Abstract
Introduction
Kidney Disease Improving Global Outcomes guidelines indicate that glucocorticoids and immunosuppressants comprise the first therapeutic regimens after 4 to 6 months of treatment for high-risk primary membranous nephropathy (PMN). However, some patients cannot achieve complete or partial remission at 6 months. This study aimed to evaluate the efficacy of traditional immunotherapy combined with hydroxychloroquine (HCQ), a well-known immune regulator, in patients with PMN.
Methods
This was a single-center, open-label, prospective study. We recruited 72 patients with nephrotic syndrome and PMN proven by renal biopsy from May 2020 to June 2024. We compared changes in proteinuria, serum albumin levels, estimated glomerular filtration rate (eGFR), and relapse rate at 3, 6, 9, and 12 months follow-up in 41 patients who received glucocorticoid and immunosuppressant, and in 31 who received HCQ plus standard-of-care.
Results
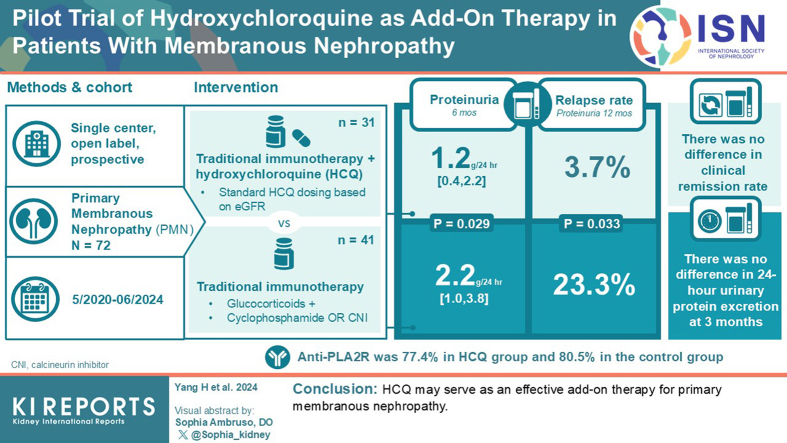
Baseline characteristics showed no statistical significance between the 2 groups. However, the HCQ group showed significantly reduced proteinuria compared to standard-of-care group. A reduced proteinuria was seen at 6 months (1.2 [0.4–2.2] vs. 2.2 [1.0–3.8] g/d, P = 0.029) and the relapse rate with 12 months follow-up was also significantly decreased in the HCQ group compared to the standard-of-care group (3.7% vs. 23.3%, P = 0.033).
Conclusions
HCQ may serve as an effective add-on therapy for PMN.
Keywords: add-on therapy, glucocorticoid, hydroxychloroquine, immunosuppressants, primary membranous nephropathy
Graphical abstract
PMN, a glomerular disease, is the most common cause of adult nephrotic syndrome.1 PMN pathogenesis occurs via the formation of subepithelial immune complexes, leading to diffuse thickening of the glomerular basement membrane.2 It is mostly diagnosed using renal biopsy for determining the stage of PMN under electronic microscopy. In the past 20 years, M-type phospholipase A2 receptor (PLA2R)3 has been identified as a useful, novel noninvasive biomarker of diagnosing PMN for those patients with biopsy contradiction or have no need for immunosuppressive therapy.4,5 The prognosis of PMN is heterogeneous, with one-third of patients experiencing spontaneous remission, one-third maintaining persistent proteinuria, and the remaining one-third progressing to decline of renal function ultimately developing to end-stage renal disease.6,7
For patients initially diagnosed with PMN, studies recommend supportive care based on renin-angiotensin-aldosterone system inhibitors (RAASi), and lifestyle changes to reduce proteinuria and increase the chance of spontaneous remission.8,9 The Kidney Disease: Improving Global Outcomes guidelines recommend assessing risk of renal function progression and starting immunosuppressive therapy as early as possible in high-risk patients with nephrotic syndrome.4 The combination of alkylating agents and glucocorticoids is an effective treatment option for patients with PMN, and studies have shown that it can reduce the progression of renal function in patients.10 Calcineurin inhibitors (CNIs), another first-line medication for PMN, are less toxic than cyclophosphamide (CTX) and glucocorticoids. A clinical meta-analysis reported that the 6-month and 12-month overall response rates were 79.9% and 78.6% respectively for the CNIs group, and 64.7% and 77.4% respectively for the CTX group. CNIs have superior short-term effectiveness and safety compared to CTX. However, CNIs lead to a higher risk of PMN recurrence.11 Therefore, new drugs are needed to increase the remission rate of PMN and reduce adverse reactions of glucocorticoids and immunosuppressants.
HCQ was originally developed as an antimalarial drug.12 It has become the first-line treatment for rheumatic immune diseases, such as systemic lupus erythematosus and rheumatoid arthritis.13 Owing to its immunomodulatory effects, HCQ exerts its effects on various kidney diseases. HCQ improves renal remission rate in lupus nephritis, reduces the risk of relapse, and delays the progression of renal dysfunction.14 HCQ can target toll-like receptor-9 (TLR-9) and inhibit macrophage activation, thereby reducing renal fibrosis.15 A randomized controlled trial16 confirmed that HCQ reduced urinary protein levels in patients with IgA nephropathy with less adverse reactions. Another clinical study found that for patients with PMN receiving RAASi treatment, the addition of HCQ for 6 months reduced urinary protein levels more significantly, and improved the clinical remission rate.17 However, whether HCQ can play an effective role in patients who require immunosuppressive treatment requires further study.
We designed and conducted a prospective study to evaluate the efficacy and safety of HCQ in patients with PMN who were receiving immunosuppressive therapy.
Methods
Trial Design
This was a single-center, prospective, open-label controlled trial conducted between May 2020 and June 2024 at Shengjing Hospital of China Medical University. A total of 72 patients were screened and enrolled in this study. All patients received RAASi supportive therapy; 41 received glucocorticoids combined with immunosuppressive therapy named as standard-of-care, and 31 received HCQ in addition to standard-of-care. All patients were fully informed and allocated to one of the 2 groups based on their preferences. This study complied with the principles of the Declaration of Helsinki. All the participants provided written informed consent. The research protocol was reviewed and approved by the hospital’s ethics committee and registered with the China Clinical Trial Registry (ChiCTR2300076346).
Participants
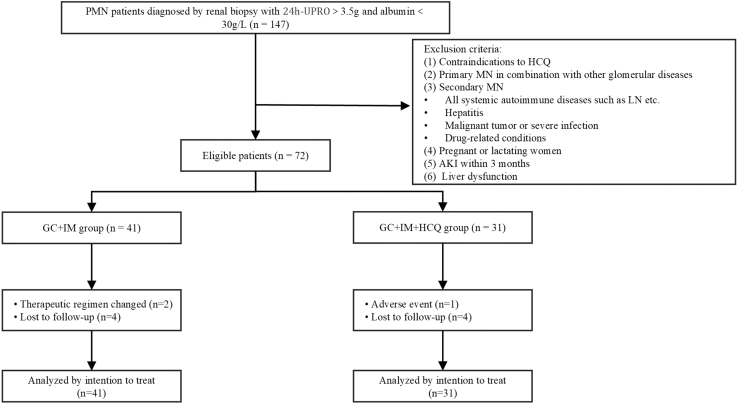
The inclusion criteria were as follows: (i) PMN diagnosed by renal biopsy, (ii) age between 18 and 75 years, (iii) no treatment with glucocorticoids and immunosuppressants before enrollment, (iv) eGFR > 30 ml/min per 1.73 m2, (v) 24-hour urinary protein excretion > 3.5 g, and (vi) serum albumin < 30 g/l. The exclusion criteria were as follows: (i) contraindications to HCQ (allergy or visual field defect); (ii) PMN in combination with other glomerular diseases; (iii) secondary MN including all systemic autoimmune diseases such as lupus nephritis, hepatitis, malignant tumors or severe infection, and drug-related conditions; (iv) pregnant or lactating women; (v) acute kidney injury within 3 months; (vi) liver dysfunction.
Interventions and Follow-Up
The control group received glucocorticoids and immunosuppressive treatments. According to the guideline recommendations,4 for patients with high-risk assessment and concurrent nephrotic syndrome, the selection of immunosuppressive therapy is based on the combination of glucocorticoids with CTX or CNIs. After sufficient discussion with patients about the risks, benefits, and cost-effectiveness of these immunosuppressants, the selection of baseline immunosuppressant was based on the patients’ own preferences. The initial dose of i.v. infusion of CTX is 0.5 to 0.75 g/m2/mo, and the maximum dose is 1 g/mo. The initial treatment was administered once a month for 6 months. It was then adjusted once every 3 months and maintained for 6 months. The total dosage was 9 g to 12 g. The initial dose of oral prednisone was 1 mg/kg/d for 2 months, thereafter, tapered gradually over 6 months until discontinuation. The initial dose of tacrolimus (TAC) was 0.05 to 0.1 mg/kg/d, administered orally in equal doses 12 hours apart. The dose was adjusted to maintain a target blood concentration of 5 to 8 ng/ml. Prednisone (0.5 mg/kg/d) was administered during TAC treatment. After 8 weeks of treatment, the dose was reduced by 5 mg every 2 weeks. When the dose reached 10 mg/d, it was maintained for 2 months. The dose was reduced to 2.5 mg every 2 weeks until discontinuation.
For the HCQ group, oral HCQ was added to the standard-of-care. The HCQ dose was adjusted based on the patient's initial eGFR. When eGFR > 45 ml/min per 1.73 m2, the dose was 0.2 g, twice a day; when eGFR was 30 to 45 ml/min per 1.73 m2, the dose was 0.1 g, 2 to 3 times a day; when eGFR < 30 ml/min per 1.73 m2, the dose was 0.1 g once daily.
Patients continued to receive RAASi supportive therapy after enrollment. Blood pressure was controlled to less than 130/80 mm Hg with tolerant RAASi or additional antihypertensive agents. Patients with nephrotic syndrome often have elevated blood lipid levels and are at risk of thrombosis. The use of statins to lower blood lipid levels was permitted in this study, and anticoagulants were administered according to the patient's clinical condition.
All clinical data were collected from patients’ medical records during the visit and follow-up. Age, sex (male/female), visit time, mean arterial pressure, 24-hour urinary protein excretion, serum albumin, serum creatinine, eGFR (according to the Chronic Kidney Disease-Epidemiology Collaboration formula), transaminases, total cholesterol, hemoglobin, anti-PLA2R antibody, and renal pathology data were collected. Fundus examination was completed prior to HCQ treatment and then the retina and visual field were routinely evaluated every 3 to 6 months. Any adverse reactions during follow-up were reported promptly and treated appropriately. All patients were followed-up for collecting relevant clinical data at 3 months, 6 months, and 12 months after treatment.
Outcomes
The primary outcome of this study was the clinical response rate during follow-up. Proteinuria remission is a recognized surrogate indicator of long-term outcome in patients with PMN. Clinical responses included complete response and partial response. Complete response was defined as urinary protein excretion < 0.3 g/d. Partial response was defined as a reduction in proteinuria > 50% of baseline and < 3.5 g/d. Nonresponse was defined as a decrease in proteinuria < 50% of baseline. Secondary outcomes included changes in 24-hour urinary protein excretion, serum albumin, eGFR, anti-PLA2R antibody, and recurrence rates during follow-up. Relapse was defined as urinary protein excretion of >3.5 g/d and an increase of > 50% in patients who achieved clinical remission. Treatment failure was defined as the development of renal insufficiency (decline of eGFR > 50% of the baseline), or failure to achieve clinical remission.
Safety
Adverse events were defined as uncomfortable symptoms and abnormal clinical indicators occurring during treatment and follow-up. Severe adverse events were defined as any adverse medical incident that resulted in mortality, life-threatening conditions, necessitated hospitalization, sustained or severe disability, or loss of ability.
Statistical Analyses
Based on the results of previous studies and the results of preliminary experiments of this research group, we assumed that the alpha value was 0.05 (2-tailed test), the power (1−β) was 0.90, the dropout rate was 15%, and that each group required 31 patient cases. Normally distributed data were expressed as mean ± SD, and continuous variables were compared using the t test. Nonnormally distributed data were expressed as medians (Q25 and Q75), and continuous variables were compared using the Wilcoxon rank-sum test. Categorical data were expressed as counts and percentages, and nominal variables were compared using the chi-square test. Cumulative response rates were calculated using the Kaplan-Meier method and assessed using the log-rank or Breslow test. Two-tailed P < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS version 26.0 (SPSS, Armonk, NY).
Results
Baseline Characteristics of the Patients
A total of 147 patients were screened between May 2020 and June 2024. After exclusion according to the relevant criteria, 72 patients were enrolled and completed the follow-up. All participants continued to receive RAASi treatment, and their blood pressure status was monitored. In total, 41 patients were administered standard-of-care. Four patients were lost during the 1-year follow-up period, of which 1 was lost to follow-up at 4 months, and the other 3 were treated and followed-up for more than 6 months. Two patients relapsed at 12 months and their treatment regimens were changed. Thirty-one patients received HCQ in addition to standard-of-care. In this group, 4 patients were lost to follow-up after 6 months of treatment. One patient developed visual abnormalities at 3 months and HCQ treatment was suspended. The enrollment process of this study is depicted in a flowchart (Figure 1).
Figure 1.
Flow diagram of the trial evaluating the effect of hydroxychloroquine in patients with membranous nephropathy. AKI, acute kidney injury; GC, glucocorticoid; HCQ, hydroxychloroquine; IM, immunosuppressant; LN, lupus nephritis; MN, membranous nephropathy; PMN, primary membranous nephropathy; UPRO, urinary protein.
The clinical characteristics of the enrolled patients are presented in Table 1. The control group (n = 41) had an average age of 53.3 years and included 26 men. The average age of patients (n = 31) in the HCQ group was 54.9 years and included 25 men. The positive rate of serum PLA2R antibodies was 80.5% for the control group, in which 29 patients received CTX and 12 patients received TAC. The positive rate of serum PLA2R antibodies was 77.4% for the HCQ group, in which 24 patients received CTX and 7 patients received TAC. Other clinical indices included 24-hour urinary protein excretion, serum albumin, eGFR, total cholesterol, pathological features of PMN, type of immunosuppressant, statin administration, and follow-up duration. There were no statistically significant differences in these clinical conditions between the 2 groups.
Table 1.
Baseline characteristics
| Parameters | GC + IM group (n = 41) | GC + IM + HCQ group (n = 31) | P value |
|---|---|---|---|
| Age (yr) | 53.3 ± 9.5 | 54.9 ± 13.7 | 0.581 |
| Sex (male/female) | 26/15 | 25/6 | 0.111 |
| Time before treatment (mo) | 3.0 (1.0–8.5) | 4.0 (1.0–9.0) | 0.831 |
| MAP (mm Hg) | 104.7 ± 9.7 | 103.8 ± 12.3 | 0.723 |
| Urinary protein (g/d) | 7.1 (5.2–8.7) | 7.6 (5.1–10.8) | 0.387 |
| Serum albumin (g/l) | 21.9 ± 3.9 | 22.5 ± 3.9 | 0.510 |
| eGFR (ml/min per 1.73 m2) | 95.3 ± 17.5 | 93.8 ± 17.0 | 0.727 |
| Anti-PLA2R antibody positive, n (%) | 33 (80.5) | 24 (77.4) | 0.751 |
| Level of anti-PLA2R antibody (RU/ml) | 96.3 (30.8–221.4) | 63.5 (29.5–135.4) | 0.172 |
| Cholesterol (mmol/l) | 8.1 ± 2.3 | 7.2 ± 1.8 | 0.084 |
| Histological stage of PMN | |||
| Stage I | 1 | 1 | 0.841 |
| Stage II | 32 | 16 | 0.035 |
| Stage III + IV | 8 | 14 | 0.037 |
| Pathological features of PMN, n (%) | |||
| Glomerulosclerosis | 26 (63.4) | 23 (74.2) | 0.331 |
| Renal tubular atrophy | 37 (90.2) | 26 (83.9) | 0.418 |
| Renal interstitial fibrosis | 22 (53.7) | 18 (58.1) | 0.709 |
| Type of immunosuppressant | |||
| Cyclophosphamide | 29 | 24 | 0.524 |
| Tacrolimus | 12 | 7 | 0.524 |
| Statins administration, n (%) | 36 (87.8) | 23 (74.2) | 0.137 |
| Follow-up time (mo) | 14.0 ± 4.0 | 13.1 ± 5.0 | 0.440 |
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin-receptor blockers; eGFR, estimated glomerular filtration rate; GC, glucocorticoid; HCQ, hydroxychloroquine; IM, immunosuppressant; MAP, mean arterial pressure; PLA2R, M-type phospholipase A2 receptor; PMN, primary membranous nephropathy.
Primary Outcomes
After receiving standard-of-care, some patients achieved clinical remission. The clinical remission rates for the HCQ group and the control group showed no statistical significance at 3 months (61.3% vs. 63.4%, P = 0.854), 6 months (87.1% vs. 73.2%, P = 0.150), and 12 months (93.5% vs. 90.2%, P = 0.615).
Secondary Outcomes
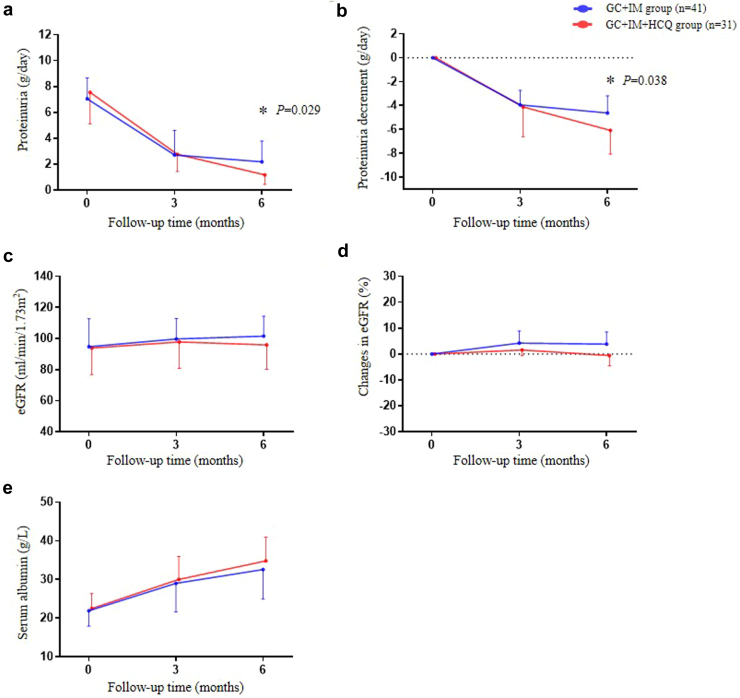
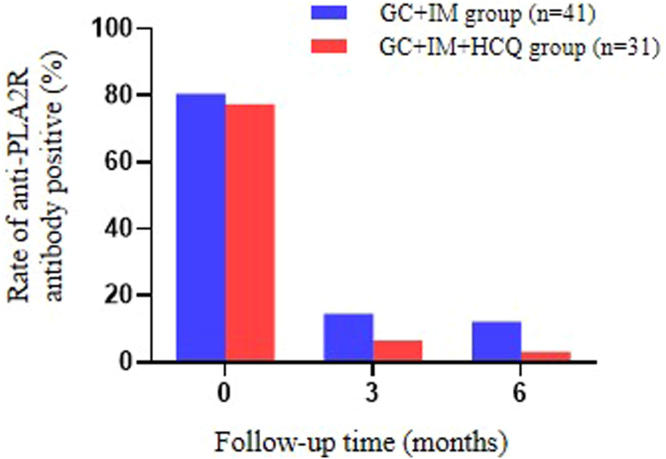
The baseline 24-hour urinary protein excretion rates for the HCQ and control groups were 7.6 (5.1–10.8) and 7.1 (5.2–8.7) g/d, respectively (P = 0.387); after treatment for 3 months, the urinary protein excretion rates were 2.8 (1.4, 5.3) and 2.7 (1.5–4.6) g/d, respectively (P = 0.968). However, after treatment for 6 months, the urinary protein excretion rates for the HCQ and control groups were 1.2 (0.4–2.2) and 2.2 (1.0, 3.8) g/d, respectively (P = 0.029; Figure 2a). The HCQ group showed a statistically significant reduction in urinary protein than the control group at 6 months (−6.1 [−4.2 to −8.1] vs. −4.6 [−3.2 to −7.0] g/d, P = 0.038; Figure 2b). During follow-up, no significant differences were observed in the patients’ eGFR (Figure 2c) and changes in eGFR were also not statistically different (Figure 2d). Serum albumin levels were gradually increased in the HCQ group compared to the control group at 6 months (Figure 2e). Among patients with positive serum anti-PLA2R antibodies, the antibodies for both groups were significantly reduced after treatment, and there was no significant difference in antibody positive rate between the 2 groups at 6 months (3.2% vs. 12.2%, P = 0.173; Figure 3).
Figure 2.
Effects of HCQ add-on therapy on PMN with 6 months follow-up. (a) 24-hour urinary protein excretion. (b) Temporal change magnitude of proteinuria decreases after the treatment. (c) Level of eGFR. (d) Changes in eGFR. (e) Levels of serum albumin. eGFR, estimated glomerular filtration rate; GC, glucocorticoid; HCQ, hydroxychloroquine; IM, immunosuppressant. ∗P < 0.05, GC + IM group versus GC + IM + HCQ group.
Figure 3.
Changes in the rate of anti-PLA2R antibody positivity after 6 months of HCQ treatment. The positive rate of anti-PLA2R antibody was approximately 80% before treatment. Then the positive rate gradually decreased at 3 and 6 months following the treatment. GC + IM + HCQ group showed a lower trend compared to GC + IM and the lowest level in HCQ add-on group at 6 month after the treatment. GC, glucocorticoid; HCQ, hydroxychloroquine; IM, immunosuppressant.
Relapse
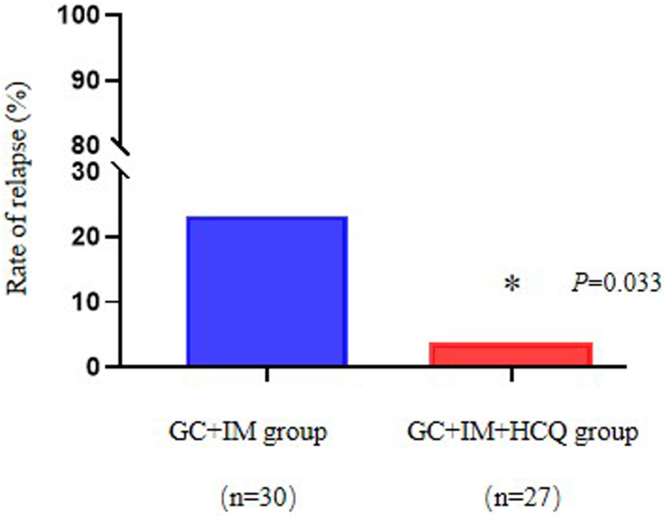
During follow-up, 8 patients relapsed after achieving clinical remission, which included 1 patient in the HCQ group and 7 patients in the control group. The relapse rate of patients in the HCQ group was significantly reduced compared to the standard-of-care group (3.7% vs. 23.3%, P = 0.033; Figure 4). All relapsed cases occurred after 6 months of treatment and were switched to alternative suppressive therapy.
Figure 4.
Relapse rate of nephrotic syndrome in 2 groups at 12 months of follow-up. The recurrence rate in the GC + IM + HCQ group was significantly decreased than GC + IM group at 12 months follow-up period. ∗P < 0.05, GC + IM + HCQ group versus GC + IM group. GC, glucocorticoid; HCQ, hydroxychloroquine; IM, immunosuppressant.
Safety Evaluation
During follow-up, 19 patients reported adverse events (Table 2). There was no statistically significant difference in the incidence of adverse events between the HCQ and control groups (16.1% vs. 34.1%, P = 0.086). No serious adverse events were observed for either group during the follow-up. The most common adverse events were infections (including upper respiratory and urinary tract infections), abnormal liver function, rash, and gastrointestinal discomfort. Patients’ symptoms improved after appropriate treatment with no interruption in treatment or follow-up. One patient in the HCQ group developed visual field defects 3 months after treatment; therefore, HCQ treatment was suspended and withdrawn from the study.
Table 2.
Adverse events reported during the study
| Events | GC + IM group (n = 41) | GC + IM + HCQ group (n = 31) |
|---|---|---|
| n (%) | ||
| Number of events | ||
| 0 | 27 (66) | 26 (84) |
| 1 | 13 (32) | 4 (13) |
| ≥2 | 1 (2) | 1 (3) |
| Upper respiratory infection | 1 (2) | 0 |
| Pneumonia | 2 (5) | 0 |
| Urinary tract infection | 3 (7) | 1 (3) |
| Lymphopenia | 2 (5) | 0 |
| Glucose intolerance | 1 (2) | 0 |
| Hepatic enzymes increased | 2 (5) | 2 (6) |
| Thrombocytopenia | 1 (2) | 0 |
| Rash | 1 (2) | 2 (6) |
| Nausea | 1 (2) | 0 |
| Fecal occult blood | 1 (2) | 1 (3) |
| Visual field defect | 0 | 1 (3) |
GC, glucocorticoid; HCQ, hydroxychloroquine; IM, immunosuppressant.
Subgroup Analysis Based on Urinary Protein Levels
We found a difference in urinary protein excretion between the 2 groups after 6 months of treatment. To further explore the heterogeneity of the effect of HCQ at different urinary protein levels, we conducted a subgroup analysis based on baseline urinary protein levels. The study divided the patients into 2 subgroups: 3.5 to 8 g/d and > 8 g/d of urinary protein excretion. There was no difference between patients with baseline urinary protein levels of < 8 g/d (P = 0.933) and > 8 g/d (P = 0.667) in the HCQ and control groups. After 6 months of treatment, the HCQ group showed a greater decrease in urinary protein excretion when the level was < 8 g/d (P = 0.076; Supplementary Figure S1a) and > 8 g/d (P = 0.178; Supplementary Figure S1b).
Discussion
In the present study, we compared the efficacy and safety of standard-of-care (glucocorticoid combined immunosuppressive treatment) and HCQ add-on therapy in patients with high-risk PMN. Achieving clinical remission in patients with PMN is associated with steady improvement in long-term renal outcomes.18 Successful treatment of PMN is typically defined by reduction in proteinuria, including complete response or partial response. Low-level proteinuria is associated with a slower decline in glomerular filtration rate.19 Therefore we also consider proteinuria as a surrogate end point for PMN. Proteinuria in the HCQ group was significantly lower than that in the control group after 6 months of treatment. In addition, the magnitude of proteinuria reduction in HCQ group was greater compared to the control group. Simultaneously, the probability of disease relapse during the follow-up period was significantly less in the HCQ group than in the control group. The safety of HCQ was revealed with the study presenting a low number of adverse events and no serious adverse events.
Immunotherapy for PMN remains controversial. Considering that one-third of patients experience spontaneous remission,20 we started immunosuppressive therapy after RAASi treatment to avoid enrolling those patients with potential spontaneous remission. In the present study, we started patient enrollment in May 2020, shortly after the publication of the MENTOR trial,21 when rituximab (RTX) was not yet available at our institution. Therefore, for the study we only enrolled patients with PMN who were treated with TAC or CTX. Since the MENTOR trial published, RTX has become one of the first line of biologics for treating high-risk PMN.4 The MENTOR trial demonstrated that RTX showed noninferiority in the remission rate compared to cyclosporine in patients with PMN at 12 months (60% vs. 52%) and showed superiority in maintaining proteinuria remission up to 24 months (60% vs. 20%). Following the MENTOR study, the STARMEN trial and RI-CYCLO trial revealed similar efficacy rate of RTX in PMN.22,23 Together, these studies demonstrated that RTX is a kind of novel effective biologic with some limitations in the induction treatment for PMN. Based on our results of HCQ as an effective add-on therapy to TAC or CTX treatment in PMN, we extrapolate that HCQ could also be a complementary therapeutic agent for patients treated with RTX to allow them to reach a higher remission and lower relapse rates. Verifying this hypothesis would call for future studies with larger cohorts of patients with PMN.
Beyond PMN, for decades, HCQ has been used to treat various autoimmune diseases as a low-cost, broad-spectrum drug.12,24 HCQ has been proven to improve the disease remission rate and decrease relapse in lupus nephritis and is recommended by multiple guidelines as a fundamental supportive treatment for systemic lupus erythematosus and lupus nephritis.4,25 In recent years, several clinical trials have found that HCQ can reduce proteinuria levels in patients with IgA nephropathy.16,26,27 Therefore, in this study, we investigated whether HCQ could achieve similar effects of reducing proteinuria in PMN. So far, only Cheng et al.17 reported that HCQ, as an adjuvant therapy, reduced proteinuria and PLA2R antibody levels in patients with low-risk PMN receiving RAASi treatment. However, for patients with PMN not obtaining remission of proteinuria after maximum RAASi supportive treatment and requiring immunosuppressive therapy, the effect of HCQ has not been reported. In this study, we firstl explored the efficacy and safety of HCQ as add-on agent over standard-of-care in patients with high-risk PMN with nephrotic syndrome. We found that the HCQ group showed a significant reduction of proteinuria compared to the control group after 6 months of treatment. Our study also found that the relapse rate of patients in the HCQ group was significantly lower than that of patients in the control group during the 12 months of follow-up.
However, the mechanisms of the renoprotective role of HCQ in PMN remains an incomplete understanding. HCQ is a weak alkaline compound that can enter cell lysosomes and interfere with enzymatic activity by increasing the pH.28 HCQ inhibits the antigen presentation process by accumulating in the lysosomes of antigen-presenting cells, thereby increasing the pH of these organelles, which is critical for disease development.29, 30, 31 The activation of TLRs in macrophages and neutrophils, which in turn induces the production and secretion of inflammatory cytokines, plays an important role in the pathogenesis of rheumatic immune diseases.13 Considering that the activation of TLR and subsequent downstream signaling occurs only in the acidification zone, studies have found that HCQ can produce inhibitory effects on TLR3, TLR7, and TLR9 by interfering with the endosomal acidification process. In addition, HCQ can prevent endosomal TLR activation by directly interacting with TLR ligands and preventing them from binding to endosomal TLRs.12 A recent clinical study32 also found that HCQ can reduce some of the disease-causing antibodies in patients with type 1 diabetes and improve blood sugar status. HCQ may exert its antiproteinuria effect by regulating antigen-presenting cells and inhibiting the activation of T cells, thereby reducing the production of autoimmune antibodies.
In general, HCQ is considered to have a favorable safety profile. The main adverse events were rashes, gastrointestinal distress, and retinopathy.33 A meta-analysis of randomized controlled trials found a higher risk of skin hyperpigmentation in HCQ compared placebo, whereas the increase in other adverse events was not statistically significant.34 The adverse effects of HCQ in ophthalmology is dose and duration dependent. Retinopathy was rare at a recommended dose of <5 mg/kg actual weight for less than 5 of use.35 In the present study, only 1 patient developed visual field changes after 3 months of HCQ treatment, and this symptom gradually improved after discontinuation of the drug. Even though side effects of HCQ are limited, recent study suggests that monitoring HCQ blood levels could supplement the assessment of treatment compliance for patients with systemic lupus erythematosus by aiding in monitoring of medication adherence and reducing potential adverse drug reactions.36 However, given that patients with PMN do not have the same level of adherence issues as those with systemic lupus erythematosus and can achieve complete remission through shorter-term treatment, we do not see measuring of HCQ blood levels as critical for patients with PMN; and believe that periodic monitoring of retinal toxicity suffices for HCQ as an add-on therapy for PMN.
The research for novel treatments of PMN is propelled through progress in the diagnostic tools for the disease, and PLA2R has in recent years demonstrated strong utility in this function. However, beyond diagnosis, despite certain evidence showing that lower baseline levels of PLA2R antibody may link to higher remission of proteinuria, there has not been adequate support for the correlation between PLA2R levels and disease severity or time course.5,37 Furthermore, PLA2R levels vary depending on the timing of antibody testing, and the scientific community has yet to come to a consensus around the optimal testing time.38 Thus, per the 2021 Kidney Disease: Improving Global Outcomes guideline, renal biopsy is still required for those patients with PMN who need immunosuppressive therapy regardless of PLA2R level.4 Our enrolled patients are all PMN proven by renal biopsy.
Our study has some limitations: patient population was homogenous, given that the study took place at a single center in China, and had a shorter follow-up period (12 months). Since the reporting of data in this study, we have continued to follow-up on these patients to assess long-term impacts. In addition, in the future, the results of our study will benefit from verification in diverse ethnic populations and through utilizing randomized controlled trial design across multiple test centers.
In conclusion, for patients with PMN who require glucocorticoids and immunosuppressive treatment, the addition of HCQ may effectively reduce urinary protein excretion and decrease relapse rate of PMN. HCQ may serve as a valuable add-on therapy for PMN.
Disclosure
All the authors declared no competing interests.
Acknowledgments
This research was supported by Chinese Nature Science Foundation 82170740 (HZ), 82100743 (JL), Applied Basic Research Program of Liaoning Province 2022JH2/101300048 (HZ), Liao Ning Revitalization Talents Program XLYC2002081 (HZ), Pandeng Scholar of Liaoning Province 2013222 (HZ), Outstanding Scientific Fund of Shengjing Hospital of China Medical University 202206 (HZ). This work was also supported by the Intramural Research Program, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Maryland, USA. We thank Qijun Wu, a statistical expert, the Chief of Clinical Epidemiology Department for consultation of study design.
Footnotes
Figure S1. Changes in proteinuria from baseline in subclass of PMN with GC + IM or GC + IM + HCQ treatment during the follow-up period. (a) 24-hour total proteinuria with 3.5 to 8 g/d. (b) 24-hour total proteinuria > 8 g/d.
Supplementary Material
Figure S1. Changes in proteinuria from baseline in subclass of PMN with GC + IM or GC + IM + HCQ treatment during the follow-up period. (a) 24-hour total proteinuria with 3.5 to 8 g/d. (b) 24-hour total proteinuria > 8 g/d.
References
- 1.Glassock R.J. The pathogenesis of idiopathic membranous nephropathy: a 50-year odyssey. Am J Kidney Dis. 2010;56:157–167. doi: 10.1053/j.ajkd.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 2.Couser W.G. Primary membranous nephropathy. Clin J Am Soc Nephrol. 2017;12:983–997. doi: 10.2215/CJN.11761116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beck L.H., Bonegio R.G.B., Lambeau G., et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361:11–21. doi: 10.1056/NEJMoa0810457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int. 2021;100:S1–S276. doi: 10.1016/j.kint.2021.05.021. [DOI] [PubMed] [Google Scholar]
- 5.Ramachandran R., Jha V., Bose B. Key points in managing PLA2R-associated membranous nephropathy. Kidney Int Rep. 2024;9:2320–2322. doi: 10.1016/j.ekir.2024.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schieppati A., Mosconi L., Perna A., et al. Prognosis of untreated patients with idiopathic membranous nephropathy. N Engl J Med. 1993;329:85–89. doi: 10.1056/NEJM199307083290203. [DOI] [PubMed] [Google Scholar]
- 7.Polanco N., Gutiérrez E., Covarsí A., et al. Spontaneous remission of nephrotic syndrome in idiopathic membranous nephropathy. J Am Soc Nephrol. 2010;21:697–704. doi: 10.1681/ASN.2009080861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van den Brand J.A.J.G., van Dijk P.R., Hofstra J.M., Wetzels J.F.M. Long-term outcomes in idiopathic membranous nephropathy using a restrictive treatment strategy. J Am Soc Nephrol. 2014;25:150–158. doi: 10.1681/ASN.2013020185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rojas-Rivera J.E., Ortiz A., Fervenza F.C. Novel treatments paradigms: membranous nephropathy. Kidney Int Rep. 2023;8:419–431. doi: 10.1016/j.ekir.2022.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van de Logt A.-E., Hofstra J.M., Wetzels J.F. Pharmacological treatment of primary membranous nephropathy in 2016. Expert Rev Clin Pharmacol. 2016;9:1463–1478. doi: 10.1080/17512433.2016.1225497. [DOI] [PubMed] [Google Scholar]
- 11.Qiu T.T., Zhang C., Zhao H.W., Zhou J.W. Calcineurin inhibitors versus cyclophosphamide for idiopathic membranous nephropathy: a systematic review and meta-analysis of 21 clinical trials. Autoimmun Rev. 2017;16:136–145. doi: 10.1016/j.autrev.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Nirk E.L., Reggiori F., Mauthe M. Hydroxychloroquine in rheumatic autoimmune disorders and beyond. EMBO Mol Med. 2020;12 doi: 10.15252/emmm.202012476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schrezenmeier E., Dörner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol. 2020;16:155–166. doi: 10.1038/s41584-020-0372-x. [DOI] [PubMed] [Google Scholar]
- 14.Parikh S.V., Almaani S., Brodsky S., Rovin B.H. Update on lupus nephritis: core curriculum 2020. Am J Kidney Dis. 2020;76:265–281. doi: 10.1053/j.ajkd.2019.10.017. [DOI] [PubMed] [Google Scholar]
- 15.Zheng H., Zhang Y., He J., et al. Hydroxychloroquine inhibits macrophage activation and attenuates renal fibrosis after ischemia-reperfusion injury. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.645100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu L.-J., Yang Y.-Z., Shi S.-F., et al. Effects of hydroxychloroquine on proteinuria in IgA nephropathy: a randomized controlled trial. Am J Kidney Dis. 2019;74:15–22. doi: 10.1053/j.ajkd.2019.01.026. [DOI] [PubMed] [Google Scholar]
- 17.Cheng Y.-J., Cheng X.-Y., Zhang Y.-M., et al. Effects of hydroxychloroquine on proteinuria in membranous nephropathy. J Nephrol. 2022;35:1145–1157. doi: 10.1007/s40620-021-01182-z. [DOI] [PubMed] [Google Scholar]
- 18.Cattran D.C., Kim E.D., Reich H., Hladunewich M., Kim S.J., Toronto Glomerulonephritis Registry group Membranous nephropathy: quantifying remission duration on outcome. J Am Soc Nephrol. 2017;28:995–1003. doi: 10.1681/ASN.2015111262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson A., Cattran D.C., Blank M., Nachman P.H. Complete and partial remission as surrogate end points in membranous nephropathy. J Am Soc Nephrol. 2015;26:2930–2937. doi: 10.1681/ASN.2015010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hladunewich M.A., Troyanov S., Calafati J., Cattran D.C., Metropolitan Toronto Glomerulonephritis Registry The natural history of the non-nephrotic membranous nephropathy patient. Clin J Am Soc Nephrol. 2009;4:1417–1422. doi: 10.2215/CJN.01330209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fervenza F.C., Appel G.B., Barbour S.J., et al. Rituximab or cyclosporine in the treatment of membranous nephropathy. N Engl J Med. 2019;381:36–46. doi: 10.1056/NEJMoa1814427. [DOI] [PubMed] [Google Scholar]
- 22.Fernández-Juárez G., Rojas-Rivera J., Logt A.V., et al. The STARMEN trial indicates that alternating treatment with corticosteroids and cyclophosphamide is superior to sequential treatment with tacrolimus and rituximab in primary membranous nephropathy. Kidney Int. 2021;99:986–998. doi: 10.1016/j.kint.2020.10.014. [DOI] [PubMed] [Google Scholar]
- 23.Scolari F., Delbarba E., Santoro D., et al. Rituximab or cyclophosphamide in the treatment of membranous nephropathy: the RI-CYCLO randomized trial. J Am Soc Nephrol. 2021;32:972–982. doi: 10.1681/ASN.2020071091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rainsford K.D., Parke A.L., Clifford-Rashotte M., Kean W.F. Therapy and pharmacological properties of hydroxychloroquine and chloroquine in treatment of systemic lupus erythematosus, rheumatoid arthritis and related diseases. Inflammopharmacology. 2015;23:231–269. doi: 10.1007/s10787-015-0239-y. [DOI] [PubMed] [Google Scholar]
- 25.Fanouriakis A., Kostopoulou M., Alunno A., et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis. 2019;78:736–745. doi: 10.1136/annrheumdis-2019-215089. [DOI] [PubMed] [Google Scholar]
- 26.Yang Y.-Z., Chen P., Liu L.-J., et al. Comparison of the effects of hydroxychloroquine and corticosteroid treatment on proteinuria in IgA nephropathy: a case-control study. BMC Nephrol. 2019;20:297. doi: 10.1186/s12882-019-1488-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu M., Bian X., Wang L., Li G. The effect of hydroxychloroquine on residual proteinuria in patients with immunoglobulin A nephropathy: a retrospective study based on propensity score matching. Front Med (Lausanne) 2022;9 doi: 10.3389/fmed.2022.922365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martinez G.P., Zabaleta M.E., Di Giulio C., Charris J.E., Mijares M.R. The role of chloroquine and hydroxychloroquine in immune regulation and diseases. Curr Pharm Des. 2020;26:4467–4485. doi: 10.2174/1381612826666200707132920. [DOI] [PubMed] [Google Scholar]
- 29.Ziegler H.K., Unanue E.R. Decrease in macrophage antigen catabolism caused by ammonia and chloroquine is associated with inhibition of antigen presentation to T cells. Proc Natl Acad Sci U S A. 1982;79:175–178. doi: 10.1073/pnas.79.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomé R., Issayama L.K., DiGangi R., et al. Dendritic cells treated with chloroquine modulate experimental autoimmune encephalomyelitis. Immunol Cell Biol. 2014;92:124–132. doi: 10.1038/icb.2013.73. [DOI] [PubMed] [Google Scholar]
- 31.Kowatsch M.M., Lajoie J., Mwangi L., et al. Hydroxychloroquine reduces T cells activation recall antigen responses. PLoS One. 2023;18 doi: 10.1371/journal.pone.0287738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Libman I., Bingley P.J., Becker D., et al. Hydroxychloroquine in Stage 1 type 1 diabetes. Diabetes Care. 2023;46:2035–2043. doi: 10.2337/dc23-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fernandez A.P. Updated recommendations on the use of hydroxychloroquine in dermatologic practice. J Am Acad Dermatol. 2017;76:1176–1182. doi: 10.1016/j.jaad.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 34.Eljaaly K., Alireza K.H., Alshehri S., Al-Tawfiq J.A. Hydroxychloroquine safety: a meta-analysis of randomized controlled trials. Travel Med Infect Dis. 2020;36 doi: 10.1016/j.tmaid.2020.101812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marmor M.F., Kellner U., Lai T.Y., Melles R.B., Mieler W.F. American Academy of Ophthalmology. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 revision) Ophthalmology. 2016;123:1386–1394. doi: 10.1016/j.ophtha.2016.01.058. [DOI] [PubMed] [Google Scholar]
- 36.Chakrabarti K., McCune W.J. Advances in the clinical use of hydroxychloroquine levels. Curr Opin Rheumatol. 2022;34:151–157. doi: 10.1097/BOR.0000000000000872. [DOI] [PubMed] [Google Scholar]
- 37.De Vriese A.S., Glassock R.J., Nath K.A., Sethi S., Fervenza F.C. A proposal for a serology-based approach to membranous nephropathy. J Am Soc Nephrol. 2017;28:421–430. doi: 10.1681/ASN.2016070776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barbour S.J., Fervenza F.C., Induruwage D., et al. Anti-PLA2R antibody levels and clinical risk factors for treatment nonresponse in membranous nephropathy. Clin J Am Soc Nephrol. 2023;18:1283–1293. doi: 10.2215/CJN.0000000000000237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Changes in proteinuria from baseline in subclass of PMN with GC + IM or GC + IM + HCQ treatment during the follow-up period. (a) 24-hour total proteinuria with 3.5 to 8 g/d. (b) 24-hour total proteinuria > 8 g/d.