Video
Introduction
Gastroparesis is a chronic debilitating disease of the stomach associated with profound decreases in patient quality of life.1 Despite a rising prevalence, therapeutic options remain markedly limited. Although gastric peroral endoscopic myotomy (G-POEM) has emerged as a novel therapy for gastroparesis, success rates are variable and therapeutic options for patients that fail G-POEM are limited.2
We propose a novel endoscopic therapy for refractory gastroparesis, using a modified endoscopic sleeve gastroplasty (ESG) technique to promote gastric tubularization after failed primary G-POEM.
Case presentation
Following institutional review board approval, retrospective data from 2 patients who elected to undergo modified ESG for gastric tubularization for the treatment of refractory transplant–associated gastroparesis was obtained.
Patient 1 was a 65-year-old man with severe post lung transplant–associated gastroparesis that previously failed dietary, promotility medical therapy, pyloric botulinum toxin injection, and G-POEM. Post G-POEM, the patient's minimal symptoms were unchanged (pre– and post–G-POEM gastroparesis cardinal symptom index of 0.8); however, ongoing significant gastric retention on gastric scintigraphy was noted post–G-POEM, with 2- and 4-hour retention percentages noted at 37% and 89%, respectively (vs 98% 2-hour and 94% 4-hour retention rates pre-GPOEM). Twenty-four-hour pH impedance testing demonstrated increased rates of weak acid reflux despite twice daily proton pump inhibitor use, and the patient's course was further complicated by multiple rounds of acute lung allograft dysfunction felt to be secondary to severe gastroparesis and subsequent aspiration. The patient declined post pyloric feed or percutaneous gastrojejunostomy tube placement and was deemed a nonsurgical candidate. Following detailed informed consent process and multidisciplinary discussion, patient 1 elected to undergo modified endoscopic sleeve gastroplasty to promote gastric tubularization for the treatment of refractory gastroparesis following previous pyloromyotomy.
Patient 2 was a 29-year-old man with severe post kidney transplant–associated medically refractory gastroparesis status post failed dietary, medical, pyloric botulinum injection, and G-POEM for gastroparesis. Patient 2 noted severe postprandial abdominal bloating, distension, nausea, and vomiting with a total gastroparesis cardinal symptom index of 3.8 post G-POEM (3.7 pre G-POEM) with minimal change in gastric scintigraphy (GES). Patient noted minimal ability to tolerate any oral intake without severe symptoms and received full nutrition through gastrojejunostomy feedings with minimal oral intake only for comfort. Patient 2 expressed a desire to avoid surgical interventions and, following a detailed informed consent process and multidisciplinary discussion, elected to undergo a modified endoscopic sleeve gastroplasty for the treatment of refractory gastroparesis post previous pyloromyotomy.
Endoscopic methods
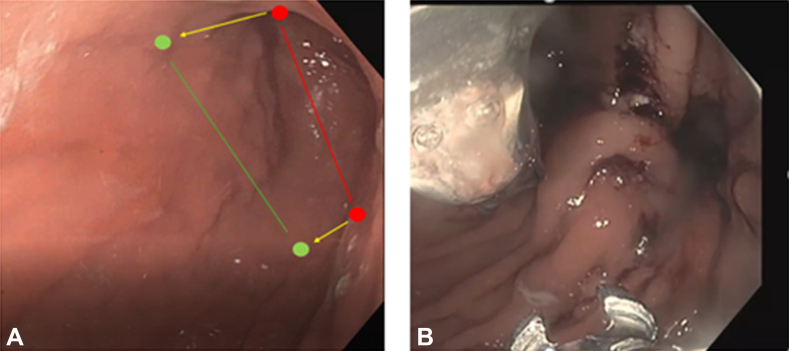
Following initial endoscopic inspection of the stomach, a double-channel therapeutic endoscope (GIF-2TH180, Olympus, Tokyo, Japan) fitted with an endoscopic suturing device (Overstitch, Boston Scientific, Boston, Mass, USA) was inserted into the stomach. Video 1 (available online at www.videogie.org) demonstrates the modified endoscopic sleeve gastroplasty technique following gastric pyloromyotomy. To limit excessive restriction and possible weight loss, sutures did not extend past the 11-o'clock and 6-o'clock positions along the lesser and greater curvature of the stomach, respectively, with a goal total volume reduction of less than 50% (Fig. 1).
Figure 1.
A,Red circles denote classic initial and end suture extent, with the red line denoting the approximate resultant luminal narrowing. Green circles denote modified ESG initial and end suture extent at the 11 o’clock and 6 o’clock positions with the green line denoting the less resultant volume restriction of the open accordion's modified ESG. B, Endoscopic view of initial suture placement. Note the size of the lumen restriction at <50%.
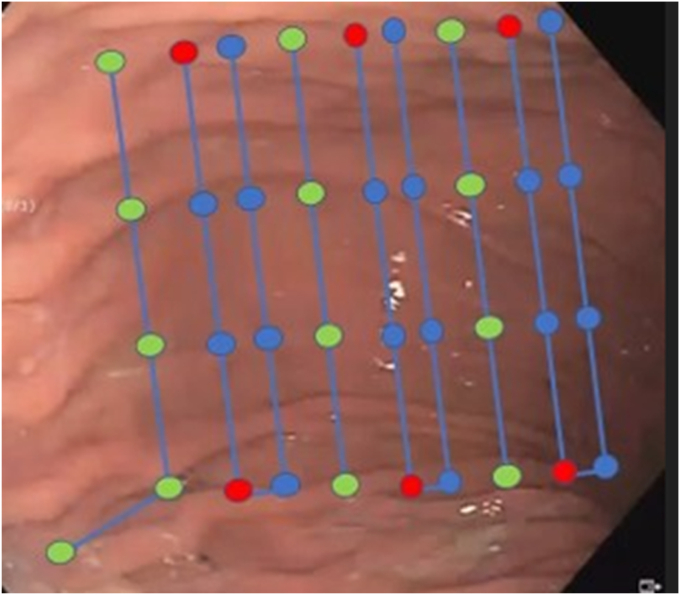
The endoscopic suturing pattern is denoted by Figure 2. Six running endoscopic sutures utilizing a U-I-U-I-U-I pattern were used. Each “U” suture used placement of 7 to 8 suture bites, and an accompanied reinforcement suture was placed following each U suture cinch. The final “I” suture was elongated in a “J” shape to exclude the fundus but avoid direct fundal suturing.
Figure 2.
Endoscopic sleeve suture pattern used in modified endoscopic sleeve. Each colored node denotes a single suture bite. Each suture is performed in the superior to inferior direction. Blue nodes denote “U”-shaped patterns. Each “U” suture is reinforced by a single running “reinforcement suture” at each red node. Green nodes denote location of “I”-shaped suture patterns. Note the final “I” suture pattern was elongated in a J shape to exclude the fundus but avoid direct fundal suturing.
The mean procedure duration was 65 minutes, and both patients were admitted for postoperative observation. There were no intraprocedural adverse events or serious adverse events. One patient developed postoperative urinary retention that was treated supportively. Postoperatively, the diet was advanced slowly with aggressive nausea and pain control with 2 weeks of full liquid diet followed by 2 weeks of soft diet with resumption of gastroparetic diet after 4 weeks. A nutritionist was used to monitor dietary practices post modified ESG to ensure adequate caloric intake. Gastroparesis cardinal symptom index scores (GCSI) and GES were obtained 2 months post procedure. No patient was on prokinetic medications at the time of the follow-up GES study or post follow-up symptom evaluation.
Results
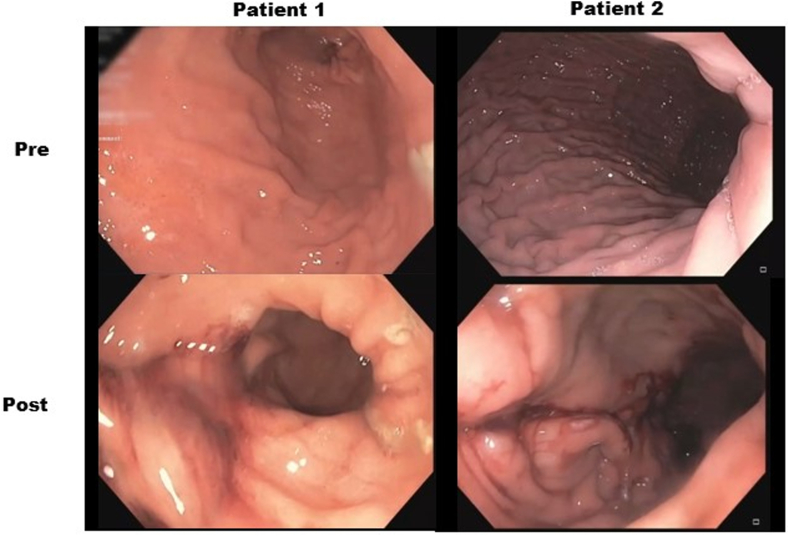
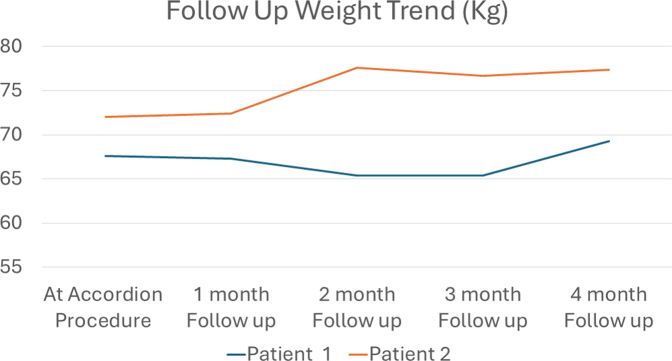
Following the procedure, the relative percent change in GCSI improved by mean 69.0%, with most of the reduction noted in the postprandial fullness subcategory (mean reduction 65.4%). Mean percent relative change in GES improved at 2 and 4 hours by 15.5% and 29.7%, respectively (Table 1). No weight loss was noted for either patient on 2- and 4-month follow-up (Supplemental Fig. 1, available online at www.videogie.org). Patient 2 noted the ability to tolerate soft foods without a significant increase in symptoms post modified ESG compared with near complete aphagia earlier. Figure 3 demonstrates the postoperative appearance of each respective sleeve.
Table 1.
Preprocedural and postprocedural changes in gastroparesis cardinal symptom index scores and gastric scintigraphy results
| Pre–open accordion | Post–open accordion | Relative % change | |
|---|---|---|---|
| Patient 1 | |||
| GCSI total | 0.58 | 0.083 | −85.7 |
| Nausea/vomiting subdomain | 0 | 0 | 0 |
| Fullness subdomain | 1.75 | 0.25 | −85.7 |
| Bloating subdomain | 0 | 0 | 0 |
| Gastric scintigraphy study | |||
| 2-h retention, % | 60 | 55 | −8.3 |
| 4-h retention, % | 31 | 21 | −32.3 |
| Patient 2 | |||
| GCSI total | 3.67 | 1.75 | −52.3 |
| Nausea/vomiting subdomain | 3 | 2.5 | −16.7 |
| Fullness subdomain | 5 | 2.75 | −45.0 |
| Bloating subdomain | 2 | 0 | −100 |
| Gastric scintigraphy study | |||
| 2-h retention, % | 97 | 75 | −22.7 |
| 4-h retention, % | 89 | 65 | −27.0 |
GCSI, Gastroparesis cardinal symptom index scores.
Figure 3.
Volume restriction noted following modified endoscopic sleeve gastroplasty for the treatment of refractory gastroparesis.
Conclusion
To our knowledge, this is the first case series of modified endoscopic sleeve gastroplasty for the treatment of medically refractory gastroparesis post previous pyloromyotomy. This “open accordion”–like change in the stomach via modified ESG (accordion shape) following previous gastric pyloromyotomy failure (open end) was associated with improvements in both subjective symptom scores and objective gastric emptying rates without any associated negative impact on weight.
The physiological basis for our modified ESG for the treatment of gastroparesis draws heavily from surgical sleeve gastrectomy (SG), although with several distinct differences. Somewhat paradoxically, faster gastric emptying and intestinal filling has been noted post SG, counter to classic teaching that SG's weight loss promotion is predominately through gastric body restriction.3 In addition to altering GI motility, parallel alterations in GI hormones have been noted post SG, primarily attributed to the surgical removal of the ghrelin-laden gastric fundus.3 Data from the use of SG for treatment of refractory gastroparesis have noted an increase in gastric emptying time related to a reduction in the gastric lumen diameter following sleeve gastrectomy without a significant influence on weight.4,5 However, sleeve gastrectomy for the treatment of gastroparesis has not been adopted broadly, likely related to the invasive nature of the procedure.
In contrast to SG, ESG has not shown changes in GI hormones, likely due to the common practice of avoiding fundal suturing.6, 7, 8 Fundal suturing has been associated with higher procedural morbidity without contributing to weight loss.9 We elected to avoid direct fundal suturing and instead focused on exaggerated “J” suture shape at the apex of the sleeve to promote gastric fundal exclusion. This was performed to limit procedural morbidity and avoid mucosal disruption of the ghrelin-laden fundus to avoid promotion of weight loss.9,10 It is unclear if complete fundal exclusion akin to surgical sleeve in ESG would further influence gastric emptying times and warrants future investigation.
Traditional ESG has been noted to induce persistent changes in gastric retention with higher 2-hour, T1/2-, and 4-hour gastric retention rates compared with SG, although the changes were relatively modest.7 Interestingly, Vargas et al7 noted that despite the reduction in gastric emptying time, gastric body motility was preserved within the gastric imbrication. Using Poiseuille's Law of laminar flow, we opine that severe restrictions to the size of the gastric body via traditional ESG may produce decreases in gastric emptying rates; however, the improvement in forward flow noted with less gastric restriction in our modified ESG with previous pyloromyotomy may be secondary to compensatory changes in other factors (such as compensatory increases in gastric pressure) that leads to an apparent increase in flow despite the reduction in cross-sectional area. It is unclear whether the accelerated emptying noted in both of our patients only occurs in combination of a modified ESG and previous G-POEM or if a modified ESG promoting gastric tubularization could lead to accelerated gastric emptying in isolation.11
Several other important differences in our modified ESG versus traditional ESG require further clarification.10 Although comparative reports have noted no significant difference in suture pattern effect on weight loss outcomes, it is unclear if variations in suture patterns could result in greater influence on both gastric hormone secretion or gastric emptying.10 The MERIT trial predominantly used a series of “U”-shaped sutures for gastric tubularization, with additional optional reinforcement sutures used to strengthen the plications.12 Our study utilized alternative “U” and “I” suture patterns with serial reinforcement sutures. Additionally, although no fundal suturing was performed, as noted above, the final suture was elongated to exclude the fundus without direct fundal suturing; whether our pattern results in differences in gastric emptying compared with the more widely performed predominate “U” pattern is unknown.
Medically refractory gastroparesis remains a significant therapeutic challenge with limited therapeutic options. Gastroparesis represents a heterogeneous disease with various pathophysiologic pathways contributing to its development, including impaired gastric accommodation, dysmotility of the gastric body, aberrant antral contractility, and pylorospasm. Although novel pyloric-directed therapies have gained widespread use, managing patients who do not respond to treatments for pylorospasm is sorely needed. In this initial pilot proof of concept study, a modified endoscopic sleeve gastroplasty following an endoscopic pyloromyotomy procedure is associated with improvements in GCSI scores and improvements in gastric emptying rates in refractory gastroparesis without significant influence on weight. Further longer-term and larger prospective studies are needed to confirm these preliminary results.
Disclosure
Dr Podboy is an iTHRIV Scholar. The iTHRIV Scholars Program is supported in part by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Numbers UL1TR003015 and KL2TR003016.
Supplementary data
A novel application of endoscopic sleeve gastroplasty for the treatment of refractory gastroparesis
Supplemental Fig. S1.
Denotes follow up weight of each patient post open accordion.
References
- 1.Camilleri M., Kuo B., Nguyen L., et al. ACG clinical guideline: gastroparesis. Am J Gastroenterol. 2022;117:1197–1220. doi: 10.14309/ajg.0000000000001874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Podboy A., Hwang J.H., Nguyen L.A., et al. Gastric per-oral endoscopic myotomy: current status and future directions. World J Gastroenterol. 2019;25:2581–2590. doi: 10.3748/wjg.v25.i21.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Melissas J., Leventi A., Klinaki I., et al. Alterations of global gastrointestinal motility after sleeve gastrectomy. Ann Surg. 2013;258:976–982. doi: 10.1097/SLA.0b013e3182774522. [DOI] [PubMed] [Google Scholar]
- 4.Samuel B., Atiemo K., Cohen P., et al. The effect of sleeve gastrectomy on gastroparesis: a short clinical review. Bariatr Surg Pract Patient Care. 2016;11:84–89. [Google Scholar]
- 5.Lee A.M., Fuchs K.H., Varga G., et al. Sleeve gastrectomy for treatment of delayed gastric emptying-indications, technique, and results. Langenbecks Arch Surg. 2020;405:107–116. doi: 10.1007/s00423-020-01856-5. [DOI] [PubMed] [Google Scholar]
- 6.Fayad L., Adam A., Schweitzer M., et al. Endoscopic sleeve gastroplasty versus laparoscopic sleeve gastrectomy: a case-matched study. Gastrointest Endosc. 2019;89:782–788. doi: 10.1016/j.gie.2018.08.030. [DOI] [PubMed] [Google Scholar]
- 7.Vargas E.J., Rizk M., Gomez-Villa J., et al. Effect of endoscopic sleeve gastroplasty on gastric emptying, motility and hormones: a comparative prospective study. Gut. 2023;72:1073–1080. doi: 10.1136/gutjnl-2022-327816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lopez-Nava G., Negi A., Bautista-Castaño I., et al. Gut and metabolic hormones changes after endoscopic sleeve gastroplasty (ESG) vs. laparoscopic sleeve gastrectomy (LSG) Obes Surg. 2020;30:2642–2651. doi: 10.1007/s11695-020-04541-0. [DOI] [PubMed] [Google Scholar]
- 9.Farha J., McGowan C., Hedjoudje A., et al. Endoscopic sleeve gastroplasty: suturing the gastric fundus does not confer benefit. Endoscopy. 2021;53:727–731. doi: 10.1055/a-1236-9347. [DOI] [PubMed] [Google Scholar]
- 10.Sarkar A., Tawadros A., Andalib I., et al. Safety and efficacy of endoscopic sleeve gastroplasty for obesity management in new bariatric endoscopy programs: a multicenter international study. Ther Adv Gastrointest Endosc. 2022;15 doi: 10.1177/26317745221093883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson C.C., Trasolini R., Jirapinyo P. Bariatric endoscopic antral myotomy: first-in-human proof of concept of a novel therapeutic method to delay gastric emptying and induce weight loss. iGIE. 2023;2:102–106. [Google Scholar]
- 12.Abu Dayyeh B.K., Bazerbachi F., Vargas E.J., et al. Endoscopic sleeve gastroplasty for treatment of class 1 and 2 obesity (merit): a prospective, multicentre, randomised trial. Lancet. 2022;400:441–451. doi: 10.1016/S0140-6736(22)01280-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A novel application of endoscopic sleeve gastroplasty for the treatment of refractory gastroparesis