Introduction
Membranous glomerulonephritis (MGN) is an autoimmune kidney disease resulting from damage to podocytes and the glomerular basement membrane, due to accumulation of immune complexes and leading to urinary loss of serum proteins.1,2 Clinically, this leads to hypoalbuminemia, proteinuria, fluid retention, edema, and eventually loss of kidney function. Most patients have autoantibodies to phospholipase A2 receptor (PLA2R) with smaller proportions of patients developing autoantibodies to other antigens. Standard-of-care immunosuppressive therapies include calcineurin inhibitors, alkylating agents with glucocorticoids, and anti-CD20-mediated B cell depletion (rituximab).3, 4, 5 Alkylating agents generally result in an 80% to 90% rate of remission1 and rituximab-induced remissions can last over 2 years. All treatments result in patients being immunocompromised. Alkylating agents with high dose glucocorticoids have many additional side effects.1 A small percentage of patients are refractory to standard-of-care therapies and patient insurance does not consistently cover B cell depleting therapies. Alternative strategies to treatment are needed.
Case Presentation
Case 1
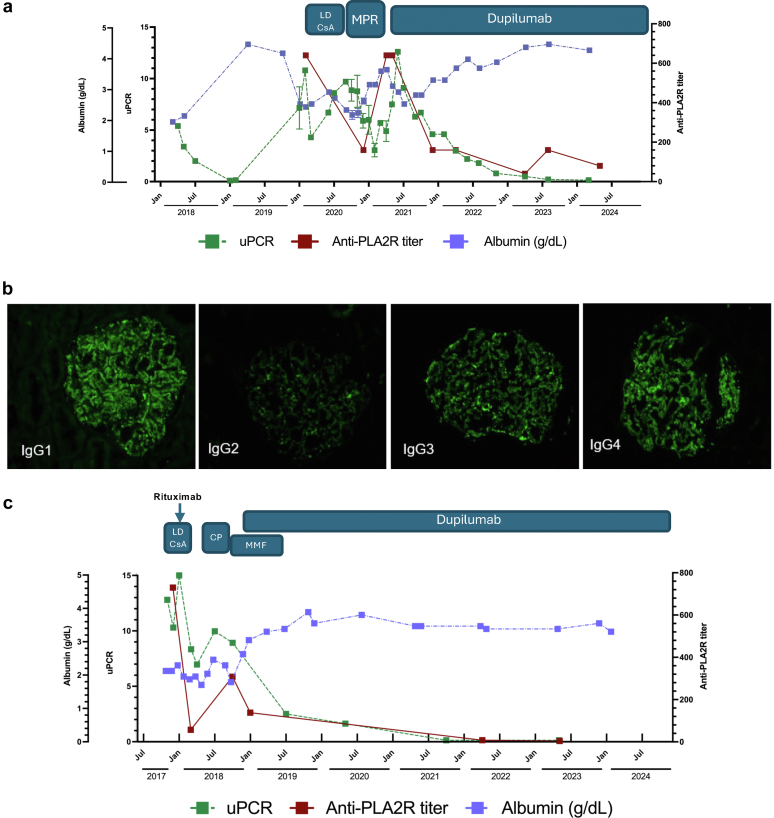
A 53-year-old male with a history of atopic disease (asthma, atopic dermatitis [AD], rhinitis, food allergy) was diagnosed with MGN in 2018 by biopsy after the patient presented with lower extremity swelling. Findings on the biopsy were positive for finely granular glomerular capillary wall staining for IgG (2+) which is consistent with MGN. The patient, however, was initially negative for anti-PLA2R autoantibodies by biopsy immunofluorescence and serum analysis. Bloodwork revealed new onset hypercholesterolemia, and urinalysis revealed a urine protein-to-creatinine ratio > 5. Antibodies to a panel of viral and autoantigens including HIV, hepatitis B, hepatitis C, DNA, myeloperoxidase, proteinase-3, glomerular basement membrane, and anti-nuclear antibody were undetectable. Levels of serum C3 and C4 were also normal. Treatment with angiotensin-converting enzyme inhibitors and diuretics was initiated, and disease remitted until the beginning of 2020 without the use of immunosuppression (Figure 1a). Thereafter, disease flared with urine protein-to-creatinine ratio exceeding 10. At this time, high titers of anti-PLA2R were present. The patient was initially treated with low-dose cyclosporine resulting in a partial response that waned over 4 to 5 months. As a result, treatment with cyclophosphamide and glucocorticoids (a modified Ponticelli regimen) was initiated. At completion of the modified Ponticelli regimen, a partial response of decreased autoantibodies and proteinuria was noted; however, both parameters rapidly worsened and hypoalbuminemia recurred. As the patient withdrew from steroids, there was a severe flare of AD. Treatment with dupilumab (300 mg biweekly) was initiated. In addition to significant improvement in the patient’s AD, continued treatment with dupilumab correlated with decreased anti-PLA2R titers, decreased proteinuria, and recovery of serum protein concentrations to the normal range (above 3.5 g/dl) (Figure 1a). Importantly, compared to multiple debilitating side effects of previous therapies, dupilumab treatment was well-tolerated. The patient remained on dupilumab for AD, and proteinuria continued to decrease for over 30 months, with MGN now considered to be in remission.
Figure 1.
MGN therapeutic response to dupilumab. (a) and (c) Data shown are for total serum albumin levels, uPCR, and anti-PLA2R autoantibody titers (shown as inverse of the dilution) at the indicated time points for (a) Case 1 and (c) Case 2. Time span of the indicated therapies is shown above the graph. (b) Diagnosis of Case 2 using immunofluorescence with isotype-specific antibodies on kidney biopsies. CP, cyclophosphamide; LD CsA, low-dose cyclosporin/tacrolimus; MGN, membranous glomerulonephritis; MMF, mycophenolate; MPR, modified Ponticelli regimen, uPCR, urine protein-to-creatinine ratios.
Case 2
A 21-year-old female with a history of childhood-onset mild persistent asthma, severe AD, and Celiac disease presented in the fall of 2017 with lower extremity edema. Her presentation was soon followed by an asthma exacerbation requiring high dose glucocorticoids; however, lower extremity edema persisted. This led to a diagnosis of nephrotic syndrome, and she was also found to have Hashimoto’s thyroiditis. anti-nuclear antibody, antineutrophil cytoplasmic autoantibody, anti-proteinase 3, and antimyeloperoxidase were negative. Complement levels (C3 and C4) were normal. CH50 levels were slightly above the normal range. A kidney biopsy showed IgG subepithelial immune complex deposits, predominantly IgG4 with codominant IgG1 and IgG3, and positive anti-PLA2R staining, further supporting an MGN diagnosis (Figure 1b). Serum anti-PLA2R level was 730 units/ml (normal: <14). She was initially treated with tacrolimus, followed by rituximab therapy (Figure 1c). Anti-PLA2R levels initially declined but rose again within 3 months. Subsequent cyclophosphamide therapy was complicated by leukopenia. Her AD improved during this time; however, her anti-PLA2R levels remained high and started to rise after completing cyclophosphamide. Following the course of cyclophosphamide, she was started on mycophenolate. Her AD significantly worsened and was resistant to topical treatments leading to the initiation of dupilumab. During treatment with dupilumab, mycophenolate was discontinued, and serum mycophenolic acid levels decreased to undetectable levels. The patient’s edema, AD, and hypoalbuminemia showed improvements within 30 days of starting treatment with dupilumab. Anti-PLA2R levels decreased, although this could be partially attributable to mycophenolate, which was maintained at the beginning of dupilumab treatment, and eventually became undetectable. She has remained in remission with dupilumab therapy without any flares of AD or evidence of MGN recurrence for over 4 years.
Discussion
IL-4 was first characterized as a B cell growth factor, supporting B cell growth, differentiation, and antibody isotype switching in culture and in vivo.S1 IL-4 was subsequently linked to allergic diseases.S2 The testing and successful trials of dupilumab (Dupixent), an IL-4Rα neutralizing antibody, led to the standard-of-care use of this biologic to treat moderate to severe AD. The use of dupilumab has now been expanded to cases of asthma and allergic rhinitis. The standard treatment course is a bi-weekly home injectable that is very well-tolerated with few side effects.S3,S4
The therapeutic benefit of IL-4R blockade in autoimmune diseases is less well-established. IL-4 has numerous effects on B cells, including protecting them from Fas- or antigen-receptor activated apoptosis and increasing expression of numerous surface receptors, including MHC class II; T cell costimulatory markers; and CD23, a marker of transitional B cells.S1 Overexpression of IL-4 results in increased autoimmunity.S5 Several reports have suggested that IL-4 contributes to breaking tolerance in B cells and contributes to cell fate during positive and negative selection.S6,S7 In antibody-mediated diseases of adjuvant-free models, blockade of IL-4 can be therapeutic.S8,S9 It is also possible that IL-4 might have direct effects on podocytes,S10 suggesting that IL-4R blockade could be functioning at multiple levels. With that perspective, Case 1 clearly began showing clinical improvement before reduction of autoantibodies. Regardless, dupilumab has been used in autoimmune diseases, including IgG4 diseases, pemphigoid, and alopecia areata with clinical improvement.S11–S13 Similar to this report, 2 children (male, ages 9 and 13) with AD complicated by nephrotic syndrome were treated with dupilumab and saw improvements in AD symptoms and symptoms of nephrotic syndrome that allowed decrease of immunosuppressant therapies.S14 Our case studies take that further by showing sustained remission when dupilumab is the only maintenance immunosuppressive.
With that perspective, dupilumab, with a high safety profile and low incidence of side effects, may offer an attractive alternative to rituximab, which causes B cell depletion and may not be covered by insurance, and immunosuppressive and cytotoxic regimens that frequently have severe side effects (Table 1). It is not clear if the efficacy might be restricted to patients with concurrent atopy or if atopy results in a distinct endotype of MGN. Further cases and clinical trials will provide information on the broader utility of this treatment approach.
Table 1.
Teaching points
|
|
|
MGN, membranous glomerulonephritis.
Disclosure
All the authors declared no conflicting interests.
Patient Consent
The authors declare that they have obtained consent from the patients discussed in the report.
Acknowledgments
This work was supported by the Brown Center for Immunotherapy. AP was supported by the National Institutes of Health Grant T32 AI060519. AC was supported by National Institutes of Health Grant T32 HL007910.
Footnotes
Supplementary References.
Supplementary Material
Supplementary References.
References
- 1.Couser W.G. Primary membranous nephropathy. Clin J Am Soc Nephrol. 2017;12:983–997. doi: 10.2215/CJN.11761116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anders H.J., Kitching A.R., Leung N., Romagnani P. Glomerulonephritis: immunopathogenesis and immunotherapy. Nat Rev Immunol. 2023;23:453–471. doi: 10.1038/s41577-022-00816-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramachandran R., Hn H.K., Kumar V., et al. Tacrolimus combined with corticosteroids versus modified Ponticelli regimen in treatment of idiopathic membranous nephropathy: randomized control trial. Nephrol (Carlton) 2016;21:139–146. doi: 10.1111/nep.12569. [DOI] [PubMed] [Google Scholar]
- 4.Ramachandran R., Yadav A.K., Kumar V., et al. Two-year follow-up study of membranous nephropathy treated with tacrolimus and corticosteroids versus cyclical corticosteroids and cyclophosphamide. Kidney Int Rep. 2017;2:610–616. doi: 10.1016/j.ekir.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Remuzzi G., Chiurchiu C., Abbate M., Brusegan V., Bontempelli M., Ruggenenti P. Rituximab for idiopathic membranous nephropathy. Lancet. 2002;360:923–924. doi: 10.1016/S0140-6736(02)11042-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary References.