Abstract
We present here a novel approach to identify T-cell antigens from any infectious agent by use of a library of purified recombinant proteins. Essential features of this strategy include (i) a highly efficient cDNA cloning system which negatively selects against nonrecombinant transformants by making use of the bacterial EcoK restriction system, (ii) affinity staining of cDNA clones expressing recombinant proteins, and (iii) a procedure of simultaneous purification of recombinant proteins from large numbers of isolated clones (representing the protein library) in a single step from pools consisting of up to 24 individual clones. The feasibility of the screening system was confirmed by constructing a protein library of the human parasite Schistosoma mansoni. The recombinant antigens of this library were used to stimulate CD4+ T cells derived from the axillary lymph nodes of mice vaccinated with irradiated cercariae. In initial screening experiments, we detected parasite-specific proliferation and gamma interferon (IFN-γ) secretion in response to several pools of cDNA clones. Further analysis of one particular pool revealed that only one of its constituents stimulated considerable IFN-γ secretion by CD4+ T cells and that the expressed antigen is identical to a small fragment of myosin heavy chain.
Over the last few years, cell-mediated immune responses have been shown to be responsible for protective immunity against a wide range of parasites and intracellular bacteria (10). Consequently, identification of the corresponding T-cell epitopes in these models represents a pivotal step in the design of new and effective vaccines.
Several groups have already managed to clone potent T-cell antigens from various organisms. Conventionally, these recombinant antigens have been identified by screening cDNA expression libraries with sera from infected patients (24, 34), sera from experimentally exposed animals (2), or sera raised against biochemically fractionated antigens from the infective pathogen (11). The selected cDNA clones were subsequently tested for their T-cell-stimulatory capacities. However, this combination of antibody-based screening and T-cell assays permits the identification only of T-cell antigens, which also carry B-cell epitopes on the same molecule (18). Alternatively, crude supernatants (31) or semipurified fusion proteins (30) from bacteriophage expression libraries have been used directly in T-cell proliferation assays, thereby avoiding screening with antisera. However, only soluble antigens may be identified by these techniques (34), while many recombinant proteins form insoluble aggregates (27). Furthermore, in most screening assays only T-cell clones (9, 14, 20) or T-cell hybrids (22) have been used as responder cells. This might be due to the easy availability of large homogeneous cell populations from cultivated cell lines, and it also avoids background stimulation of non-T cells in heterogeneous cell populations by bacterial endotoxin contamination of crude antigen preparations. None of these strategies mentioned above has proven to be applicable to routine work. Even more important, there is no standardized method available for screening a whole cDNA library solely on the basis of T-cell reactivity that is applicable to polyclonal cell populations from lymph nodes (LN), blood, or spleen for systematic identification of the relevant T-cell antigens of a given pathogen.
We present here a novel screening approach which allows simultaneous expression and affinity purification of large numbers of randomly selected recombinant proteins from any organism suitable for stimulation studies with T cells. The antigens of the so-called protein library can be easily copurified from pools consisting of up to 24 different clones and then tested with lymphocytes derived from infected or immunized hosts. We demonstrated the feasibility of our approach by the construction of a Schistosoma mansoni protein library, leading to the identification of a fragment of myosin heavy chain as a highly reactive T-cell epitope in mice vaccinated with irradiated cercariae.
MATERIALS AND METHODS
Animals and parasites.
NMRI, MF1, and C57BL/6 mice were obtained from the breeding facilities of the Biochemisches Institut, Justus-Liebig-Universität Giessen, Giessen, Germany, and the Department of Biology, University of York, York, United Kingdom. Puerto Rican isolates of S. mansoni were maintained by passage through NMRI or MF1 mice and Biomphalaria glabrata snails. Infections were performed percutaneously by standard techniques (25). Soluble worm antigens (SWAP) were prepared as previously described (16).
Construction of the vector plasmid.
Incubations with DNA-modifying enzymes were performed according to the instructions of the manufacturer (New England Biolabs or Promega). DNA sequences were determined by standard techniques. Synthetic oligonucleotides were purchased from Pharmacia. The EcoK recognition sequence within the ampicillin resistance gene of pQE-12 (Qiagen) was destroyed by site-directed PCR mutagenesis without changing the amino acid sequence of the encoded β-lactamase enzyme. After filling in of the original EcoRI site in the promoter region, the oligonucleotides AATTAACGAATTCGTGCTA and AATTTAGCACGAATTCGTT were inserted between the BamHI and HindIII sites of the vector, thus forming a new cloning site. Cloning steps were performed in Escherichia coli TG1 (EcoK negative) in order to obtain EcoK-unmethylated plasmid DNA.
Preparation of cDNA.
A λgt10 cDNA library from adult S. mansoni was constructed by standard protocols (21). Second-strand cDNA synthesis was performed with random (N6) primers as well as with oligo(dT) primers. Phage DNA was purified from a plate lysate of the whole library by affinity chromatography (Qiagen), and cDNA inserts were PCR amplified. For each reaction, 0.25 μg of λgt10 DNA template, 25 pmol of primers, 250 μM deoxynucleoside triphosphates, 2.5 mM MgCl2, and 3 U of Taq polymerase (Promega) were used in a total volume of 50 μl. Cycles were 94°C for 30 s, 60°C for 1 min, and 72°C for 2 min, with 20 repeats. Purification and desalting of the amplified cDNA were performed on S-500 columns (Pharmacia). Fractions with apparent fragment sizes of between 200 and 3,000 bp were pooled and digested with EcoRI.
The protein library.
PCR-amplified cDNA was inserted into the EcoRI restriction site of pEcoK-5 and introduced into E. coli M15 (EcoK positive). For each reaction, 0.01 pmol of unmethylated plasmid DNA and 1 to 2 μg of purified cDNA were ligated. Screening for cDNA-containing colonies was performed by PCR analysis of randomly isolated clones. Screening for antigen expression was performed by colony blotting onto nitrocellulose membranes with an Ni-nitrilotriacetic acid (NTA)–alkaline phosphatase conjugate according to the instructions of the manufacturer (Qiagen). Positive colonies were picked from the original bacterial plates and rescreened.
Subcloning of calpain and FABP cDNAs.
The cDNA of schistosome calpain (accession no. M67499) was subcloned by inserting the EcoRI/BglII fragment of plasmid pRizk1C (1) into a derivative of pEcoK-5. The 280-amino-acid fragment expressed by pDS-calpain/EB contained the T-cell epitope recognized by the previously described Th1 cell clone B (9). Plasmid pDS-FABP, expressing the complete coding sequences of fatty acid binding protein (FABP) (Sm14; accession no. M60895), was described elsewhere (13).
Purification of recombinant antigens.
For individual recombinants, bacterial broth was inoculated 1:10 with overnight cultures of the respective E. coli cDNA clones. For preparation of antigen pools, up to 50 separately grown overnight cultures of cDNA clones from the protein library were mixed and diluted 1:10 with bacterial broth. After 30 min, protein expression was induced by treatment with 2 mM isopropyl-β-d-thiogalactopyranoside for 3 to 4 h. Bacterial cells were pelleted and lysed in 6 M guanidine hydrochloride–100 mM Na2HPO4, pH 8.0. TALON affinity matrix (Clontech) was added to the supernatant and after 1 h was washed three times with 8 M urea–100 mM Na2HPO4–50 mM NaCl at pH 8.0 and once with the same solution at pH 7.5. Elution of recombinant antigens was performed by addition of 50 mM EDTA. Eluted individual or pooled antigens were analyzed for purity and molecular mass on Tricine-sodium dodecyl sulfate gels (23). Protein concentrations were determined by a bicinchoninic acid protein assay (Pierce), and samples were dialyzed against phosphate-buffered saline (PBS) or culture medium.
T-cell proliferation and cytokine assays.
C57BL/6 mice (6 to 8 weeks old) were vaccinated via the shaved abdomen with 500 S. mansoni cercariae attenuated by 20 krads of irradiation with the 60Co source at the Strahlenzentrum, Universität Giessen, Germany, or at Cookridge Hospital, Leeds, United Kingdom. On day 4 after immunization, single-cell suspensions of the axillary LN were prepared, and the pooled LN cells from 10 mice were incubated with CD4+ magnetic beads and separated on VS+ MACS columns (Miltenyi). Eluted cell populations were 92 to 97% CD4+, as determined by flow cytometry with CD4+-fluorescein isothiocyanate antibodies (Pharmingen). Splenocytes from naive mice were irradiated with 3,000 rads and used as antigen-presenting cells (APC) for CD4+ populations. A total of 1 × 105 LN cells alone or 1 × 105 CD4+ cells with 4 × 105 APC were cultivated in 96-well plates in 200 μl/well as described previously (15). Cultures were stimulated with soluble antigens and with individual or pooled recombinant antigens as indicated in the figure legends. After 72 h, 120 μl of supernatant was removed and tested for the presence of gamma interferon (IFN-γ) and interleukin-4 (IL-4) by corresponding two-site enzyme-linked immunosorbent assays (ELISAs) as described previously (16). Cells were pulsed with [3H]thymidine (18.5 kBq per well; ICN) and harvested 18 h later. Isotope incorporation was determined by liquid scintillation counting.
The calpain-specific Th1 cell clone B was maintained and used for stimulation assays as described previously (9). In brief, once the clone was established, it was stimulated every 3 to 4 weeks with antigen and APC. During the intervening period, it was expanded in medium supplemented with 35 U of recombinant human IL-2 (generously provided by Cetus Corp., Emeryville, Calif.) per ml. For proliferation assays and cytokine analysis, resting clone B cells were incubated at 2 × 105 cells/well with 5 × 105 APC in a final volume of 200 μl in the presence of antigen, as indicated in the figure legends. Supernatants were removed for IFN-γ determination after 48 h. Proliferation was measured by overnight incorporation of [3H]thymidine.
ELISA.
Multiple-vaccination serum was obtained from a C57BL/6 mouse exposed three times to irradiated cercariae 14 days after the third vaccination. Microtiter plates (Maxisorp; Nunc) were coated overnight at 4°C with recombinant IrV-5 (kindly provided by Karl Hoffmann) or clone 2.3 at 4 μg/ml (50 μl/well) diluted in PBS. The plates were washed five times with PBS plus 0.05% Tween 20 (PBST) and probed for 2 h at room temperature with the serum samples diluted 1:100 in PBST (50 μl/well). After five washes, the plates were probed for 2 h at room temperature with peroxidase-labelled rabbit anti-mouse immunoglobulin G (Sigma) diluted 1:2,000 in PBST. After a further five washes, 50 μl of 3,3′,5,5′-tetramethylbenzidine substrate solution (Kirkegaard & Perry Laboratories) was added to each well. Fifteen minutes later, absorbance was quantified at 630 nm with a Dynatech MR500 ELISA reader.
RESULTS
Construction of a vector for highly efficient cDNA cloning.
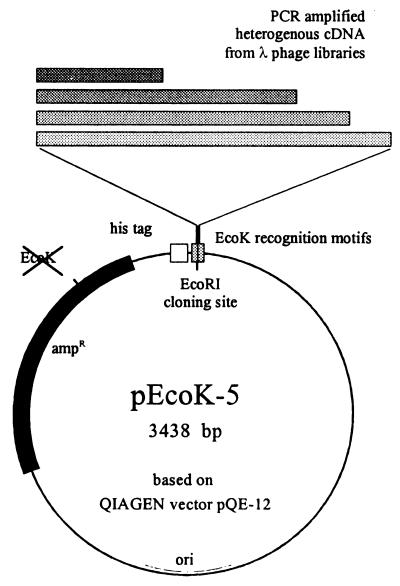
The cDNA inserts of a previously constructed library of adult S. mansoni worms in bacteriophage λgt10 (EB) were PCR amplified and ligated into plasmid pEcoK-5. This plasmid is a derivative of expression vector pQE-12 (Qiagen) and was designed to allow positive selection of recombinant clones by means of the bacterial EcoK restriction system. The original EcoRI site in the promoter region and the EcoK signal in the ampicillin resistance gene of pQE-12 were destroyed, and a new EcoRI cloning site, flanked by the two motifs of the new EcoK recognition sequence, was created between the BamHI and HindIII sites of the multiple cloning site (Fig. 1). After insertion of cDNA, the EcoK signal is destroyed, thus allowing replication of the recombinant plasmid while restricting the wild-type vector (7, 32). By using unmethylated plasmid DNA for insertion of heterogeneous PCR-amplified schistosome cDNA, up to 84% of all colonies contained cDNA inserts upon transformation of EcoK-positive bacteria (E. coli M15), whereas the EcoK-negative E. coli strain TG1 yielded only 5 to 6% recombinants (Table 1).
FIG. 1.
Essential features of the cloning and expression vector, pEcoK-5. Heterogeneous PCR-amplified cDNA was inserted into a unique EcoRI site flanked by the recognition motifs of the EcoK restriction system (the original EcoK signal in the ampicillin resistance (ampR) gene was destroyed previously without changing the amino acid sequence of the encoded enzyme). Clones expressing recombinant antigens could easily be identified and isolated after detection of the N-terminal histidine tags fused to each protein.
TABLE 1.
Positive selection by EcoK restrictiona
| Expt | cDNA-positive coloniesb
|
Selection factorc | |
|---|---|---|---|
| E. coli M15 | E. coli TG1 | ||
| 1 | 0.78 | 0.05 | 15.6 |
| 2 | 0.84 | 0.06 | 15.2 |
cDNA-containing colonies after transformation of two different E. coli strains were detected by PCR analysis. Typical results of two independent experiments are shown.
Relative numbers of cDNA-positive colonies in relation to total numbers of randomly analyzed colonies.
Selection factors were calculated as the ratio of values for EcoK-positive (M15) and EcoK-negative (TG1) E. coli strains.
Creation of a recombinant protein library.
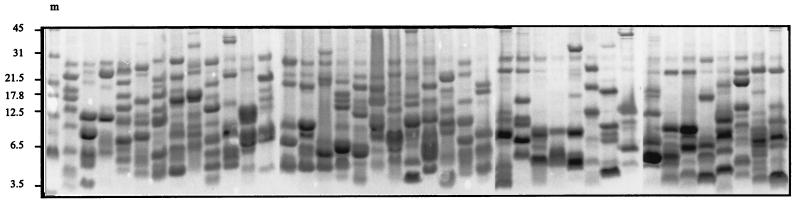
Colonies stably expressing inserted open reading frames could easily be identified by use of an Ni-NTA conjugate covalently linked to alkaline phosphatase (Qiagen). This complex bound specifically to the N-terminal histidine residues of the recombinant proteins. Selective staining of recombinant fusion proteins by the Ni-NTA conjugate is possible due to the fact that small histidine-tagged peptides, up to ca. 30 amino acids in length, resulting from cDNAs inserted in inverse orientation or in the wrong reading frame are quickly degraded in the bacteria (Qiagen). By colony blotting, we obtained approximately 6 to 9% positively stained colonies after cDNA transformation, all of which expressed recombinant antigens as verified by protein analysis of isolated clones. The molecular masses of the expressed antigens ranged between 2 and 45 kDa, whereas the cDNA insert sizes ranged from ca. 100 to more than 2,000 bp, as determined by PCR analysis (data not shown). A total of 960 cDNA clones were isolated and stored in microwell plates. Sequence analysis of 96 randomly chosen cDNA clones revealed the presence of large proportions of schistosome-specific sequences (33.8% of all cDNA inserts) as well as unknown sequences (43.2%) in the protein library, while their redundancy was quite low. Among the schistosome proteins identified were cathepsin B, tropomyosin, FABP, and cyclophilin B. Large numbers of recombinant antigens were expressed in pools consisting of up to 24 different individual clones and purified with TALON affinity matrix (Clontech). After copurification, gel electrophoresis revealed that distinct protein bands could be observed for each of the clones, with different band intensities according to the expression levels and the growth rates of the individual cDNA clones (Fig. 2).
FIG. 2.
Recombinant antigens purified from 960 randomly isolated cDNA expression clones of the S. mansoni protein library. For each of the 40 lanes, 24 cultures were pooled randomly. Protein purification of these pools were performed by using TALON affinity matrix (Clontech) under denaturing conditions. The amount of protein per lane corresponds to 4 ml of bacterial culture. Lane m, protein size markers (in kilodaltons).
Stimulation of CD4+ cells from vaccinated mice with recombinant antigens.
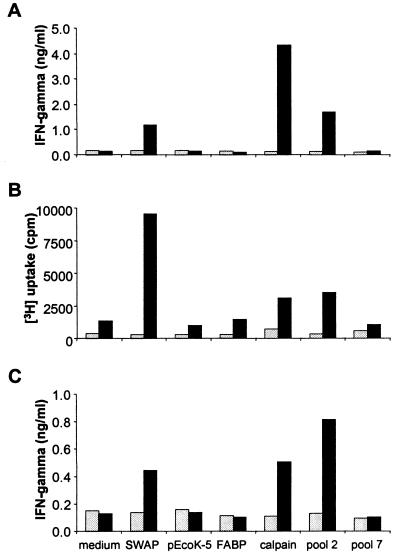
SWAP stimulated proliferation and cytokine synthesis of LN cells (Fig. 3A) and CD4+ populations (Fig. 3B and C) derived from mice vaccinated with irradiated cercariae but not from naive animals. Consistent with results of previous experiments showing induction of Th1-type immune responses after vaccination with radiation-attenuated larvae (16), the production IFN-γ was much higher than of IL-4 (data not shown). Of the two recombinant schistosome antigens, FABP and calpain, tested, only calpain stimulated high levels of LN cell and CD4+-T-cell activity (Fig. 3). Levels of proliferation and cytokine production for preparations from bacteria transformed with the empty cloning vector, pEcoK-5, were always indistinguishable from values obtained with medium alone, thus indicating the absence of detectable mitogenic contamination in the recombinant protein samples. In this study, 264 recombinant antigens from the S. mansoni protein library were copurified into 22 different pools, each containing 12 cDNA clones. We observed highly significant stimulation of T cells in the presence of some pools (>4.5-fold-increased levels of IFN-γ secreted by CD4+ cells for pool 2 and two other pools), whereas others showed no signals at all (pool 7 and four other pools), despite the presence of similar protein amounts in the preparations (Fig. 3). The remaining pools induced intermediate responses, with IFN-γ levels increased to 1.5- to 3.5-fold above background values (data not shown).
FIG. 3.
Stimulation of murine lymphocytes with recombinant antigens. (A) IFN-γ production by unfractionated LN cells. (B) Proliferation of purified CD4+ cells. (C) IFN-γ production by CD4+ cells. Axillary LN cells from naive (light bars) and vaccinated (dark bars) mice were cultivated with medium alone or in the presence of 15 μg of SWAP per ml. FABP and calpain were used at 10 μg/ml. A total of 264 antigens derived from the protein library were purified into 22 pools, each containing 12 randomly isolated cDNA clones. These were used for stimulation assays at concentrations corresponding to ca. 4 ml of bacterial culture per well (final antigen concentrations of about 10 to 50 μg/ml). The results shown for pools 2 and 7 represent typical results for different pools. Bacteria carrying the empty cloning vector, pEcoK-5, were included in the purification protocol to check for copurified bacterial contamination. The data represent the mean values of stimulation assays performed in triplicate.
Stimulation of CD4+ cells with mixtures of calpain and FABP.
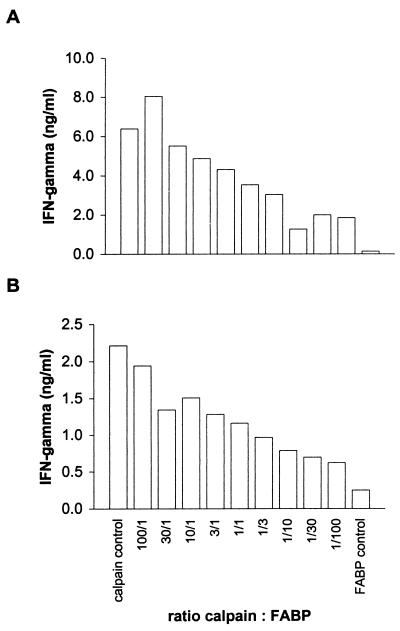
Since recombinant FABP did not elicit any cellular response at all, it was used as a negative control in further experiments. To evaluate if specific T-cell responses (i.e., to calpain) can be observed even in the presence of a large excess of irrelevant antigens (i.e., FABP), calpain and FABP were copurified from pooled bacterial cultures after inoculation with the cDNA expression clones at ratios ranging from 100:1 to 1:100. These mixtures were used at equal dilutions to stimulate the calpain-specific T-cell clone B and freshly isolated CD4+ cells from vaccinated mice. In both cases, calpain-induced IFN-γ was detectable even at a 100-fold excess of FABP when compared to the negative control with FABP alone (Fig. 4). It should be noted that due to the lower expression level of the calpain cDNA clone, the resulting ratio of calpain to FABP in the T-cell assays was even lower than the theoretically expected value of 1:100. Therefore, these results indicate that it should be possible to screen pools of cDNA clones each containing even more than the 12 clones used in the above-described experiments (Fig. 3).
FIG. 4.
Stimulation of T cells with mixtures of recombinant antigens. (A) IFN-γ production by the calpain-specific Th1 clone B. (B) IFN-γ production by CD4+ cells from the LN of vaccinated mice. Calpain and FABP were copurified after cocultivation of both expression cDNA clones at different ratios, ranging between 100:1 and 1:100. Antigens were used at dilutions corresponding to 3.5 ml of bacterial culture per ml. Due to different levels of expression of these cDNA clones, final antigen concentrations ranged from 6.7 μg/ml (calpain control) to 22.0 μg/ml (FABP control), with the mixtures in between.
Stimulation of CD4+ cells by a fragment of myosin heavy chain.
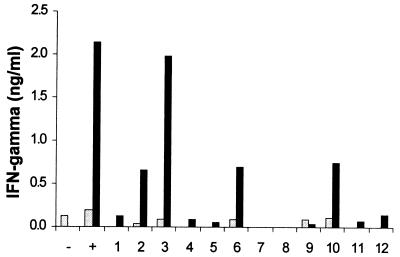
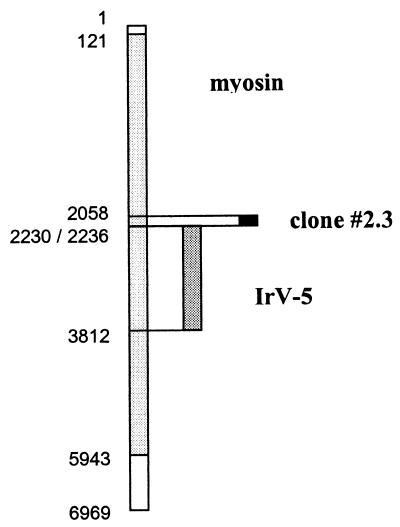
Since pool 2 antigens induced IFN-γ secretion from CD4+ cells at levels comparable to SWAP or recombinant calpain, the identities of the cDNA clones contained in this pool were investigated further. After expression and purification of the 12 individual antigens, clone 2.3 in particular exhibited high levels of IFN-γ secretion by CD4+ cells from vaccinated mice (Fig. 5), whereas cells from naive mice showed no stimulation at all. IFN-γ production was also induced to a lesser extent by clones 2.2, 2.6, and 2.10. The importance of these three clones as candidate T-cell antigens remains to be confirmed. DNA sequence analysis revealed that the cDNA insert of clone 2.3 was identical to a 178-bp fragment of schistosome myosin heavy chain (33). Interestingly, the 62-kDa IrV-5 fragment of myosin heavy chain has previously been associated with protective immunity in the radiation-attenuated vaccine model (26). It should be noted, however, that the sequences of clone 2.3 and IrV-5 do not overlap except in two codons (Fig. 6). The individual cDNA clones of several other pools showing positive effects on T cells are currently being analyzed, and there might be further T-cell epitopes present among the 264 cDNA clones screened so far.
FIG. 5.
Stimulation of CD4+ cells from naive (light bars) and vaccinated (dark bars) mice with the 12 individual antigens of pool 2. The protein amounts corresponded to 0.2 ml of bacterial culture per well. Negative control (−), medium alone; positive control (+), 10 μg of calpain per ml. The data represent the mean values of stimulation assays performed in triplicate.
FIG. 6.
Alignment of the DNA sequence of myosin heavy chain (accession no. L01634) with those of the cloned fragments IrV-5 (accession no. X65591) and cDNA clone 2.3. The positions of the first and the last base pairs within the 6,969-bp myosin sequence are shown. The coding region of myosin ranges from bp 121 to 5943.
Antibody reactivity to clone 2.3.
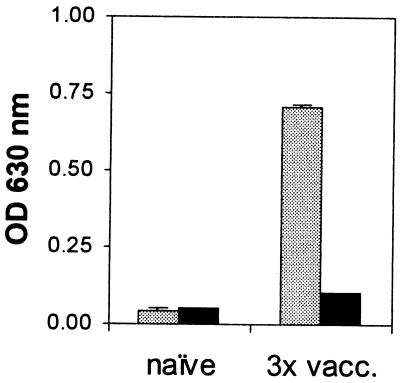
Consistent with the fact that multiple exposures to the irradiated vaccine induce a specific antibody response against the IrV-5 fragment (26), in an ELISA experiment the multiple-vaccination serum strongly recognized IrV-5 but only marginally recognized clone 2.3 (Fig. 7). A Western blot of recombinant IrV-5, as well as calpain, gave a strong signal when probed with serum from a multiply vaccinated mouse (data not shown). However, this serum did not recognize the clone 2.3 protein. Furthermore, by colony blotting we could not detect clone 2.3 with this serum, although the calpain expression clone, pDS-calpain/EB, was readily detected (data not shown).
FIG. 7.
Antibody reactivity to clone 2.3 (dark bars) and IrV-5 (light bars). Antisera were obtained from naive mice or after three exposures to irradiated cercariae (3× vacc.). The data represent the mean values and standard errors of assays performed in duplicate. OD, optical density.
DISCUSSION
We here report the first screening protocol for identification of T-cell antigens, combining high-level expression of recombinant antigens with an application to heterogeneous T-cell populations, thus providing the technology for the development of powerful vaccines. Our new strategy is based on the production of libraries containing hundreds, or thousands, of proteins of an organism and on the use of these purified proteins for T-cell proliferation and cytokine assays. In contrast to previously published methods (9, 14, 20), our protocol allows the use of polyclonal T-cell populations from infected or immunized hosts for stimulation assays, i.e., without prior establishing T-cell clones or T-cell lines. This method was used to identify T-cell antigens in mice vaccinated against schistosomiasis, where the protective immune response depends on the presence of antigen-specific CD4+ T cells (6). However, the screening system described should be applicable to any other model of infectious diseases where cell-mediated immune responses play a key role, such as murine leishmaniasis in resistant mice (4) or Listeria infections (29). Furthermore, it also is pertinent to CD4+-T cell-dependent reactions in autoimmune diseases, tumor immunology, allogeneic transplantations, and allergic reactions (12, 19).
Although they are indispensable for construction of comprehensive cDNA libraries, conventional bacteriophage vectors are not appropriate for large-scale production of individual recombinant proteins. Plasmid-based expression systems are more suitable in this respect (27). However, they have the disadvantage of depending upon large quantities of cDNA for library construction due to the low transformation efficiency of naked DNA. In order to construct a powerful protein expression library, the positive features of both vector systems were combined. Because of the limited amounts of parasite material, adult schistosome cDNA was PCR amplified from a phage library, although we are aware that PCR amplification of heterogeneous cDNA might bias the original content of the library. In this respect, to avoid as far as possible PCR-related changes in the original frequency of certain transcripts, as well as to minimize the likelihood of point mutations, we restricted the reaction to a maximum of 20 PCR cycles. Short cDNA fragments with only little sequence information were removed by gel filtration. To increase the yield of recombinant plasmids, EcoK restriction was used as a positive-selection system (Fig. 1). Consistent with the results of others (7, 32), this led to an enrichment of cDNA-positive clones by a factor of 16 compared with the transformation of EcoK-negative cells (Table 1). Moreover, the total yields of cDNA-positive colonies of up to 84% in our hands were even higher than those described previously (7, 32). In contrast to most other vector systems (5), here only a minimum of vector-encoded sequences was added to the inserted parasite cDNA. There were just 10 additional amino acids, including a tag of 6 histidine residues, at the N terminus of each recombinant antigen and translation stops in every reading frame two to five codons behind the inserted cDNA.
Prior selection of individual protein-expressing cDNA clones by histidine tag-specific staining was performed, since the cDNA was inserted in a random orientation into the plasmid. Therefore, only one of six clones could statistically contain cDNA inserted in the correct reading frame. In order to keep bacterial contamination at a minimum, antigen-expressing cDNA clones were initially selected from the excess of unproductive clones of the primary gene library by using an Ni-NTA–alkaline phosphatase conjugate. The selection procedure eliminates clones containing very short translation products because they are degraded by an intrinsic proteolytic activity in E. coli. Conventionally, in order to reduce the total numbers of cDNA clones to be analyzed, preselection of immunogenic epitopes has been performed by screening cDNA libraries with immune sera (9, 20). In contrast, preselection of antigen-expressing clones with a biochemical affinity matrix as described in our work is a novel method which avoids the need for specific antibodies and therefore allows the creation of expression libraries also containing antigens that are not recognized by any serum.
By isolation of individual cDNA clones to build up a protein library, expression of randomly isolated antigens could be performed in a controlled and reproducible manner, which is in contrast to a protocol described by Mougneau and colleagues (14). Sequential fractionation and rescreening of large uncharacterized pools as performed by that group will inevitably lead to loss of rare, unstable, or even toxic epitopes present in the original cDNA, thus allowing detection of common and highly expressed antigens only. In our system, the use of a sensitive histidine tag-specific staining procedure should allow detection of any expressed recombinant protein, irrespective of the actual expression level. The recombinant antigens could be purified in roughly similar amounts (Fig. 2), but with the band intensities depending on the individual expression levels. Although most of our antigens were insoluble under physiological conditions, subsequent T-cell stimulation assays could be performed with very high sensitivity. It is very unlikely that putative interactions between different antigens would affect these assays, because any such interaction (either specific binding between two peptides or unspecific aggregation of several molecules) would be broken up during antigen processing and presentation by APC. In principle, therefore, every epitope present in our protein library should be available in amounts sufficient to detect stimulation of proliferation or cytokine production. Of course, it is conceivable that certain cDNA sequences cannot be expressed in our bacterial system at all, which is a general complication with randomly isolated expression clones and applies to any kind of cDNA library.
Importantly, the fact that in all experiments performed with preparations of bacteria carrying the empty cloning vector, pEcoK-5, the background values for cell proliferation and IFN-γ secretion were negligible (Fig. 3) argues against contamination with bacterial endotoxin. Using two putatively protective antigens, FABP and calpain, as controls for our work with murine LN and CD4+ cells, we detected considerable induction of T-cell proliferation and cytokine secretion with recombinant calpain only (Fig. 3). This confirmed its proposed function as a major T-cell antigen in the protective immune response against schistosome infection. Calpain was the first S. mansoni candidate vaccine identified solely on the basis of its T-cell reactivity (9), and vaccination with recombinant calpain resulted in significant immune protection of treated mice (8). The second control antigen used, FABP, has been described to be highly protective in mice and rabbits (28), but all attempts to reproduce these data have failed (reference 3 and our unpublished observations). Because it did not stimulate detectable levels of lymphocyte proliferation or cytokine secretion, it was used as a negative control in our experiments.
In order to detect signals from even weakly stimulatory antigens, the first screening of the protein library was performed with pools consisting of 12 individual recombinants only. If T-cell stimulation was observed with a particular pool, the antigens of this pool were tested individually again. In this way, the whole protein library could be screened within a short time. However, in assays to determine the sensitivity of the approach, recombinant calpain induced a significant IFN-γ response even in the presence of a 30- to 100-fold excess of FABP. This was true not only with a calpain-specific Th1 cell clone (Fig. 4A) as responder but also with total CD4+ T cells from the LN of vaccinated mice (Fig. 4B). Since copurification of more than 12 different antigens could easily be performed (Fig. 2), further experiments examining 960 cDNA clones purified from 40 pools, each consisting of 24 cDNA clones, are being performed.
The utility of our new approach to detect T-cell antigens is demonstrated by the successful identification of a fragment of myosin heavy chain as the major reactive component in one of the pools tested (Fig. 5). This result fits with previous data revealing an important role for myosin in protective immunity against schistosomiasis. A different fragment of myosin, IrV-5, has been identified by screening a cDNA library with immune sera (26); however, our myosin fragment, clone 2.3, does not overlap with IrV-5 except in two amino acids (Fig. 6). They therefore represent two entirely different parts of the full-length molecule. Since we were not able to detect clone 2.3 with multiple-vaccination serum, in contrast to the case for recombinant IrV-5 (Fig. 7), we therefore conclude that clone 2.3 contains a potent T-cell epitope which might never have been identified by conventional antibody-based screening approaches. Analysis of additional positive pools of recombinant antigens is under way and will enable us to identify additional T-cell antigens involved in the immune reactions in the irradiated vaccine model against schistosomiasis. However, since protective immunity is directed against lung stage parasites (6, 16), a cercaria or schistosomulum protein library, rather than an adult worm library as used for the above-described experiments, is more likely to allow identification of potent new vaccine candidates (17) and therefore will represent an even more important tool for future experiments.
In summary, we believe that by our new approach the technological gap of an urgently needed efficient method for identifying T-cell antigens has been filled. The unsatisfactory results from independent trials with putative vaccine candidates against schistosomiasis (3) underline the need to pursue new routes for the identification of antigens other than those predominantly reactive with antibodies.
ACKNOWLEDGMENTS
This work was supported by a studentship from the Deutsche Forschungsgemeinschaft (to M.E.) and by a Wellcome Trust Career Development Fellowship (to A.P.M.).
Plasmid pRizk1C was kindly provided by Mette Strand, and recombinant IrV-5 was kindly provided by Karl Hoffmann. We are grateful to David Johnston for sequence analysis of cDNA clones for the Schistosoma Genome Project, to Sonia Anderson for lymphocyte phenotyping, to Pat Coulson for providing multiple-vaccination serum, and to Boran Altinçiçek, Ralf Füllkrug, Barbara Preiss, and Marlene Stein for excellent technical assistance.
REFERENCES
- 1.Andresen K, Tom T D, Strand M. Characterization of cDNA clones encoding a novel calcium-activated neutral proteinase from Schistosoma mansoni. J Biol Chem. 1991;266:15085–15090. [PubMed] [Google Scholar]
- 2.Beattie I A, Swaminathan B, Ziegler H K. Cloning and characterization of T-cell-reactive protein antigens from Listeria monocytogenes. Infect Immun. 1990;58:2792–2803. doi: 10.1128/iai.58.9.2792-2803.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergquist N R, Colley D G. Schistosomiasis vaccines: research and development. Parasitol Today. 1998;14:99–104. doi: 10.1016/s0169-4758(97)01207-6. [DOI] [PubMed] [Google Scholar]
- 4.Bogdan C, Gessner A, Solbach W, Röllinghoff M. Invasion, control and persistence of Leishmania parasites. Curr Opin Immunol. 1996;8:517–525. doi: 10.1016/s0952-7915(96)80040-9. [DOI] [PubMed] [Google Scholar]
- 5.Christensen L S, Boye M. Establishment of a genomic bank of bovine herpesvirus 1 using a novel positive selection plasmid vector. J Virol Methods. 1992;36:277–282. doi: 10.1016/0166-0934(92)90058-l. [DOI] [PubMed] [Google Scholar]
- 6.Coulson P S. The radiation-attenuated vaccine against schistosomes in animal models: paradigm for a human vaccine? Adv Parasitol. 1997;39:271–336. doi: 10.1016/s0065-308x(08)60048-2. [DOI] [PubMed] [Google Scholar]
- 7.De Backer O, Chomez P, de Plaen E. Positive selection of recombinant plasmids based on the EcoK restriction activity of Escherichia coli K-12. Gene. 1994;150:197–198. doi: 10.1016/0378-1119(94)90885-0. [DOI] [PubMed] [Google Scholar]
- 8.Hota-Mitchell S, Siddiqui A A, Debakan G A, Smith J, Tognon C, Podesta R B. Protection against Schistosoma mansoni infection with a recombinant baculovirus-expressed subunit of calpain. Vaccine. 1997;15:1631–1640. doi: 10.1016/s0264-410x(97)00081-9. [DOI] [PubMed] [Google Scholar]
- 9.Jankovic D, Aslund L, Oswald I P, Caspar P, Champion C, Pearce E, Coligan J E, Strand M, Sher A, James S L. Calpain is the target antigen of a Th1 clone that transfers protective immunity against Schistosoma mansoni. J Immunol. 1996;157:806–814. [PubMed] [Google Scholar]
- 10.Jankovic D, Sher A. Initiation and regulation of CD4+ T-cell function in host-parasite models. Chem Immunol. 1996;63:51–65. [PubMed] [Google Scholar]
- 11.Kohlstädt S, Couissinier-Paris P, Bourgois A, Bouchon B, Piper K, Kolbe H, Dessein A J. Characterization of a schistosome T cell-stimulating antigen (Sm10) associated with protective immunity in humans. Mol Biochem Parasitol. 1997;84:155–165. doi: 10.1016/s0166-6851(96)02787-9. [DOI] [PubMed] [Google Scholar]
- 12.Liblau R S, Singer S M, McDevitt H O. Th1 and Th2 CD4+ T cells in the pathogenesis of organ-specific autoimmune diseases. Immunol Today. 1995;16:34–38. doi: 10.1016/0167-5699(95)80068-9. [DOI] [PubMed] [Google Scholar]
- 13.Moser D, Tendler M, Griffiths G, Klinkert M Q. A 14-kDa Schistosoma mansoni polypeptide is homologous to a gene family of fatty acid binding proteins. J Biol Chem. 1991;266:8447–8454. [PubMed] [Google Scholar]
- 14.Mougneau E, Altare F, Wakil A E, Zheng S, Coppola T, Wang Z E, Waldmann R, Locksley R M, Glaichenhaus N. Expression cloning of a protective Leishmania antigen. Science. 1995;268:563–566. doi: 10.1126/science.7725103. [DOI] [PubMed] [Google Scholar]
- 15.Mountford A P, Coulson P S, Pemberton R M, Smythies L E, Wilson R A. The generation of interferon-gamma-producing T lymphocytes in skin-draining lymph nodes, and their recruitment to the lungs, is associated with protective immunity to Schistosoma mansoni. Immunology. 1992;75:250–256. [PMC free article] [PubMed] [Google Scholar]
- 16.Mountford A P, Harrop R, Wilson R A. Antigens derived from lung-stage larvae of Schistosoma mansoni are efficient stimulators of proliferation and gamma interferon secretion by lymphocytes from mice vaccinated with irradiated cercariae. Infect Immun. 1995;63:1980–1986. doi: 10.1128/iai.63.5.1980-1986.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mountford A P, Harrop R. Vaccination against schistosomiasis: the case for lung-stage antigens. Parasitol Today. 1998;14:109–114. doi: 10.1016/s0169-4758(97)01169-1. [DOI] [PubMed] [Google Scholar]
- 18.Mustafa A S, Oftung F, Deggerdal A, Gill H K, Young R A, Godal T. Gene isolation with human T lymphocyte probes. Isolation of a gene that expresses an epitope recognized by T cells specific for Mycobacterium bovis BCG and pathogenic mycobacteria. J Immunol. 1988;141:2729–2733. [PubMed] [Google Scholar]
- 19.Neophytou P I, Roep B O, Arden S D, Muir E M, Duinkerken G, Kallan A, de Vries R R P, Hutton J C. T-cell epitope analysis using subtracted expression libraries (TEASEL): application to a 38-kDa autoantigen recognized by T cells from an insulin-dependent diabetic patient. Proc Natl Acad Sci USA. 1996;93:2014–2018. doi: 10.1073/pnas.93.5.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saavedra R, de Meuter F, Decourt J L, Herion P. Human T cell clone identifies a potentially protective 54-kDa protein antigen of Toxoplasma gondii cloned and expressed in Escherichia coli. J Immunol. 1991;147:1975–1982. [PubMed] [Google Scholar]
- 21.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 22.Sanderson S, Campbell D J, Shastri N. Identification of a CD4+ T cell-stimulating antigen of pathogenic bacteria by expression cloning. J Exp Med. 1995;182:1752–1757. doi: 10.1084/jem.182.6.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schägger H, von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 24.Skeiky Y A W, Guderian J A, Benson D R, Bacelar O, Carvalho E M, Kubin M, Badaro R, Trinchieri G, Reed S G. A recombinant Leishmania antigen that stimulates human peripheral blood mononuclear cells to express a Th1-type cytokine profile and to produce interleukin 12. J Exp Med. 1995;181:1527–1537. doi: 10.1084/jem.181.4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smithers S R, Terry R J. The infection of laboratory hosts with cercariae of Schistosoma mansoni and the recovery of the adult worms. Parasitology. 1965;55:695–700. doi: 10.1017/s0031182000086248. [DOI] [PubMed] [Google Scholar]
- 26.Soisson L M, Masterson C P, Tom T D, McNally M T, Lowell G H, Strand M. Induction of protective immunity in mice using a 62-kDa recombinant fragment of a Schistosoma mansoni surface antigen. J Immunol. 1992;149:3612–3620. [PubMed] [Google Scholar]
- 27.Stüber D, Matile H, Garotta G. System for high level production in E. coli and rapid purification of recombinant proteins: application to epitope mapping, preparation of antibodies and structure-function analysis. In: Lefkovits I, Pernis B, editors. Immunological methods. New York, N.Y: Academic Press; 1990. pp. 121–152. [Google Scholar]
- 28.Tendler M, Brito C A, Vilar M M, Serra-Freire N, Diogo C M, Almeida M S, Delbem A C, da Silva J F, Savino W, Garratt R C, Katz N, Simpson A S. A Schistosoma mansoni fatty acid-binding protein, Sm14, is the potential basis of a dual-purpose anti-helminth vaccine. Proc Natl Acad Sci USA. 1996;93:269–273. doi: 10.1073/pnas.93.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Unanue E R. Studies in listeriosis show the strong symbiosis between the innate cellular system and the T cell response. Immunol Rev. 1997;158:11–25. doi: 10.1111/j.1600-065x.1997.tb00988.x. [DOI] [PubMed] [Google Scholar]
- 30.Villarreal-Ramos B, Sanchez-Garcia J, Stoker N, Timms E, Chomer D, Raff K, Mitchison N A. Screening gene expression libraries for epitopes recognized in Mycobacterium leprae by mouse T cells. Eur J Immunol. 1991;21:2621–2624. doi: 10.1002/eji.1830211047. [DOI] [PubMed] [Google Scholar]
- 31.Warren R L, Lu D, Sizemore D R, Baron L S, Kopecko D J. Method for identifying microbial antigens that stimulate specific lymphocyte responses: application to Salmonella. Proc Natl Acad Sci USA. 1990;87:9823–9827. doi: 10.1073/pnas.87.24.9823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waye M M Y, Verhoeyen M E, Jones P T, Winter G. EcoK selection vectors for shotgun cloning into M13 and deletion mutagenesis. Nucleic Acids Res. 1985;13:8561–8571. doi: 10.1093/nar/13.23.8561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weston D, Schmitz J, Kemp W M, Kunz W. Cloning and sequencing of a complete myosin heavy chain cDNA from Schistosoma mansoni. Mol Biochem Parasitol. 1993;58:161–164. doi: 10.1016/0166-6851(93)90100-c. [DOI] [PubMed] [Google Scholar]
- 34.Wilson M E, Young B M, Paetz Andersen K, Weinstock J V, Metwali A, Ali K M, Donelson J E. A recombinant Leishmania chagasi antigen that stimulates cellular immune responses in infected mice. Infect Immun. 1995;63:2062–2069. doi: 10.1128/iai.63.5.2062-2069.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]