Abstract
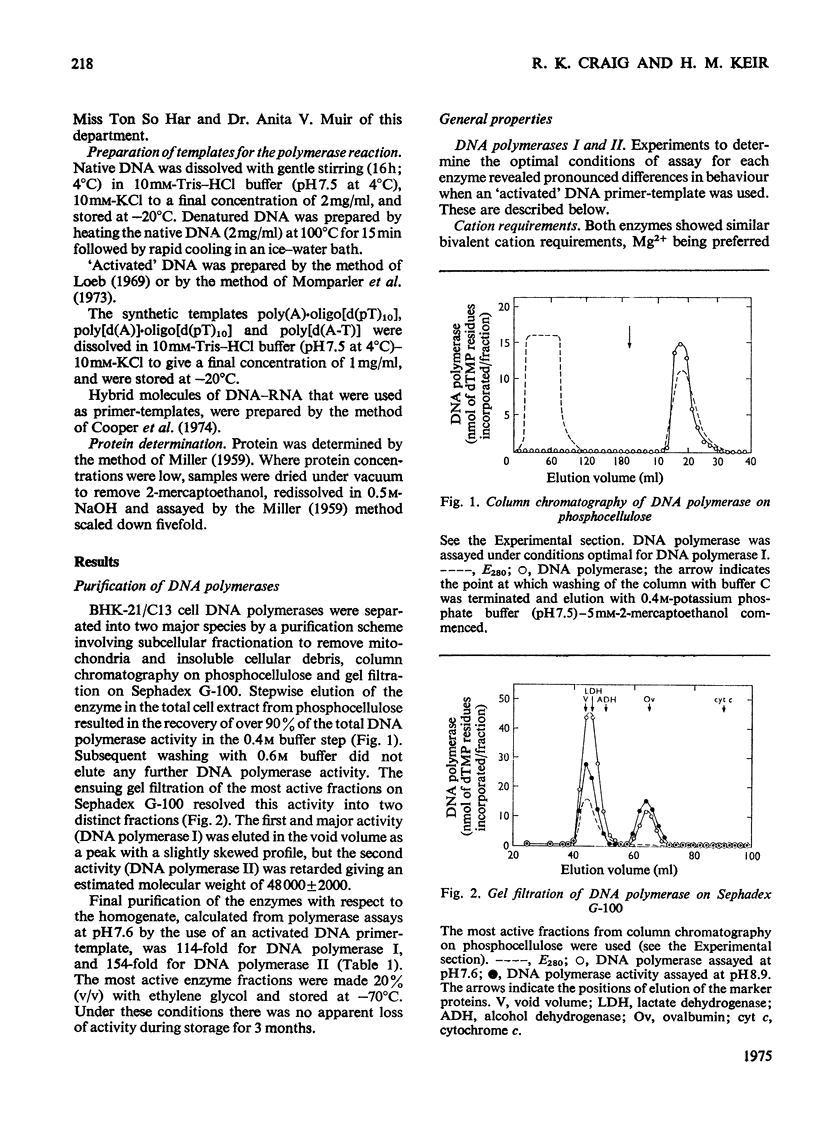
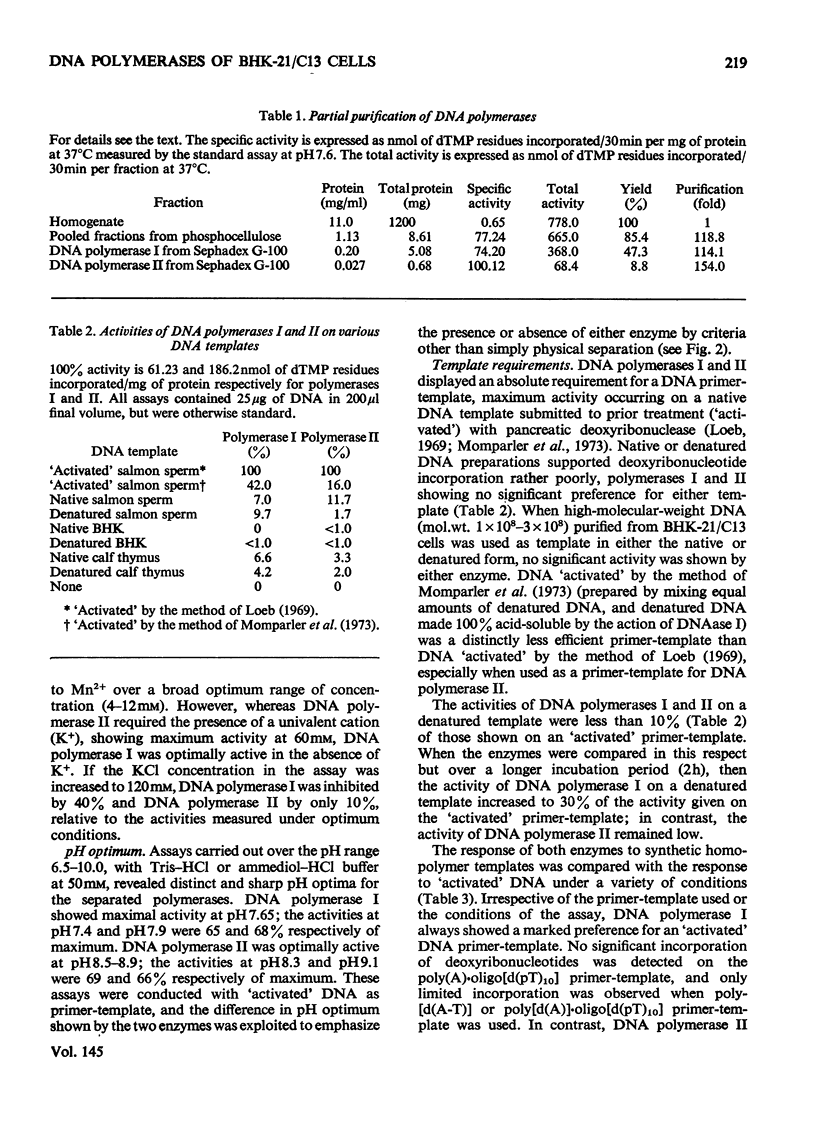
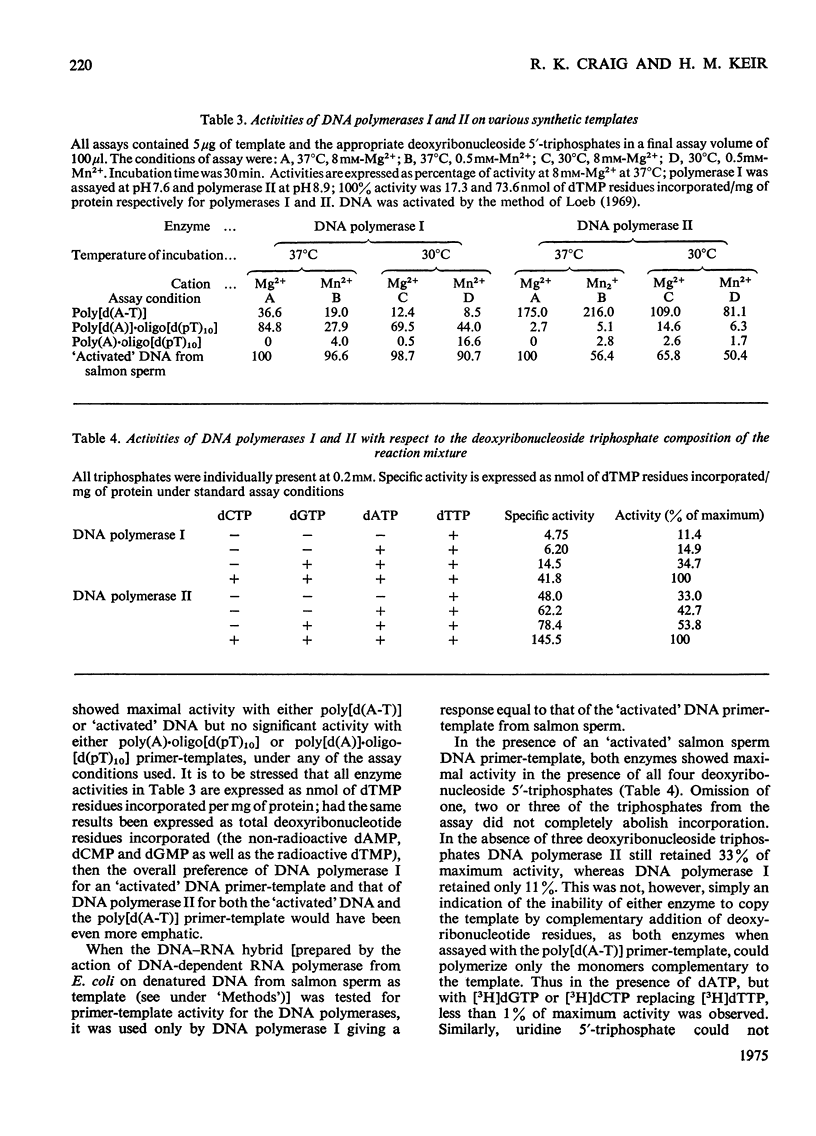
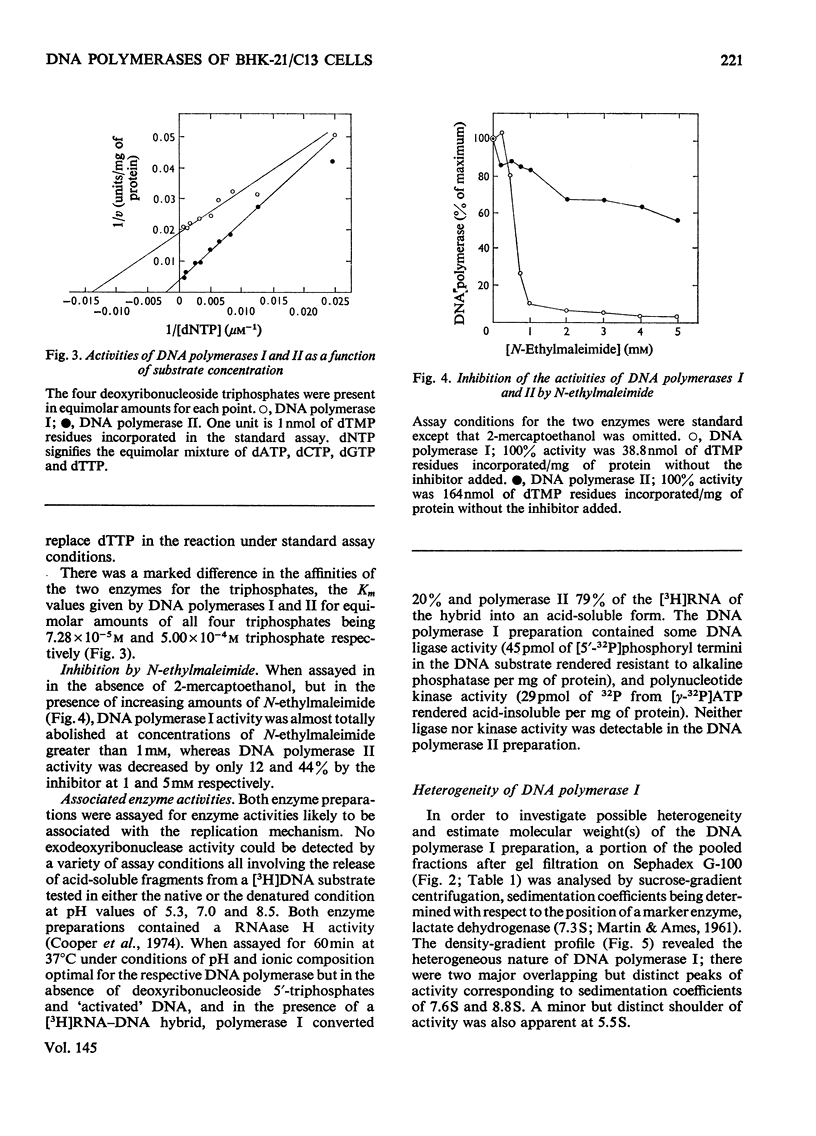
DNA polymerase from BHK-21/C13 cells were separated into two species, DNA polymerase I corresponding to the heterogeneous enzyme with sedimentation coefficient of 6-8S, and DNA polymerase II, corresponding to the enzyme with sedimentation coefficient of 3.3S. DNA polymerase I was purified 114-fold and DNA polymerase II 154-fold by a simple extraction procedure followed by column chromatography on phosphocellulose and gel filtration through Sephadex G-100. The purified enzymes differed markedly in respect of pH optimum, stimulation and inhibition by K+, Km for the deoxyribonucleoside 5'-triphosphates, stability to heating at 45 degrees C, and inhibition by N-ethylmaleimide. The preferred primer-template for both enzymes was "activated" DNA (DNA submitted to limited degradation by pancreatic deoxyribonuclease); native or thermally denatured DNA templates were relatively very poorly copied. When certain synthetic templates were tested, substantial differences were revealed between the two enzymes. Poly[d(A-T)] was poorly used by polymerase I but was superior to "activated" DNA for polymerase II. Poly[d(A)]-oligo[d(pT)10] was used efficiently by polymerase I but not by polymerase II. Poly(A)-oligo[d(pT)10] was not an effective primer-template although polymerase I could use it to a limited extent when Mn2+ replaced Mg2+ in the polymerase reaction and when the temperature of incubation was lowered from 37 degrees to 30 degrees C. When only one or two or three triphosphates were supplied in the reaction mixture, the activity of polymerase I was more severly diminished than that of polymerase II.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter-Gabbard K. L. A simple method for the large-scale preparation of sucrose gradients. FEBS Lett. 1972 Jan 15;20(1):117–119. doi: 10.1016/0014-5793(72)80031-0. [DOI] [PubMed] [Google Scholar]
- CUNNINGHAM L. W., CLOUSE R. W., FORD J. D. HETEROGENEITY OF THE CARBOHYDRATE MOIETY OF CRYSTALLINE OVALBUMIN. Biochim Biophys Acta. 1963 Oct 29;78:379–381. doi: 10.1016/0006-3002(63)91652-4. [DOI] [PubMed] [Google Scholar]
- Chang L. M., Bollum F. J. Deoxynucleotide-polymerizing enzymes of calf thymus gland. V. Homogeneous terminal deoxynucleotidyl transferase. J Biol Chem. 1971 Feb 25;246(4):909–916. [PubMed] [Google Scholar]
- Chang L. M., Bollum F. J. Low molecular weight deoxyribonucleic acid polymerase from rabbit bone marrow. Biochemistry. 1972 Mar 28;11(7):1264–1272. doi: 10.1021/bi00757a023. [DOI] [PubMed] [Google Scholar]
- Chang L. M. Development of terminal deoxynucleotidyl transferase activity in embryonic calf thymus gland. Biochem Biophys Res Commun. 1971 Jul 2;44(1):124–131. doi: 10.1016/s0006-291x(71)80167-5. [DOI] [PubMed] [Google Scholar]
- Chang L. M. Low molecular weight deoxyribonucleic acid polymerase from calf thymus chromatin. I. Preparation of homogeneous enzyme. J Biol Chem. 1973 Jun 10;248(11):3789–3795. [PubMed] [Google Scholar]
- Cooper R. J., Duff P. M., Olivier A., Craig R. K., Keir H. M. Multiple ribonuclease H activities from BHK-21-C13 cells. FEBS Lett. 1974 Sep 1;45(1):38–43. doi: 10.1016/0014-5793(74)80805-7. [DOI] [PubMed] [Google Scholar]
- Craig R. K., Costello P. A., Keir H. M. Dexyribonucleic acid polymerases of BHK-21/C13cells. Relationship to the physiological state of the cells, and to synchronous indution of synthesis of deoxyribonuleic acid. Biochem J. 1975 Feb;145(2):233–240. doi: 10.1042/bj1450233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig R. K., Keir H. M. Deoxyribonucleic acid poymerase of BHK-21/C13 cells. Heterogeneity, molecular asymmetry and subcellular distribution of the enzymes. Biochem J. 1975 Feb;145(2):225–232. doi: 10.1042/bj1450225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. One-step growth curve of Western equine encephalomyelitis virus on chicken embryo cells grown in vitro and analysis of virus yields from single cells. J Exp Med. 1954 Feb;99(2):183–199. doi: 10.1084/jem.99.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOGH J., FOGH H. A METHOD FOR DIRECT DEMONSTRATION OF PLEUROPNEUMONIA-LIKE ORGANISMS IN CULTURED CELLS. Proc Soc Exp Biol Med. 1964 Dec;117:899–901. doi: 10.3181/00379727-117-29731. [DOI] [PubMed] [Google Scholar]
- Goldberg E. Amino acid composition and properties of crystalline lactate dehydrogenase X from mouse testes. J Biol Chem. 1972 Apr 10;247(7):2044–2048. [PubMed] [Google Scholar]
- Ichimura M., Tsukada K. Polynucleotide kinase from rat liver nuclear extracts. J Biochem. 1971 May;69(5):823–828. doi: 10.1093/oxfordjournals.jbchem.a129533. [DOI] [PubMed] [Google Scholar]
- Lazarus L. H., Kitron N. Letter: Cytoplasmic DNA polymerase: polymeric forms and their conversion into an active monomer resembling nuclear DNA polymerase. J Mol Biol. 1973 Dec 25;81(4):529–534. doi: 10.1016/0022-2836(73)90522-6. [DOI] [PubMed] [Google Scholar]
- Lindahl T., Edelman G. M. Polynucleotide ligase from myeloid and lymphoid tissues. Proc Natl Acad Sci U S A. 1968 Oct;61(2):680–687. doi: 10.1073/pnas.61.2.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb L. A. Purification and properties of deoxyribonucleic acid polymerase from nuclei of sea urchin embryos. J Biol Chem. 1969 Apr 10;244(7):1672–1681. [PubMed] [Google Scholar]
- MACPHERSON I., STOKER M. Polyoma transformation of hamster cell clones--an investigation of genetic factors affecting cell competence. Virology. 1962 Feb;16:147–151. doi: 10.1016/0042-6822(62)90290-8. [DOI] [PubMed] [Google Scholar]
- MARGOLIASH E. Amino acid sequence of chymotryptic peptides from horse heart cytochrome c. J Biol Chem. 1962 Jul;237:2161–2174. [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Meyer R. R., Simpson M. V. DNA biosynthesis in mitochondria: partial purification of a distinct DNA polymerase from isolated rat liver mitochondria. Proc Natl Acad Sci U S A. 1968 Sep;61(1):130–137. doi: 10.1073/pnas.61.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedwick W. D., Wang T. S., Korn D. Purification and properties of nuclear and cytoplasmic deoxyribonucleic acid polymerases from human KB cells. J Biol Chem. 1972 Aug 25;247(16):5026–5033. [PubMed] [Google Scholar]
- Wang T. S., Sedwick W. D., Korn D. Nuclear deoxyribonucleic acid polymerase. Purification and properties of the homogeneous enzyme from human KB cells. J Biol Chem. 1974 Feb 10;249(3):841–850. [PubMed] [Google Scholar]
- YONEDA M., BOLLUM F. J. DEOXYNUCLEOTIDE-POLYMERIZING ENZYMES OF CALF THYMUS GLAND. I. LARGE SCALE PURIFICATION OF TERMINAL AND REPLICATIVE DEOXYNUCLEOTIDYL TRANSFERASES. J Biol Chem. 1965 Aug;240:3385–3391. [PubMed] [Google Scholar]


