Abstract
Introduction
Urinary albumin-to-creatinine ratio (uACR) is an independent predictor of chronic kidney disease (CKD) progression. However there is limited evidence on the burden of CKD according to uACR categories at the population level. This study estimates future clinical and financial burden of CKD according to uACR categories using the Inside CKD microsimulation.
Methods
The Inside CKD model is an individual patient level microsimulation that emulates national populations based on demographic, epidemiological, and economic data. The analysis estimates clinical and economic outcomes over time according to the Kidney Disease: Improving Global Outcomes (KDIGO) uACR categories (A1–A3) at a population level for 31 countries and regions.
Results
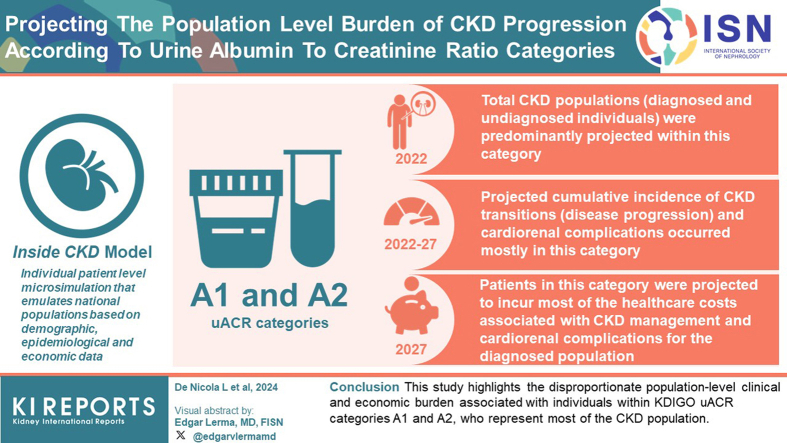
CKD populations (diagnosed and undiagnosed individuals, stages G3–G5) were projected to be predominantly within uACR categories A1 and A2 in 2022. Projected cumulative incidence of CKD stage transitions (disease progression) and cardio-renal complications (heart failure, myocardial infarction, stroke, and all-cause mortality) occurred mostly in uACR categories A1 and A2 between 2022 and 2027. Patients in uACR categories A1 and A2, who represent the largest proportion of patients with CKD, were projected to incur most of the health care costs associated with CKD management and cardio-renal complications for the diagnosed population (prevalence 2027).
Conclusion
This study highlights the disproportionate population-level clinical and economic burden associated with individuals within KDIGO uACR categories A1 and A2, who represent most of the CKD population. This awareness will help health care decision makers to appropriately allocate resources and interventions to the CKD population, including those with mild to moderately increased albuminuria, to reduce clinical and economic burden associated with CKD.
Keywords: burden of disease, cardio-renal complication, chronic kidney disease, economic burden, end-stage kidney disease, urine albumin-to-creatinine ratio
Graphical abstract
CKD is a progressive condition associated with substantial morbidity and mortality.1,2 The estimated global prevalence of CKD is 11.1%, with an estimated 850 million people affected worldwide in 2017.1,2 The prevalence of CKD is estimated to increase, due to an ageing population and an increase in risk factors (type 2 diabetes, obesity, and hypertension), with CKD predicted to become the fifth most common chronic condition by 2040.3,4
CKD is associated with a significant clinical and economic burden to society and health care systems, which increases substantially with disease progression. In developed countries the mean per-patient health care costs for CKD are estimated to range from $1600 to $53,186 per-year for stages G1 to G5.5 End-stage kidney disease (ESKD) costs are even higher, with estimates in some countries as high as $100,593 per person per year.5 CKD negatively impacts patients’ health-related quality of life,6,7 with mean EQ-5D index scores demonstrated to decrease with each progressive CKD stage. It is important to note that lower socioeconomic groups and vulnerable populations experience disproportionately high CKD prevalence, in part due to reduced access to treatments, leading to worse health outcomes.2,8
Guidelines from KDIGO recommend stratifying CKD according to estimated glomerular filtration rate (eGFR) and albuminuria, measured as uACR.9, 10, 11 This combined assessment provides improved risk stratification,12 particularly because albuminuria is an independent predictor of CKD progression as well as cardiovascular events and death.13,14 Furthermore, a recent large meta-analysis has demonstrated that albuminuria indicates the onset of 10 different adverse cardio-renal outcomes even if to a moderate degree.15
Albuminuria is stratified into 3 uACR categories, from normal to mildly increased < 30 mg/g (A1), moderately increased 30 to 300 mg/g (A2), to severely increased > 300 mg/g (A3). The risk of cardio-renal outcomes increases with higher albuminuria. Nevertheless, patients in uACR categories A1 and A2 represent the largest proportion of the CKD population and are expected to account for the greatest contribution to disease burden.12 Indeed, patients in uACR category A3 are at the highest risk on a per patient basis but only represent a small proportion of the total CKD population.16
Despite albuminuria being an independent predictor of CKD progression and cardio-renal outcomes, uACR is not routinely tested in clinical practice. A systematic review published in 2020 has identified a number of barriers to diagnosis and management of CKD in primary care, including, dissatisfaction with guidelines and a lack of time.17 A US study in 2020 showed that there was no increase in the frequency of uACR testing despite the KDIGO recommendations of 2012.18, 19, 20 The recently published KDIGO 2024 Clinical Practice Guideline discusses urine protein-to-creatinine ratio or reagent strip urinalysis for total protein as alternative options to uACR; however, these are considered inferior due to the imprecision and insensitivity in measuring total protein in urine at low concentrations, with these tests more appropriate for detecting advanced stages of CKD.21 A systematic review published in 2023 has reported that CKD screening is cost-effective in populations with diabetes and in those in high risk ethnic groups, and is likely to be more cost effective if carried out in a home setting compared with a primary care setting.22 Proactive screening, targeted to those over a certain age in the general population and/or those with risk factors, early detection, and effective intervention, have the potential to slow CKD progression.23,24 However, a multinational study has shown that up to 95.5% of patients with evidence of CKD stage G3 remain undiagnosed.25 Currently, an evidence gap exists regarding the population-level burden of CKD according to uACR categories. Improved awareness of the population-level burden of CKD would help inform evidence-based health care priorities and assist health care systems and decision makers in the allocation of resources and interventions to the area of highest unmet need.
The objective of this study was to estimate the future clinical and economic burden of CKD according to uACR categories using the validated inside CKD microsimulation model.26, 27, 28 The microsimulation incorporates multiple data sources and real-world evidence to project the full complexity of disease progression and patient outcomes.26 Specifically, we report projections for CKD prevalence, disease progression rates, cardio-renal complications, and economic burden for simulated CKD populations (both diagnosed and undiagnosed, stages G3–G5) in 31 countries and regions globally.
Methods
Model Overview
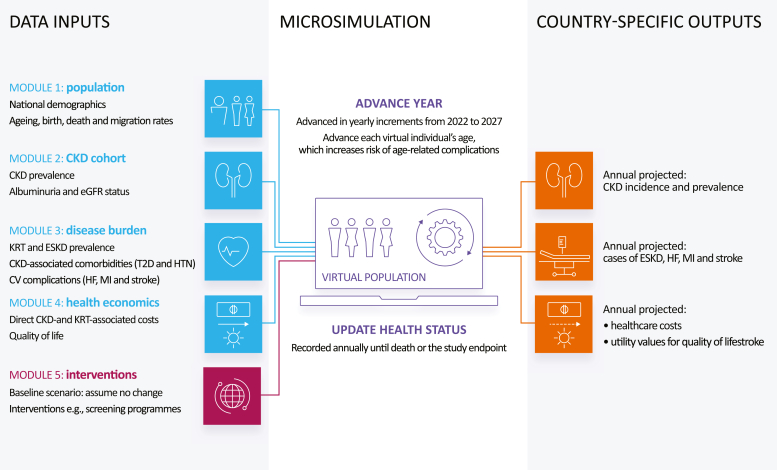
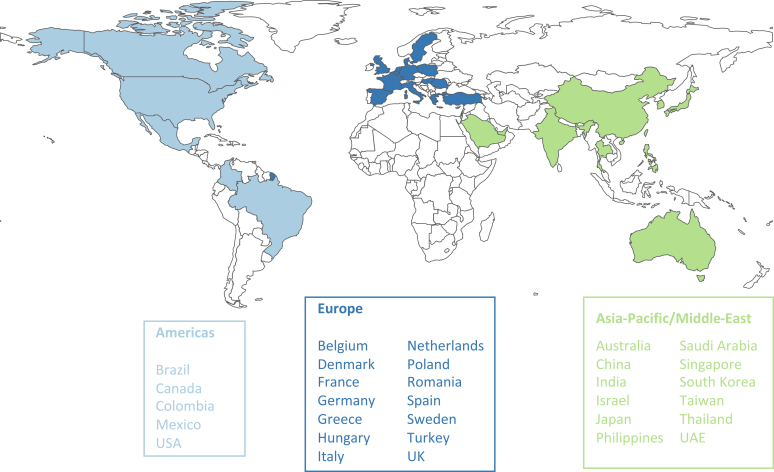
Inside CKD is an individual patient-level microsimulation that emulates national populations (CKD and non-CKD individuals) based on known demographic, epidemiological, and economic data sources (Figure 1).28,29 Previous publications outline the detailed methodology of this microsimulation.26,28, 29, 30 The current analysis estimates clinical and economic outcomes over time according to KDIGO uACR categories (A1–A3) at a national population level for 31 countries and regions in the Americas, Europe, Asia, and the Middle East (Figure 2).
Figure 1.
Overview of the Inside CKD microsimulation, adapted from Tangri et al.26 CKD, chronic kidney disease; CV, cardiovascular; eGFR, estimated glomerular filtration rate; ESKD: end-stage kidney disease; HF, heart failure; HTN, hypertension; KDIGO: Kidney Disease: Improving Global Outcomes; KRT: kidney replacement therapy; MI, myocardial infarction; SGLT2i: sodium-glucose counter-2 inhibitor; T2D: type 2 diabetes.
Figure 2.
The 31 countries and regions modeled in the Inside CKD microsimulation. CKD, chronic kidney disease.
The microsimulation generated 20 million virtual individuals (Monte-Carlo simulations) for each participating country or region that represented the general population (CKD and non-CKD individuals). Populations were then rescaled during postprocessing to reflect the size of the specific country/region of interest. The individuals were assigned baseline characteristics, including age (0–110 years) and sex (male/female), based on national statistics or the United Nations World Population Prospects database.31 Clinical characteristics including eGFR, uACR and rates of cardio-renal complications, and mortality, were assigned based on data from published national health or epidemiological surveys. These clinical characteristics identified individuals with a CKD status, for example, no CKD or CKD stage G1 to G5. The virtual individuals were cycled through the microsimulation annually over a period of 6 years (2022–2027). The projected risk of developing CKD, or disease progression in those already diagnosed with CKD, was determined by a decline in eGFR and an increase in uACR and modeled in accordance with the epidemiological data. The relative risk of cardio-renal complications and all-cause death increased with disease progression.
This microsimulation model was validated (internally and externally) across 5 aspects:
-
•
Consideration of face validity, whereby microsimulation assumptions, structure, data sources, and results were evaluated in relation to the specified aims and objectives.
-
•
Verification (or internal validation) that the model code performed as intended; assessed by 2 coders working independently.
-
•
External validation compared microsimulation data to real world data, by simulating events that had occurred.
-
•
Predictive validity compared prospectively observed events with simulated events.
-
•
Cross validity compared the results from the microsimulation with other models that addressed similar issues.32
In addition, sensitivity analyses were performed to evaluate the robustness of the results with changes to model inputs, and have been reported previously.26,28 The model was shown to be robust through external validation and sensitivity analyses, which confirmed the observed trends.
Outcomes
Prevalence of CKD and Distribution Over KDIGO Risk Categories
To model projected clinical and economic burden associated with CKD by uACR categories, the prevalent population considered for these outcomes were required to be restricted to only to those with CKD with eGFR stage G3 to G5, given an observed paucity of data availability for patients with preserved eGFR.
Country- or region-specific CKD prevalence data were derived from representative, individual-level data from cross-sectional studies. A probability of experiencing slow (slope < 4 ml/min per 1.73 m2) or fast (> 4 ml/min per 1.73 m2) progression was assigned to individuals based on estimates from the DISCOVER CKD global observational study.33 CKD progression, as defined by eGFR, was capped at a background rate of eGFR decline equivalent to a non-CKD population.34 In addition, albuminuria levels were derived from distributions each year, which could then trigger a potential CKD progression or regression. Where individual-level data were not available, for instance, if specific age categories were missing, smoothing algorithms were applied to the aggregated data to calculate distributions of uACR and eGFR to obtain more granular estimates; further detail is available in a previous publication.26 The microsimulation assumed that no policy changes or new treatment options were introduced over the modeled time horizon.26
CKD Progression (Cumulative Incidence)
Transitions between eGFR-defined stages were modeled at all stages (diagnosed and undiagnosed, G1–G5); however, only transitions from CKD stage “G3 to G4” and “G4 to G5” are reported here. Annual declines in eGFR were estimated using data from the DISCOVER CKD global observational study, a retrospective international CKD real world cohort.33 Covariates associated with slow and rapid progressors were incorporated and for comorbidities and complications, as described previously.26 The model recorded when a patient progressed from one KDIGO eGFR or uACR category to the next. The degree of change over time corresponded with age, sex, eGFR, uACR, complications, and CKD status; and a constant gradient was assumed across all settings.26 Where a patient progresses to kidney failure at CKD stage G5, they could initiate kidney replacement therapy at progression thresholds from the published literature, and in accordance with relevant national treatment guidelines.35,36 Modeled patients were then enrolled on hemodialysis, peritoneal dialysis, or would receive a kidney transplant.35,36
Cardio-Renal Complications (Cumulative Incidence)
In the CKD population (diagnosed and undiagnosed individuals, CKD stages G3–G5), the cumulative incidence of cardio-renal complications (heart failure, myocardial infarction, and stroke) and death from any cause, were projected over the model time horizon. All-cause mortality (irrespective of cardiovascular events) was adjusted for age and sex, based on national life tables. Every individual in microsimulation had a probability of dying from CKD or other unspecified causes. The probability of dying from any cause by CKD stage is derived from published estimates.10
The relative risk of developing cardio-renal complications varied according to the severity of CKD and was applied to individual’s, depending upon their KDIGO eGFR and uACR categories.
Economic Burden
Health care costs associated with CKD management and cardio-renal complications were estimated for patients diagnosed with CKD (stages G3–G5). Costs for those with CKD (stages G1–G2) were not available for all countries; and where costs were available, it was not clear if they were for CKD specifically or for comorbidities such as hypertension. The limited availability of data is discussed in more detail in Jha et al.30 Because those with CKD (stages G1–G2) often do not receive formal treatment, the costs were not included here to avoid an overestimation of total costs. The cost analysis was conducted from the perspective of the public national health service within each country. Where this was not appropriate, the model was adjusted to the commercial setting; both commercial and Medicare costs were calculated for the US. Health state–specific costs for CKD progression and cardio-renal complications were applied to the prevalence of CKD (total number of cases) to quantify the total estimated economic burden of disease. Country- and region-specific direct health care cost inputs were sourced from a published cost library derived from the Inside CKD global research program.30 Briefly, best estimates of CKD-associated costs were identified in cooperation with local research organizations by conducting country- or region-specific pragmatic literature searches of cost data available in English or local languages. All cost data were validated by local clinical experts in conjunction with the Inside CKD scientific steering committee. Cost estimates were adjusted to 2022 prices according to the gross domestic product deflator data from the International Monetary Fund.37 The inflated costs were standardized to US dollars according to implied purchasing power parity rates.28,29,37 The model was written in C++ and is proprietary codebase owned by Health Lumen.
Results
CKD Prevalence (Stages G3–G5) per uACR Category
The microsimulation projected the annual prevalence of CKD per uACR categories (A1–A3) for the CKD population (diagnosed and undiagnosed individuals, stages G3–G5). The prevalent population analyzed for cardio-renal, and economic outcomes (G3–G5, A1–A3) were predominantly within uACR categories A1 and A2 in all 31 countries and regions in 2022 (unweighted average: A1 = 86.4% [SD: 5.3], A2 = 11.3% [SD: 4.4], and A3 = 2.3% [SD: 2.6]; Table 1). This analyzed population equated to a combined total number of individuals for all countries and regions of 117,407,055 in category A1, 16,675,972 in A2, and 4,002,976 in A3. There was minimal variation in the projected annual prevalence of CKD per uACR categories (A1–A3) for the CKD population over time (2022–2027, Table 1).
Table 1.
Projected annual prevalence of CKD per KDIGO uACR category (A1–A3) for the CKD population (diagnosed and undiagnosed individuals with CKD stage G3–G5) for 31 countries and regions
| Country or region | 2022 |
2027 |
||||
|---|---|---|---|---|---|---|
| KDIGO uACR category |
KDIGO uACR category |
|||||
| A1 | A2 | A3 | A1 | A2 | A3 | |
| Australia | 689,457 (83.4) | 121,329 (14.7) | 16,279 (2) | 881,701 (84.6) | 142,600 (13.7) | 17,648 (1.7) |
| Belgium | 910,888 (88.8) | 110,558 (10.8) | 4881 (0.5) | 912,732 (89.6) | 101,537 (10) | 4156 (0.4) |
| Brazil | 12,466,041 (90.1) | 439,190 (3.2) | 926,566 (6.7) | 14,917,592 (92) | 452,856 (2.8) | 851,556 (5.2) |
| Canada | 2,650,628 (87.9) | 346,786 (11.5) | 17,501 (0.6) | 2,873,276 (88.9) | 343,443 (10.6) | 15,354 (0.5) |
| China | 17,644,768 (84.0) | 2,948,980 (14.0) | 418,069 (2) | 18,237,856 (85) | 2,866,320 (13.4) | 350,048 (1.6) |
| Colombia | 1,799,524 (93.2) | 122,993 (6.4) | 7383 (0.4) | 2,307,014 (95.1) | 114,137 (4.7) | 5596 (0.2) |
| Denmark | 430,501 (88.2) | 54,786 (11.2) | 2754 (0.6) | 431,117 (90.7) | 42,633 (9) | 1699 (0.4) |
| France | 2,842,465 (88.3) | 357,276 (11.1) | 17,726 (0.6) | 3,301,144 (90.3) | 338,178 (9.3) | 14,636 (0.4) |
| Germany | 1,928,253 (83.8) | 358,389 (15.6) | 15,547 (0.7) | 2,389,024 (85.7) | 382,113 (13.7) | 15,287 (0.5) |
| Greece | 760,321 (92.5) | 58,785 (7.1) | 3305 (0.4) | 815,502 (94.3) | 47,552 (5.5) | 2119 (0.2) |
| Hungary | 685,913 (89.0) | 81,182 (10.5) | 3333 (0.4) | 665,493 (91.9) | 56,737 (7.8) | 1719 (0.2) |
| India | 15,079,553 (88.1) | 1,739,954 (10.2) | 288,960 (1.7) | 16,881,620 (89.2) | 1,793,081 (9.5) | 261,234 (1.4) |
| Israel | 230,729 (74.5) | 64,902 (21.0) | 14,099 (4.6) | 249,618 (77.7) | 59,877 (18.6) | 11,766 (3.7) |
| Italy | 1,861,480 (91.6) | 148,752 (7.3) | 21,832 (1.1) | 2,273,290 (92.5) | 165,151 (6.7) | 20,047 (0.8) |
| Japan | 10,698,297 (82.9) | 1,988,375 (15.4) | 225,082 (1.7) | 12,736,884 (84.8) | 2,081,118 (13.9) | 203,297 (1.4) |
| Saudi Arabia | 1,718,975 (86.8) | 215,277 (10.9) | 45,516 (2.3) | 2,255,402 (86.5) | 292,455 (11.2) | 58,223 (2.2) |
| Mexico | 3,227,137 (74.8) | 518,897 (12.0) | 568,571 (13.2) | 4,271,956 (77.9) | 595,492 (10.9) | 615,519 (11.2) |
| Netherlands | 963,233 (88.4) | 120,885 (11.1) | 6027 (0.6) | 1,091,707 (90.8) | 106,202 (8.8) | 4449 (0.4) |
| Philippines | 2,692,211 (90.1) | 95,646 (3.2) | 199,658 (6.7) | 3,251,729 (91.9) | 101,783 (2.9) | 186,520 (5.3) |
| Poland | 1,968,375 (85.0) | 282,728 (12.2) | 64,768 (2.8) | 2,167,015 (89.4) | 217,801 (9) | 39,509 (1.6) |
| Romania | 807,468 (96.3) | 30,115 (3.6) | 1321 (0.2) | 1,071,136 (97.3) | 28,702 (2.6) | 1001 (0.1) |
| Singapore | 293,314 (79.8) | 62,167 (16.9) | 12,119 (3.3) | 357,273 (80.3) | 74,176 (16.7) | 13,310 (3) |
| South Korea | 1,492,040 (85.7) | 217,471 (12.5) | 30,878 (1.8) | 2,402,381 (86.3) | 337,206 (12.1) | 43,665 (1.6) |
| Spain | 3,203,256 (92.3) | 253,712 (7.3) | 14,656 (0.4) | 3,727,288 (93.1) | 260,239 (6.5) | 14,022 (0.4) |
| Sweden | 464,171 (90) | 43,500 (8.4) | 8265 (1.6) | 506,060 (92.4) | 35,997 (6.6) | 5819 (1.1) |
| Taiwan | 681,519 (85.7) | 99,378 (12.5) | 13,958 (1.8) | 1,085,083 (86.8) | 145,791 (11.7) | 18,512 (1.5) |
| Thailand | 6,973,213 (86.7) | 916,820 (11.4) | 152,365 (1.9) | 7,956,610 (87.3) | 1,006,918 (11.1) | 147,635 (1.6) |
| Turkey | 2,467,801 (78.1) | 593,417 (18.8) | 100,259 (3.2) | 2,782,003 (80.2) | 591,903 (17.1) | 96,441 (2.8) |
| UAE | 68,705 (87.3) | 8159 (10.4) | 1830 (2.3) | 90,824 (87.8) | 10,529 (10.2) | 2064 (2) |
| UK | 4,846,478 (87.9) | 635,699 (11.5) | 31,116 (0.6) | 4,941,774 (89.2) | 571,316 (10.3) | 24,772 (0.4) |
| USA | 14,860,340 (77.1) | 3,639,866 (18.9) | 768,353 (4) | 16,588,567 (79.6) | 3,588,214 (17.2) | 657,631 (3.2) |
| Total, n | 117,407,055 | 16,675,972 | 4,002,976 | 134,420,672 | 16,952,061 | 3,705,252 |
| Percentage unweighted average, % (SD) | 86.4 (5.3) | 11.3 (4.4) | 2.3 (2.6) | 88.0 (5.0) | 10.1 (4.2) | 1.8 (2.2) |
| Percentage weighted average, % | 85.0 | 12.1 | 2.9 | 86.7 | 10.9 | 2.4 |
CKD, chronic kidney disease; KDIGO, Kidney Disease: Improving Global Outcomes; uACR, urinary albumin-creatinine ratio.
CKD Progression in the CKD Population Stages G3 to G5 per uACR Category
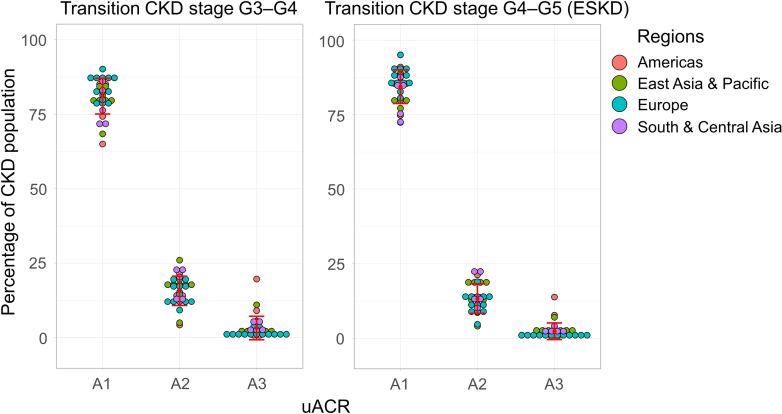
The microsimulation modeled the transitions from CKD stage “G3 to G4” and “G4 to G5” (onset of ESKD) across the CKD population (diagnosed and undiagnosed individuals, stages G3–G5) per uACR categories (A1–A3) between 2022 and 2027. Majority of the cumulative transitions from CKD stage G3 to G4 occurred in uACR categories A1 and A2 in all countries and regions (unweighted average: A1 = 72.3% [SD: 9.6], A2 = 22.7% [SD: 8.1], and A3 = 5.0% [SD: 6.3]; Figure 3 and Table 2). The total number of individuals, in all countries and regions, transitioning from CKD stage G3 to G4 were 5,032,950, 1,240,558, and 220,028, for uACR categories A1 to A3, respectively (Table 2). Likewise, the cumulative incidence of transitions from CKD stage G4 to G5 (ESKD) predominantly occurred in uACR categories A1 and A2 in all countries and regions (unweighted average: A1 = 84.4% [SD: 5.3], A2 = 13.2% [SD: 4.8], and A3 = 2.4% [SD: 2.7]; Figure 3 and Table 2). The total number of individuals, in all countries and regions, transitioning from CKD stage G4 to G5 (ESKD) were 1,059,364, 195,124, and 32,182, for uACR categories A1–A3, respectively (Table 2). The cumulative incidence of transitions from CKD stage “G3 to G4” and “G4 to G5” (onset of ESKD) for each individual country or region are presented in Supplementary Table S1.
Figure 3.
Projected cumulative transitions from CKD stage G3 to G4 and G4 to G5 (ESKD) (2022–2027) per KDIGO uACR category in the diagnosed CKD population for 31 countries and regions. CKD, chronic kidney disease; KDIGO: Kidney Disease: Improving Global Outcomes; uACR, urinary albumin-creatinine ratio.
Table 2.
Projected cumulative cardio-renal events and transitions from CKD stage G3 to G4 and G4 to G5 (ESKD) (2022–2027) per KDIGO uACR category (A1–A3) for the diagnosed and undiagnosed CKD population (CKD stages G3–G5) for 31 countries and regions
| Event | KDIGO uACR category |
|||||
|---|---|---|---|---|---|---|
| A1 |
A2 |
A3 |
||||
| Total number events | Mean (SD), % | Total number events | % (SD) | Total number events | % (SD) | |
| Transition–CKD stage G3 to G4 | 5,083,822 | 72.8 (9.8) | 1,247,778 | 22.2 (8.1) | 220,478 | 5.0 (6.3) |
| Transition–CKD stage G4 to G5 (ESKD) | 1,069,945 | 84.4 (5.3) | 196,093 | 13.2 (4.8) | 32,230 | 2.4 (2.7) |
| Heart failure | 6,419,107 | 87.7 (5.1) | 821,997 | 10.4 (4.3) | 179,413 | 1.9 (2.2) |
| Myocardial infarction | 6,535,131 | 86.8 (5.2) | 888,679 | 11.1 (4.4) | 176,867 | 2.0 (2.3) |
| Stroke | 6,045,011 | 80.8 (7.6) | 1,265,500 | 15.8 (6.2) | 259,392 | 3.3 (3.9) |
| Death (all-cause) | 47,271,478 | 78.7 (8.2) | 10,630,456 | 17.0 (6.4) | 3,088,878 | 4.3 (4.8) |
CKD, chronic kidney disease; ESKD, end-stage kidney disease; KDIGO, Kidney Disease: Improving Global Outcomes.
Mean percentages calculated as an unweighted average.
Cardio-Renal Complications in the CKD Population Stages G3 to G5 per uACR Category
The microsimulation projected the cumulative incidence of cardio-renal events for the CKD population (diagnosed and undiagnosed individuals, CKD stages G3–G5) per uACR categories (A1–3) between 2022 and 2027. The cardio-renal events predominantly occurred in uACR categories A1 and A2 in all countries and regions; cumulative incidence of cardio-renal complications for each individual country or region are presented in Supplementary Tables S2 and S3.
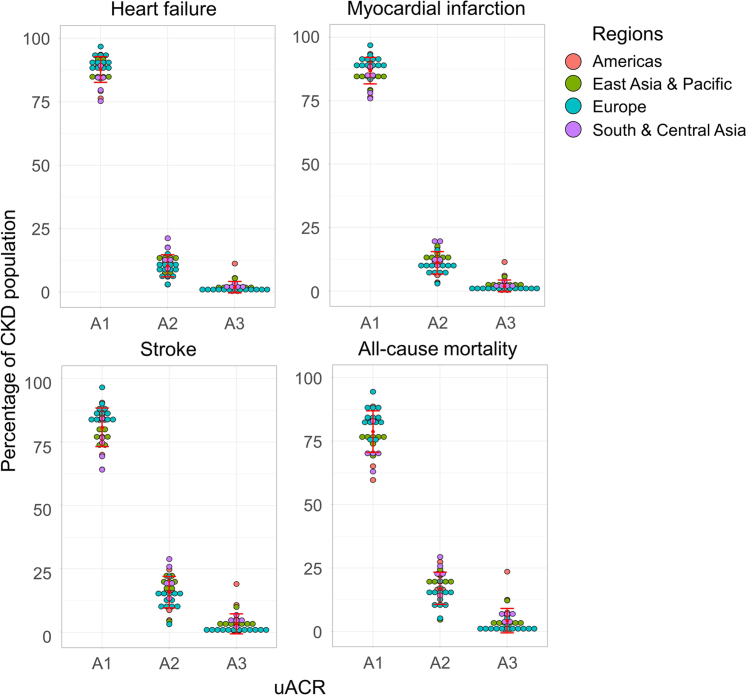
Overall, unweighted mean proportions of heart failure, myocardial infarction, and stroke events were projected to occur predominately in patients in uACR category A1 (87.7%, 86.8%, and 80.8%, respectively), with the remainder in category A2 (10.4%, 11.1%, and 15.8%, respectively) and category A3 (1.9%, 2.0%, and 3.3%, respectively) across all countries (Figure 4 and Table 2). Across the 31 countries considered or regions, the total numbers of incidents by end point were:
-
•
Heart failure: A1 = 6,419,107; A2 = 821,997; and A3 = 179,413.
-
•
Myocardial infarction: A1 = 6,535,131; A2 = 888,679; and A3 = 176,867.
-
•
Stroke: A1 = 6,045,011; A2 = 1,265,500; and A3 = 259,392.
Figure 4.
Projected cumulative incidence of heart failure, myocardial infarction, stroke, and all-cause mortality (2022–2027) per KDIGO uACR category in the diagnosed CKD population (CKD stages G3–G5) for 31 countries and regions. CKD, chronic kidney disease; KDIGO, Kidney Disease: Improving Global Outcomes; uACR, urinary albumin-creatinine ratio.
A similar pattern was observed with all-cause mortality (Figure 4 and Table 2) with comparatively small proportion of deaths projected to occur in patients with severely increased albuminuria (A1: 78.7%, A2: 17.0%, and A3: 4.3%) with a total of 3.1 million deaths in those with A3 disease versus 47.3 million and 10.6 million in those with A1 and A2 disease, respectively.
Economic Burden in the Diagnosed CKD Population Stages G3–G5 per uACR Category
The microsimulation projected the health care costs associated with CKD management and cardio-renal complications (heart failure, myocardial infarction, and stroke) for patients diagnosed with CKD (stages G3–G5) per uACR categories (A1–A3) based on the prevalent population in 2027. In line with clinical prognosis, the health care costs were predominantly attributed to patients in uACR categories A1 and A2 in all countries and regions. The CKD management costs (stages G3–G5) were predominantly associated with uACR category A1 in all countries and regions (A1 = $183.3 billion [82.4%], A2 = $33.1 billion [14.9%], and A3 = $5.95 billion [2.7%]; Table 3). The complication costs were mainly associated with both uACR categories A1 and A2 in all countries and regions (A1 = $570.0 billion [46.4%], A2 = $539.0 billion [43.7%], and A3 = $121.3 billion [9.8%], Table 3). The health care costs for each individual country or region are presented in Supplementary Tables S4 to S8.
Table 3.
Projected costs associated with CKD management and cardio-renal complications in the diagnosed population (CKD stages G3–G5) (prevalence – 2027) per uACR category for 31 countries and regions
| Cost |
KDIGO uACR categories |
|||
|---|---|---|---|---|
| A1 |
A2 |
A3 |
||
| Total CKD management costs, billion USD (%) | 183.30 (82.4%) | 33.13 (14.9%) | 5.95 (2.7%) | |
| Average cost per country for CKD stage, billion USD | G3a | 3.66 | 0.55 | 0.10 |
| G3b | 1.36 | 0.27 | 0.05 | |
| G4 | 0.52 | 0.18 | 0.03 | |
| G5 (pre-KRT) | 0.19 | 0.04 | 0.01 | |
| Total cardio-renal complication costs, billion USD (%) | 573.03 (46.4) | 539.03 (43.7) | 121.33 (9.8) | |
| Average cost per country for complications, billion USD | Heart failure | 5.67 | 5.55 | 1.08 |
| Myocardial infarction | 8.49 | 6.99 | 1.81 | |
| Stroke | 4.33 | 4.86 | 1.02 | |
CKD, chronic kidney disease; KDIGO, Kidney Disease: Improving Global Outcomes; KRT, kidney replacement therapy; uACR, urinary albumin-creatinine ratio.
Discussion
The clinical and economic burden of CKD increases as disease progresses and uACR is an important predictor of CKD progression and cardiovascular risk. Even so, uACR is not routinely tested in clinical practice. Limited published studies projecting clinical and economic burden by uACR categories are available to aid in evidence-based health care decision making and resource allocation. This study aimed to address this knowledge gap by estimating the future prevalence, CKD progression, rates of cardio-renal complications, and financial burden by uACR category over the subsequent 6 years using the validated inside CKD microsimulation. A microsimulation type model was chosen for the Inside CKD program as classical cohort-level state transition models do not allow for modelling of differences among individuals and can lead to misleading results when considering a nuanced set of characteristics.38 In contrast, this microsimulation is able to model each individual, allowing the entire distribution of multiple complex variables and their changes over time to be considered, rather than using population averages which would generate less accurate predictions. A microsimulation approach can incorporate an individual’s history into the model, allowing for example, an individual’s disease history and the risk of that influencing their life course to be included within the model. Microsimulation provides unparalleled breadth and granularity of inputs to achieve multinational population-level projections of disease progression and patient outcomes.26
The inside CKD microsimulation estimated the prevalence of the CKD population, comprising both diagnosed and undiagnosed individuals, stages G3 to G5 at a multinational level. The CKD population was projected to exist largely within uACR categories A1 and A2 (> 90%) in all 31 countries and regions studied. Subsequently the microsimulation estimated the cumulative incidence of cardio-renal events stratified by the number of individuals in each uACR category (stages G3–G5). Therefore, although patients within uACR categories A1 and A2 have a lower relative risk of cardio-renal events at an individual-level versus A3, they are projected to account for the majority of the clinical and economic burden at the population-level, given that they constitute a substantially larger proportion of the CKD population.
Randomized clinical trials tend to focus on patients with elevated albuminuria, in part as a prognostic enrichment strategy, that is, the selection of patients with a greater likelihood of having a disease-related end point to increase the absolute effect difference between groups without altering the relative effect.39, 40, 41 Furthermore, few studies on the clinical and economic burden of CKD stratified by uACR category are population-based. These discrepancies between trial settings and clinical practice make it inherently difficult for decision makers to interpret where the burden lies, and consequently to implement effective preventive strategies. Recent post hoc analyses of clinical trials have reported that treatment with sodium-glucose cotransporter-2 inhibitors is beneficial across disease stages; however, the number needed to be treated to prevent 1 cardiovascular or renal event is generally substantially lower in those with higher levels of uACR.42, 43, 44 This reinforces the assertion that screening and treatment of those with elevated uACR is beneficial and necessary. Targeted proactive screening, early detection, and effective intervention aimed at slowing disease progression are paramount to reducing the clinical and economic burden associated with CKD.23,24
The Inside CKD microsimulation results support interventions in the total CKD population. We highlight that, individuals within uACR categories A1 and A2 (stages G3–G5) are key drivers of cardio-renal outcomes and health care costs in the CKD population. These individuals are often seen by primary care physicians but do not receive specialist care until they reach more advanced CKD stages. Subsequently, they would be referred to a nephrologist whereby interventions to reduce cardio-renal complications may be implemented too late to deliver impactful benefits to the health care system. It is crucial for health care decision makers to communicate the requirement for primary care physicians to adopt early intervention in uACR categories A1 and A2 to control hypertension, or type 2 diabetes among others, to reduce cardio-renal outcomes, delay progression to ESKD, increase regression,45 and therefore the requirement for costly interventions, including heart-related hospitalizations, transplant, and dialysis.2,46 Interestingly, recent publications have shown that kidney protection is provided by SGLT2i across all eGFR categories; however, early intervention in patients with preserved kidney function (milder disease) provides the greatest protection over a lifetime by delaying the time to adverse outcomes.47,48 Collective trial evidence therefore agrees with real-world studies showing kidney protection related to SGLT2i when these drugs are initiated prior to onset of impaired eGFR or albuminuria.49,50
Microsimulation is subject to several assumptions and limitations. First, no major changes in the CKD management, such as new treatment options or policies, were modeled over the course of the projected years. Second, a paucity of available economic data to inform CKD management costs for patients with preserved eGFR (G1–G2) but moderate or severely increased albuminuria (A2–A3) precluded their inclusion in the analysis. Furthermore, variability and uncertainty in relation to the population size of patients within this subpopulation are considerable owing to the poor rates of testing for uACR in the general population and at-risk subpopulations.19,20 Consequently, the population-level clinical and economic burden estimated for those in A2 or A3 categories may be underrepresented in this analysis. Third, because the microsimulation was developed before the COVID-19 pandemic, effects of the pandemic were not considered and it is possible that the pandemic has affected CKD diagnosis, treatment and outcomes, meaning estimates provided here are likely to be conservative.51 A further limitation of the model is that it does not incorporate the effect of uncertainty of parameters, such as the distribution of standard errors. However, Monte Carlo errors in the projected estimates are provided. We acknowledge that albuminuria can fluctuate independently from CKD progression; indeed, this is a limitation of all national health, or epidemiological surveys based on a single urine test. However, the projected cumulative incidence of CKD progression, heart failure, myocardial infarction, stroke, and all-cause mortality modeled here differ remarkably per uACR category, therefore likely blunting the potential effect of albuminuria fluctuations. Lastly, although the best epidemiological data available for each country or region were used in microsimulation, data for many specific end points were unavailable for some countries. As such, proxy country or regional data were substituted; determined by a study-specific algorithm and informed by an expert panel. These proxies may not have been fully representative of the population in question. Furthermore, it is necessary to note that some of the country-specific inputs for the microsimulation may not be representative of the CKD population. This can be seen for specific countries where higher than expected prevalence in uACR category A3 is projected by microsimulation. For example, the epidemiological data for Mexico were derived from a cross-sectional study of 610 patients from the city of Guadalajara alone.52 The study population are of limited generalizability to the total population with 73% female, mean age 51 ± 14 years, with more than 50% of subjects reporting family antecedents of diabetes mellitus, hypertension, and obesity; and 30% of CKD. This limited epidemiological data highlights the requirement for high-quality real-world evidence in total CKD populations to explore the disease burden associated with albuminuria. Furthermore, this also emphasizes the limited uACR testing undertaken in clinical practice.53
Future research should include analyses to determine whether specific-population cohorts, including those with hypertension, cardiovascular disease, or type 2 diabetes, are at higher risk depending on albuminuria. Any such analysis would need to be supported by the collection of high quality real-world evidence for each cohort. Finally, the forecasts made by this microsimulation should be evaluated in the future to determine the accuracy of the microsimulation model.
In conclusion, this study highlights the disproportionate population-level clinical and economic burden associated with individuals within KDIGO uACR categories A1 and A2, who comprise much of the CKD population. Because the cardio-renal and economic analysis in this study was limited to CKD stages G3–G5 additional data is needed to establish the importance of albuminuria in early-stage CKD. However, the results provide insights into health care decision makers and health care systems to target resource allocation and interventions at the total CKD population. Proactive management of CKD, along with comorbid hypertension, type 2 diabetes, and cardiovascular disease by primary care physicians and specialists, has the potential to improve long-term outcomes and reduce the disease burden.
Disclosure
LDN has received honoraria for lectures and scientific consultation from Astellas, AstraZeneca, Bayer, and Novo Nordisk. RC-R has received honoraria as consultant from AstraZeneca, Boehringer Ingelheim, Janssen, Bayer, Chinook, AbbVie, Alexion, Novo Nordisk, and MedXL; and research support from AstraZeneca, Baxter, Boehringer Ingelheim, Roche, and Novo Nordisk. JFN-G has received honoraria as consultant from AbbVie, Amgen, AstraZeneca, Bayer, Bionet Medical, Boehringer Ingelheim, Eli Lilly, Mundipharma, Novo Nordisk, Sanofi-Genzyme, and Servier; and research support from AbbVie, Bionet Medical, Boehringer Ingelheim, Sanofi-Genzyme, Shire, and CSL Vifor. AP has received honoraria from AstraZeneca, Bayer, and Boehringer Ingelheim; and conference attendance support from AstraZeneca and Bayer. MN has received honoraria as a consultant from Astellas, Amicus Therapeutics, Otsuka, and Swixx; received honoraria for lectures/manuscript writing from Astellas, Novartis, Amicus, Therapeutics, Otsuka, Swixx, and Takeda; and support for meetings/travel from AstraZeneca and Amgen. IW has received honoraria as consultant from AstraZeneca, Bayer, Boehringer-Ingelheim, Eli Lilly, Novo Nordisk, and Sanofi. J-MH has received honoraria as consultant from Alexion, AstraZeneca, Bayer, Boehringer Ingelheim France, Novo Nordisk, Sanofi, Servier, and Vifor; and research support from AstraZeneca. LR and TC are/were employees of HealthLumen Limited. HealthLumen Limited received funding from AstraZeneca for conducting this study. JJGS, CC, and SB are employees and shareholders of AstraZeneca.
Acknowledgments
The authors would like to thank all participants in the Inside CKD study program and members of the Scientific Steering Committee (Supplementary Table S9). The authors thank Robert Jenkins and Rowena Jenkins of Health Economics and Outcomes Research Ltd. for providing medical writing support/editorial support, which was funded by AstraZeneca in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Data from this study was submitted as an abstract and subsequent poster presentation at the 2023 European Renal Association and European Dialysis and Transplant Association (ERA-EDTA) conference and the 2023 International Society for Pharmacoeconomics and Outcomes Research (ISPOR EU) conference. This estimation was carried out in line with The Guidelines for Accurate and Transparent Health Estimates Reporting (GATHER), the checklist is provided in Supplementary Table S10.
Funding
Inside CKD is funded by AstraZeneca. Inside CKD is a project based on microsimulation data and so no drugs were supplied or funded. All statistical analyses were funded by AstraZeneca and conducted by HealthLumen Ltd. The corresponding author had full access to all data and had final responsibility for the decision to submit for publication.
Data Availability Statement
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at: https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure
Author Contributions
LR and JJGS conceptualized and designed the study. LR and TC were responsible for data analysis. LDN, RC-R, JFN-G, AP, MN, IW, J-MH, LR, TC, JJGS, CC, and SB contributed to interpretation of the results, preparation, and review of the manuscript, as well as approval of the final manuscript for publication.
Footnotes
Table S1. Projected cumulative transitions from CKD stage G3 to G4 and G4 to G5 (ESKD) (2022–2027) per KDIGO uACR category in the diagnosed CKD population for 31 countries and regions.
Table S2. Projected cumulative incidence of heart failure and myocardial infarction (2022–2027) per KDIGO uACR category in the diagnosed CKD population (CKD stages G3–G5) for 31 countries and regions.
Table S3. Projected cumulative incidence of stroke and all-cause mortality (2022–2027) per KDIGO uACR category in the diagnosed CKD population (CKD stages G3–G5) for 31 countries and regions.
Table S4. Projected costs associated with heart failure in the diagnosed population (CKD stages G3–G5) (prevalence – 2027) per uACR category for 31 countries and regions.
Table S5. Projected costs associated with myocardial infarction in the diagnosed population (CKD stages G3–G5) (prevalence – 2027) per uACR category for 31 countries and regions.
Table S6. Projected costs associated with stroke in the diagnosed population (CKD stages G3–G5) (prevalence – 2027) per uACR category for 31 countries and regions.
Table S7. Projected costs associated with CKD management stages G3a and G3b in the diagnosed population (prevalence – 2027) per uACR category for 31 countries and regions.
Table S8. Projected costs associated with CKD management stages G4 and G5 in the diagnosed population (prevalence – 2027) per uACR category for 31 countries and regions.
Table S9. Inside CKD Scientific Steering Committee.
Table S10. GATHER checklist.
Supplementary Material
Table S1. Projected cumulative transitions from CKD stage G3 to G4 and G4 to G5 (ESKD) (2022–2027) per KDIGO uACR category in the diagnosed CKD population for 31 countries and regions. Table S2. Projected cumulative incidence of heart failure and myocardial infarction (2022–2027) per KDIGO uACR category in the diagnosed CKD population (CKD stages G3–G5) for 31 countries and regions. Table S3. Projected cumulative incidence of stroke and all-cause mortality (2022–2027) per KDIGO uACR category in the diagnosed CKD population (CKD stages G3–G5) for 31 countries and regions. Table S4. Projected costs associated with heart failure in the diagnosed population (CKD stages G3–G5) (prevalence – 2027) per uACR category for 31 countries and regions. Table S5. Projected costs associated with myocardial infarction in the diagnosed population (CKD stages G3–G5) (prevalence – 2027) per uACR category for 31 countries and regions. Table S6. Projected costs associated with stroke in the diagnosed population (CKD stages G3–G5) (prevalence – 2027) per uACR category for 31 countries and regions. Table S7. Projected costs associated with CKD management stages G3a and G3b in the diagnosed population (prevalence – 2027) per uACR category for 31 countries and regions. Table S8. Projected costs associated with CKD management stages G4 and G5 in the diagnosed population (prevalence – 2027) per uACR category for 31 countries and regions. Table S9. Inside CKD Scientific Steering Committee. Table S10. GATHER checklist.
References
- 1.Jager K.J., Kovesdy C., Langham R., Rosenberg M., Jha V., Zoccali C. A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases. Nephrol Dial Transplant. 2019;34:1803–1805. doi: 10.1093/ndt/gfz174. [DOI] [PubMed] [Google Scholar]
- 2.Shlipak M.G., Tummalapalli S.L., Boulware L.E., et al. The case for early identification and intervention of chronic kidney disease: conclusions from a Kidney Disease: improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2021;99:34–47. doi: 10.1016/j.kint.2020.10.012. [DOI] [PubMed] [Google Scholar]
- 3.Romagnani P., Remuzzi G., Glassock R., et al. Chronic kidney disease. Nat Rev Dis Primers. 2017;3 doi: 10.1038/nrdp.2017.88. [DOI] [PubMed] [Google Scholar]
- 4.Foreman K.J., Marquez N., Dolgert A., et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet. 2018;392:2052–2090. doi: 10.1016/S0140-6736(18)31694-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elshahat S., Cockwell P., Maxwell A.P., Griffin M., O’Brien T., O’Neill C. The impact of chronic kidney disease on developed countries from a health economics perspective: a systematic scoping review. PLoS One. 2020;15 doi: 10.1371/journal.pone.0230512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper J.T., Lloyd A., Sanchez J.J.G., Sörstadius E., Briggs A., McFarlane P. Health related quality of life utility weights for economic evaluation through different stages of chronic kidney disease: a systematic literature review. Health Qual Life Outcomes. 2020;18:310. doi: 10.1186/s12955-020-01559-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hussien H., Apetrii M., Covic A. Health-related quality of life in patients with chronic kidney disease. Expert Rev Pharmacoecon Outcomes Res. 2021;21:43–54. doi: 10.1080/14737167.2021.1854091. [DOI] [PubMed] [Google Scholar]
- 8.Liyanage T., Ninomiya T., Jha V., et al. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet. 2015;385:1975–1982. doi: 10.1016/S0140-6736(14)61601-9. [DOI] [PubMed] [Google Scholar]
- 9.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2012;3:1–150. [Google Scholar]
- 10.Levey A.S., de Jong P.E., Coresh J., et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011;80:17–28. doi: 10.1038/ki.2010.483. [DOI] [PubMed] [Google Scholar]
- 11.Stevens P.E., Ahmed S.B., Carrero J.J. KDIGO 2024 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2024;105:S117–S314. doi: 10.1016/j.kint.2023.10.018. [DOI] [PubMed] [Google Scholar]
- 12.Murton M., Goff-Leggett D., Bobrowska A., et al. Burden of chronic kidney disease by KDIGO categories of glomerular filtration rate and albuminuria: a systematic review. Adv Ther. 2021;38:180–200. doi: 10.1007/s12325-020-01568-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chronic Kidney Disease Prognosis Consortium, Matsushita K., van der Velde M., van der Velde M., et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gansevoort R.T., Matsushita K., van der Velde M., et al. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int. 2011;80:93–104. doi: 10.1038/ki.2010.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grams M.E., Coresh J., Matsushita K., et al. Estimated glomerular filtration rate, albuminuria, and adverse outcomes: an individual-participant data meta-analysis. JAMA. 2023;330:1266–1277. doi: 10.1001/jama.2023.17002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pollock C., James G., Garcia Sanchez J.J., et al. Healthcare resource utilisation and related costs of patients with CKD from the UK: a report from the DISCover CKD retrospective cohort. Clin Kidney J. 2022;15:2124–2134. doi: 10.1093/ckj/sfac168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neale E.P., Middleton J., Lambert K. Barriers and Enablers to detect and management of chronic kidney disease in primary healthcare: a systematic review. BMC Nephrol. 2020;21:83. doi: 10.1186/s12882-020-01731-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.James G., Garcia Sanchez J.J., Carrero J.J., et al. Low adherence to kidney disease: improving global outcomes 2012 CKD clinical practice guidelines despite clear evidence of utility. Kidney Int Rep. 2022;7:2059–2070. doi: 10.1016/j.ekir.2022.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alfego D., Ennis J., Gillespie B., et al. Chronic kidney disease testing among at-risk adults in the U.S. remains low: real-world evidence from a national laboratory database. Diabetes Care. 2021;44:2025–2032. doi: 10.2337/dc21-0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chu C.D., Xia F., Du Y., et al. Estimated prevalence and testing for albuminuria in US adults at risk for chronic kidney disease. JAMA Netw Open. 2023;6 doi: 10.1001/jamanetworkopen.2023.26230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levin A., Ahmed S.B., Carrero J.J., et al. Executive summary of the KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease: known knowns and known unknowns. Kidney Int. 2024;105:684–701. doi: 10.1016/j.kint.2023.10.016. [DOI] [PubMed] [Google Scholar]
- 22.Yeo S.C., Wang H., Ang Y.G., Lim C.K., Ooi X.Y. Cost-effectiveness of screening for chronic kidney disease in the general adult population: a systematic review. Clin Kidney J. 2024;17 doi: 10.1093/ckj/sfad137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cusick M.M., Tisdale R.L., Chertow G.M., Owens D.K., Goldhaber-Fiebert J.D. Population-wide screening for chronic kidney disease : a cost-effectiveness analysis. Ann Intern Med. 2023;176:788–797. doi: 10.7326/M22-3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCullough P.A., Brown W.W., Gannon M.R., et al. Sustainable community-based CKD screening methods employed by the National Kidney Foundation’s Kidney Early Evaluation Program (KEEP) Am J Kidney Dis Off J Natl Kidney Found. 2011;57:S4–S8. doi: 10.1053/j.ajkd.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 25.Tangri N., Moriyama T., Schneider M.P., et al. Prevalence of undiagnosed stage 3 chronic kidney disease in France, Germany, Italy, Japan and the USA: results from the multinational observational REVEAL-CKD study. BMJ Open. 2023;13 doi: 10.1136/bmjopen-2022-067386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tangri N., Chadban S., Cabrera C., Retat L., Sánchez J.J.G. Projecting the epidemiological and economic impact of chronic kidney disease using patient-level microsimulation modelling: rationale and methods of inside CKD. Adv Ther. 2023;40:265–281. doi: 10.1007/s12325-022-02353-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pimpin L., Cortez-Pinto H., Negro F., et al. Burden of liver disease in Europe: epidemiology and analysis of risk factors to identify prevention policies. J Hepatol. 2018;69:718–735. doi: 10.1016/j.jhep.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 28.Chertow G.M., Correa-Rotter R., EK U., et al. Projecting the clinical burden of chronic kidney disease at the patient level (Inside CKD): a microsimulation modelling study. EClinicalmedicine. 2024;72 doi: 10.1016/j.eclinm.2024.102614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chadban S., Arici M., Power A., et al. Projecting the economic burden of chronic kidney disease at the patient level (Inside CKD): a microsimulation modelling study. EClinicalmedicine. 2024;72 doi: 10.1016/j.eclinm.2024.102615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jha V., Al-Ghamdi S.M.G., Li G., et al. Global economic burden associated with chronic kidney disease: a pragmatic review of medical costs for the inside CKD research programme. Adv Ther. 2023;40:4405–4420. doi: 10.1007/s12325-023-02608-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.United Nations World population prospects 2019. 2020. https://population.un.org/wpp/
- 32.Eddy D.M., Hollingworth W., Caro J.J., et al. Model transparency and validation: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-7. Value Health. 2012;15:843–850. doi: 10.1016/j.jval.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 33.Pecoits-Filho R., James G., Carrero J.J., et al. Methods and rationale of the DISCOVER CKD global observational study. Clin Kidney J. 2021;14:1570–1578. doi: 10.1093/ckj/sfab046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.NHS. Digital Health Surv Engl. 2016. 2017. https://digital.nhs.uk/data-and-information/publications/statistical/health-survey-for-england/health-survey-for-england-2016
- 35.George L.K., Koshy S.K., Molnar M.Z., et al. Heart failure increases the risk of adverse renal outcomes in patients with normal kidney function. Circ Heart Fail. 2017;10 doi: 10.1161/CIRCHEARTFAILURE.116.003825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Go A.S., Yang J., Tan T.C., et al. Contemporary rates and predictors of fast progression of chronic kidney disease in adults with and without diabetes mellitus. BMC Nephrol. 2018;19:1–13. doi: 10.1186/s12882-018-0942-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.International Monetary Fund World Economic Outlook Report. 2022. https://www.imf.org/en/Publications/WEO/Issues/2022/10/11/world-economic-outlook-october-2022
- 38.Oderkirk J., Sassi F., Cecchini M., Astolfi R. Toward a new comprehensive international health and health care policy decision support tool. 2012. https://www.oecd.org/en/topics/policy-issues/the-future-of-health-systems.html
- 39.US Food and Drug Administration Enrichment strategies for clinical trials to support approval of human drugs and biological products - guidance for industry. 2019. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/enrichment-strategies-clinical-trials-support-approval-human-drugs-and-biological-products
- 40.Temple R. Enrichment of clinical study populations. Clin Pharmacol Ther. 2010;88:774–778. doi: 10.1038/clpt.2010.233. [DOI] [PubMed] [Google Scholar]
- 41.US Department of Health and Human Services Enrichment strategies for clinical trials to support determination of effectiveness of human drugs and biological products guidance for industry. 2019. https://www.fda.gov/media/121320/download
- 42.Delanaye P., Wissing K.M., Scheen A.J. Sodium-glucose cotransporter 2 inhibitors: renal outcomes according to baseline albuminuria. Clin Kidney J. 2021;14:2463–2471. doi: 10.1093/ckj/sfab096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mosenzon O., Wiviott S.D., Heerspink H.J.L., et al. The effect of dapagliflozin on albuminuria in DECLARE-TIMI 58. Diabetes Care. 2021;44:1805–1815. doi: 10.2337/dc21-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zelniker T.A., Raz I., Mosenzon O., et al. Effect of dapagliflozin on cardiovascular outcomes according to baseline kidney function and albuminuria status in patients with type 2 diabetes: a prespecified secondary analysis of a randomized clinical trial. JAMA Cardiol. 2021;6:801–810. doi: 10.1001/jamacardio.2021.0660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Borrelli S., Leonardis D., Minutolo R., et al. Epidemiology of CKD regression in patients under nephrology care. PLoS One. 2015;10 doi: 10.1371/journal.pone.0140138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Janowski J., Floege J., Fliser D., Böhm M., Marx N. Cardiovascular disease in chronic kidney disease: pathophysiological Insights and Therapeutic Options. Circulation. 2021;143:1157–1172. doi: 10.1161/CIRCULATIONAHA.120.050686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maddaloni E., Cavallari I., La Porta Y., et al. Impact of baseline kidney function on the effects of sodium-glucose co-transporter-2 inhibitors on kidney and heart failure outcomes: a systematic review and meta-analysis of randomized controlled trials. Diabetes Obes Metab. 2023;25:1341–1350. doi: 10.1111/dom.14986. [DOI] [PubMed] [Google Scholar]
- 48.McEwan P., Gabb P.D., Davis J.A., et al. The long-term effects of dapagliflozin in chronic kidney disease: a time-to-event analysis. Nephrol Dial Transplant. 2024 doi: 10.1093/ndt/gfae106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heerspink H.J.L., Karasik A., Thuresson M., et al. Kidney outcomes associated with use of SGLT2 inhibitors in real-world clinical practice (CVD-REAL 3): a multinational observational cohort study. Lancet Diabetes Endocrinol. 2020;8:27–35. doi: 10.1016/S2213-8587(19)30384-5. [DOI] [PubMed] [Google Scholar]
- 50.Melzer-Cohen C., Schechter M., Rozenberg A., et al. Long-term, real-world, kidney function changes with SGLT2i versus DPP4i type 2 diabetes without cardiovascular or kidney disease. Clin J Am Soc Nephrol. 2023 doi: 10.2215/CJN.0000000000000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Diamantidis C.J., Cook D.J., Dunning S., et al. Missing care: the initial impact of the COVID-19 pandemic on CKD care delivery. J Gen Intern Med. 2022;37:4241–4247. doi: 10.1007/s11606-022-07805-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cueto-Manzano A.M., Cortés-Sanabria L., Martínez-Ramírez H.R., Rojas-Campos E., Gómez-Navarro B., Castillero-Manzano M. Prevalence of chronic kidney disease in an adult population. Arch Med Res. 2014;45:507–513. doi: 10.1016/j.arcmed.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 53.Szczech L.A., Stewart R.C., Su H.L., et al. Primary care detection of chronic kidney disease in adults with type-2 diabetes: the ADD-CKD Study (awareness, detection and drug therapy in type 2 diabetes and chronic kidney disease) PLoS One. 2014;9 doi: 10.1371/journal.pone.0110535. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Projected cumulative transitions from CKD stage G3 to G4 and G4 to G5 (ESKD) (2022–2027) per KDIGO uACR category in the diagnosed CKD population for 31 countries and regions. Table S2. Projected cumulative incidence of heart failure and myocardial infarction (2022–2027) per KDIGO uACR category in the diagnosed CKD population (CKD stages G3–G5) for 31 countries and regions. Table S3. Projected cumulative incidence of stroke and all-cause mortality (2022–2027) per KDIGO uACR category in the diagnosed CKD population (CKD stages G3–G5) for 31 countries and regions. Table S4. Projected costs associated with heart failure in the diagnosed population (CKD stages G3–G5) (prevalence – 2027) per uACR category for 31 countries and regions. Table S5. Projected costs associated with myocardial infarction in the diagnosed population (CKD stages G3–G5) (prevalence – 2027) per uACR category for 31 countries and regions. Table S6. Projected costs associated with stroke in the diagnosed population (CKD stages G3–G5) (prevalence – 2027) per uACR category for 31 countries and regions. Table S7. Projected costs associated with CKD management stages G3a and G3b in the diagnosed population (prevalence – 2027) per uACR category for 31 countries and regions. Table S8. Projected costs associated with CKD management stages G4 and G5 in the diagnosed population (prevalence – 2027) per uACR category for 31 countries and regions. Table S9. Inside CKD Scientific Steering Committee. Table S10. GATHER checklist.
Data Availability Statement
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at: https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure