease: Improving Global Outcomes guidelines for CKD staging, as a confirmatory test in circumstances in which serum creatinine may be less accurate, and where precision is required for medication dosing.3 Specific patient characteristics were highly correlated with cystatin C testing. Individuals with testing for albuminuria, a vastly underutilized biomarker,7 were more likely to have testing for cystatin C. For example, almost half of individuals with cystatin C testing in 2014 also had albuminuria testing. Cystatin C testing was also more likely among an older population. We can speculate that clinicians treating this older patient group were concerned about the non-GFR determinants of creatinine in the setting of frailty and muscle loss. These practice patterns are consistent with the detailed scenarios described by Chen et al.4 in which non-GFR determinants may affect creatinine and inform clinical management.4
Our observation that declines in eGFRcr and declines in eGFRcys are not fully consistent builds upon previous evidence that there are frequently cross-sectional differences in the 2 measures and that the proportion of individuals with a large difference may increase over time.8 It has been shown that eGFRcr-cys is the best measure with respect to measured GFR in most settings,1,9 including when there are large discrepanciesS3 Discrepant eGFRcr and eGFRcys is a strong risk factor for mortality and other clinical outcomes,8,S4–S6 and our results show a 30% decline in eGFRcr had only moderate sensitivity for a decline of 30% in eGFRcys, underscoring the utility of testing both creatinine and cystatin C over time in order to best assess the kidney function of patients.S4
Strengths of this study include the large sample size in a unique setting in which there is a longstanding history of cystatin C testing in routine care. However, we are limited in that we can only provide provider reasons for testing and any subsequent actions, as we do not have access to medical notes. Given the unique nature of the setting for this analysis, these results may not translate to other settings in which costs of testing, including the costs for reagents, laboratory time, and reimbursement logistics, lack of health care insurance coverage, and provider awareness and comfort of using cystatin C in routine care are large factors.
This study provides a real-world account of cystatin C testing, finding testing rates reach 4% to 7% of patients with serum creatinine tests. Decline in eGFRcr and eGFRcys were only moderately concordant, underlying the potential utility in testing both markers.
Disclosures
Dr. Nitsch reports other support from UK Kidney Association outside the submitted work. Dr. Fu reports grants from Dutch Kidney Foundation, grants from Netherlands Organization of Scientific Research, and grants from Karolinska Institute during the conduct of the study. All the authors declared no competing interests.
Funding
This study was supported by the Swedish Research Council (#2023–01807), National Institute of Diabetes and Digestive and Kidney Diseases grants R01DK100446 and R01DK115534, the Swedish Heart and Lung Foundation (20230371), Region Stockholm (ALF Medicine, FoUI–986028), and a Junior Kolff Grant from the Dutch Kidney Foundation (22OK2026). Dr. Fu is supported by a VENI grant from the Netherlands Organization for Scientific Research and an internal funding grant from Karolinska Institute.
Footnotes
Supplementary Methods.
Supplementary References.
Figure S1. Proportion with cystatin C testing by calendar year among individuals with measured serum creatinine
Figure S2. Trends in proportion of the population with cystatin C testing by year, stratified by G- and A-stage.
Figure S3. Percent with cystatin C testing in 2014, stratified by eGFRcr stages and albuminuria testing status.
Table S1. Population with serum creatinine tested and proportion with testing cystatin C by year, stratified by G- and A-stage.
Table S2. Characteristics of individuals tested for creatinine and/or cystatin C in 2014, stratified by ACR testing status.
Table S3. Odd ratios and 95% CIs for cystatin C testing, overall and by ACR testing status.
Table S4A–I. Characteristics of individuals tested for creatinine and/or cystatin C in 2010–2018.
Table S5. Characteristics at first cystatin C measurement and odd ratios and 95% CIs for cystatin C re-testing.
Table S6. Definition of study covariates.
Supplementary Material
Supplementary Methods. Supplementary References. Figure S1. Proportion with cystatin C testing by calendar year among individuals with measured serum creatinine. Figure S2. Trends in proportion of the population with cystatin C testing by year, stratified by G- and A-stage. Figure S3. Percent with cystatin C testing in 2014, stratified by eGFRcr stages and albuminuria testing status.
Table S1. Population with serum creatinine tested and proportion with testing cystatin C by year, stratified by G- and A-stage. Table S2. Characteristics of individuals tested for creatinine and/or cystatin C in 2014, stratified by ACR testing status. Table S3. Odd ratios and 95% CIs for cystatin C testing, overall and by ACR testing status. Table S4A–I. Characteristics of individuals tested for creatinine and/or cystatin C in 2010–2018. Table S5. Characteristics at first cystatin C measurement and odd ratios and 95% CIs for cystatin C re-testing. Table S6. Definition of study covariates.
References
- 1.Inker L.A., Eneanya N.D., Coresh J., et al. New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med. 2021;385:1737–1749. doi: 10.1056/NEJMoa2102953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Delgado C., Baweja M., Crews D.C., et al. A unifying approach for GFR estimation: recommendations of the NKF-ASN task force on reassessing the inclusion of race in diagnosing kidney disease. J Am Soc Nephrol. 2021;32:2994–3015. doi: 10.1681/ASN.2021070988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.KDIGO K. Clinical practice guideline for the evaluation and management of chronic kidney disease: public review draft. 2023. https://kdigo.org/wp-content/uploads/2017/02/KDIGO-2023-CKD-Guideline-Public-Review-Draft_5-July-2023.pdf
- 4.Chen D.C., Potok O.A., Rifkin D., Estrella M.M. Advantages, limitations, and clinical considerations in using cystatin C to estimate GFR. Kidney360. 2022;3:1807–1814. doi: 10.34067/KID.0003202022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grubb A., Simonsen O., Sturfelt G., Truedsson L., Thysell H. Serum concentration of cystatin C, factor D and beta 2-microglobulin as a measure of glomerular filtration rate. Acta Med Scand. 1985;218:499–503. doi: 10.1111/j.0954-6820.1985.tb08880.x. [DOI] [PubMed] [Google Scholar]
- 6.Carrero J.J., Elinder C.G. The Stockholm CREAtinine Measurements (SCREAM) project: fostering improvements in chronic kidney disease care. J Intern Med. 2022;291:254–268. doi: 10.1111/joim.13418. [DOI] [PubMed] [Google Scholar]
- 7.Shin J.I., Chang A.R., Grams M.E., et al. Albuminuria testing in hypertension and diabetes: an individual-participant data meta-analysis in a global consortium. Hypertension. 2021;78:1042–1052. doi: 10.1161/HYPERTENSIONAHA.121.17323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farrington D.K., Surapaneni A., Matsushita K., Seegmiller J.C., Coresh J., Grams M.E. Discrepancies between cystatin C-based and creatinine-based eGFR. Clin J Am Soc Nephrol. 2023;18:1143–1152. doi: 10.2215/CJN.0000000000000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu E.L., Levey A.S., Coresh J., et al. Accuracy of GFR estimating equations based on creatinine, cystatin C or both in routine care. Nephrol Dial Transplant. 2024;39:694–706. doi: 10.1093/ndt/gfad219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Methods. Supplementary References. Figure S1. Proportion with cystatin C testing by calendar year among individuals with measured serum creatinine. Figure S2. Trends in proportion of the population with cystatin C testing by year, stratified by G- and A-stage. Figure S3. Percent with cystatin C testing in 2014, stratified by eGFRcr stages and albuminuria testing status.
Table S1. Population with serum creatinine tested and proportion with testing cystatin C by year, stratified by G- and A-stage. Table S2. Characteristics of individuals tested for creatinine and/or cystatin C in 2014, stratified by ACR testing status. Table S3. Odd ratios and 95% CIs for cystatin C testing, overall and by ACR testing status. Table S4A–I. Characteristics of individuals tested for creatinine and/or cystatin C in 2010–2018. Table S5. Characteristics at first cystatin C measurement and odd ratios and 95% CIs for cystatin C re-testing. Table S6. Definition of study covariates.