Abstract
Introduction
Single-nucleotide polymorphisms (SNPs) in the UMOD-PDILT genetic locus are associated with chronic kidney disease (CKD) in European populations, through their effect on urinary uromodulin (uUMOD) levels. The genetic and nongenetic factors associated with uUMOD in African populations remain unknown.
Methods
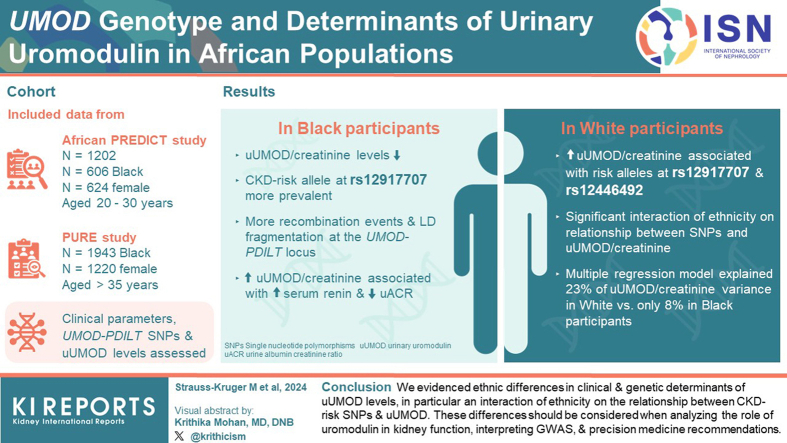
Clinical parameters, 3 selected UMOD-PDILT SNPs and uUMOD levels were obtained in 1202 young Black and White adults from the African-PREDICT study and 1943 middle aged Black adults from the PURE-NWP-SA study, 2 cross-sectional, observational studies.
Results
Absolute uUMOD and uUMOD/creatinine levels were lower in Black participants compared to White participants. The prime CKD-risk allele at rs12917707 was more prevalent in Black individuals, with strikingly more risk allele homozygotes compared to White individuals. Haplotype analysis of the UMOD-PDILT locus predicted more recombination events and linkage disequilibrium (LD) fragmentation in Black individuals. Multivariate testing and sensitivity analysis showed that higher uUMOD/creatinine associated specifically with risk alleles at rs12917707 and rs12446492 in White participants and with higher serum renin and lower urine albumin-to-creatinine ratio in Black participants, with a significant interaction of ethnicity on the relationship between all 3 SNPs and uUMOD/creatinine. The multiple regression model explained a greater percentage of the variance of uUMOD/creatinine in White adults compared to Black adults (23% vs. 8%).
Conclusion
We evidenced ethnic differences in clinical and genetic determinants of uUMOD levels, in particular an interaction of ethnicity on the relationship between CKD-risk SNPs and uUMOD. These differences should be considered when analyzing the role of uromodulin in kidney function, interpreting genome-wide association studies (GWAS), and precision medicine recommendations.
Keywords: Africa, chronic kidney disease, epidemiology, GWAS, uromodulin
Graphical abstract
CKD is a global health burden that affects an estimated 9% to 10% of the adult population worldwide.1,2 CKD shows a disproportionate burden in individuals from low- and middle-income countries. Reported prevalence rates for CKD (stage 3–5) have ranged from 10.7% to 13.9% in Sub-Saharan Africa, and 6.4% to 8.7% in South Africa specifically.3 In South Africa, renal failure was the eighth leading cause of natural death in adults aged 15 to 44 years (1.2% of deaths).4 Given the high heritability of kidney function in populations of European descent,5 genetic studies including GWAS have been crucial to gaining insights into the mechanisms driving CKD onset and development. To date, however, the vast majority of GWAS for kidney function and risk of CKD have been conducted in individuals of European and East Asian descent6 with limited research in continental African populations.
The largest and most recent GWAS for estimated creatinine-based glomerular filtration rate (eGFR) identified 424 genetic loci, explaining nearly 10% of the eGFR variance.7 Consistently, common noncoding SNPs, defined as genetic variants with a population frequency of ≥1%, at the UMOD-PDILT locus show among the strongest associations and the largest effect size on eGFR and CKD.7,8 This effect, associated with increased uromodulin production and excretion,8,9 is consistent across European and East Asian groups and is also observed in kidney function decline.6,10,11 SNPs in UMOD have also been associated with the risk of kidney stones and with hypertension.12,13 In contrast, ultrarare, mostly missense pathogenic UMOD variants lead to autosomal dominant tubulointerstitial kidney disease, in which accumulation of mutant uromodulin in the endoplasmic reticulum, causing endoplasmic reticulum stress, drives progressive kidney disease.14 This genetic evidence ranging from common risk SNPs for CKD to rare Mendelian kidney disease indicates an important role for UMOD in the genetic architecture of kidney disease.
The UMOD gene codes for uromodulin, a kidney-specific protein that is abundantly excreted in normal urine. Uromodulin, which is essentially produced by the thick ascending limb of the loop of Henle, plays multiple roles in salt reabsorption and blood pressure control, regulation of innate immunity, and protection against kidney stones and urinary tract infections.12,15 The exclusive production of uromodulin by kidney tubular cells stresses the potential value of this protein as a biomarker that can be assessed in blood and urine.15, 16, 17, 18
The CKD risk variants at the UMOD-PDILT locus are associated with uUMOD levels in the general population of European descent.8,19,20 Homozygous carriers of the CKD risk UMOD allele have approximately 2-fold higher levels of uUMOD, compared to homozygous carriers of the protective allele.9,20,21 Population studies indicated a high frequency of the CKD risk allele in most populations, probably because of a protective effect of uromodulin against urinary tract infections.22,23 A Mendelian randomization study showed that the increase in uUMOD levels driven by SNPs at the UMOD-PDILT locus have a direct, causal, and adverse effect on kidney function outcome in the general population.13
To date, there have been a limited number of studies evaluating genetic associations to kidney function parameters in continental African cohorts. In these studies, the UMOD signal was not significantly linked to eGFR in adults of African ancestry.24, 25, 26 In addition, the genetic and clinical factors influencing uromodulin excretion in continental African populations remain largely unknown. We therefore investigated 3 selected UMOD-PDILT SNPs, associated with uUMOD levels and with eGFR and CKD in cohorts of mostly European descent, as well as clinical and genetic determinants of uromodulin excretion in 2 large population-based studies originating from South Africa.
Methods
This study included 1202 Black and White individuals (n = 606 Black; n = 624 female, aged 20–30 years) from the African Prospective Study for the Early Detection and Identification of Cardiovascular Disease and Hypertension (African-PREDICT), and 1943 Black individuals (n = 1220 female, aged >35 years) from the Prospective Urban and Rural Epidemiolocal (PURE) study. Both studies included participants from the North-West Province of South Africa. The methodologies of the African-PREDICT27 and PURE28,29 studies were previously published. Data on self-reported ethnicity, sex (defined as male or female), age, and socioeconomic status were obtained using a General Health and Demographic Questionnaire (African-PREDICT) and an Adult Questionnaire (PURE). In this study, Black and White are regarded as ethnic groupings, that consider historical and cultural backgrounds. Ancestry is used when referring to a shared genetic trait. All participants gave written informed consent, and the protocols for both studies complied with institutional guidelines and the Declaration of Helsinki. African-PREDICT and the South African leg of PURE were approved by the Health Research Ethics Committee of the North-West University. The African-PREDICT study is registered at ClinicalTrails.gov (Nr. NCT03292094). This report was drafted in accordance with STROBE and STREGA guidelines for reporting observational studies. The following 3 variants in the UMOD-PDILT locus were analyzed: rs4293393 (UMOD), rs12917707 (UMOD), and rs12446492 (PDILT) (Supplementary Figure S1).18,21 We refer to the major alleles T (rs4293393), G (rs12917707), and T (rs12446492) as “CKD risk” and “UMOD increasing”21 alleles and for the minor alleles c (rs4293393), t (rs12917707), and a (rs12446492) as “CKD protective” and “UMOD lowering” alleles. A detailed methodology is presented in the Supplementary Study Design and Methodology and Supplementary References.
Results
Characteristics of the Cohorts and Distribution of uUMOD Excretion
The African-PREDICT cohort included 1202 Black and White participants, and the PURE study included 1943 Black participants. In Tables 1 and 2, we present the socioeconomic, anthropometric, blood pressure, and kidney function profiles of these 2 cohorts. The mean age of the PURE cohort is approximately 25 years older than that of the African-PREDICT cohort.
Table 1.
Baseline characteristics of 1202 participants from the African-PREDICT cohort
| Characteristics | Total group N = 1202 |
Black n = 606 | White n = 596 | Black vs. White P |
|---|---|---|---|---|
| Age, yrs | 24.5 ± 3.1 | 24.5 ± 3.2 | 24.6 ± 3.1 | 0.50 |
| Male, n (%) | 578 (48.1) | 295 (48.7) | 283 (47.5) | 0.68 |
| Socioeconomic status, n (%) | <0.001a | |||
| Low | 477 (39.7) | 359 (59.3) | 118 (19.8) | |
| Middle | 346 (28.8) | 162 (26.8) | 184 (30.9) | |
| High | 378 (31.5) | 84 (13.9) | 294 (49.3) | |
| Anthropometric measurements | ||||
| BMI (kg/m2) | 25.1 ± 5.6 | 24.6 ± 5.7 | 25.5 ± 5.2 | 0.003 |
| Waist circumference (cm) | 80.1 ± 12.5 | 77.8 ± 10.9 | 82.6 ± 13.5 | <0.001a |
| Waist to Height ratio | 0.48 ± 0.07 | 0.47 ± 0.07 | 0.48 ± 0.07 | 0.36 |
| Cardiovascular profile | ||||
| Clinic SBP (mm Hg) | 119 ± 12.1 | 120 ± 11.9 | 119 ± 12.2 | 0.15 |
| Clinic DBP (mm Hg) | 78.5 ± 7.9 | 79.6 ± 8.2 | 77.4 ± 7.5 | <0.001a |
| Kidney function | ||||
| eGFR (ml/min per 1.73 m2) | 120 ± 18.6 | 123 ± 16.0 | 116 ± 20.4 | <0.001a |
| eGFR < 60 (ml/min per 1.73 m2) | 1 (0.08) | 1 (0.17) | 0 | |
| UACR (mg/mmol) | 0.50 (0.16–2.33) | 0.51 (0.15–2.27) | 0.49 (0.17–2.34) | 0.20 |
| Urinaryspot creatinine (mg/dl) | 163 ± 91.5 | 155 ± 92.3 | 171 ± 90.1 | 0.002 |
| Urinaryspot Na+ (mmol/l) | 138 ± 66.3 | 155 ± 67.6 | 121 ± 60.2 | <0.001a |
| Urinaryspot K+ (mmol/l) | 63.7 ± 35.8 | 52.7 ± 30.5 | 74.9 ± 37.3 | <0.001a |
| Urinaryspot Na+/K+ ratio (mmol/l) | 2.9 ± 2.1 | 3.7 ± 2.3 | 2.0 ± 1.5 | <0.001a |
| Uric acidspot (mg/dl) | 51.5 ± 27.6 | 44.5 ± 26.3 | 58.7 ± 27.1 | <0.001a |
| Urine volume (l/d) | 1.22 (0.51–3.16) | 1.16 (0.46–2.66) | 1.28 (0.56–3.25) | 0.004 |
| Osmolality (mOsm/kgH2O) | 731 ± 266 | 711 ± 277 | 750 ± 253 | 0.010 |
| Uromodulin (μg/ml) | 55.0 (11.4–166.0) | 50.5 (10.4–164.0) | 59.9 (13.3–172.0) | <0.001a |
| Male | 54.0 (12.1–165.5) | 48.8 (10.7–165.5) | 60.1 (13.8–166.9) | |
| Female | 55.9 (11.2–170.6) | 52.2 (9.70–162.2) | 59.8 (12.3–176.6) | |
| Uromodulin (mg/g creatinine) | 40.9 (18.8–82.1) | 39.8 (19.4–77.0) | 42.0 (18.3–86.7) | 0.045 |
| Male | 36.9 (17.7–67.2) | 36.5 (19.3–67.6) | 37.3 (16.0–67.0) | |
| Female | 45.0 (20.0–90.0) | 43.2 (18.6–85.4) | 46.8 (20.0–91.4) | |
| Nitrite (μM) | 3.9 (1.9–10.1) | 4.0 (1.9–11.3) | 3.8 (1.9–8.4) | 0.052 |
| Nitrite (μM/mM Creatinine) | 0.3 (0.1–1.7) | 0.4 (0.1–2.0) | 0.3 (0.1–1.3) | 0.023 |
| Renin-Angiotensin-Aldosterone Profile | ||||
| Estimated NaCl intake (g/d) | 7.6 (2.5–20.1) | 7.6 (2.5–20.9) | 7.6 (2.5–19.8) | 0.74 |
| Plasma Renin activity-S (pmol/l) | 89.3 (15.4–304.8) | 63.2 (11.3–267.1) | 126.9 (39.0–344.1) | <0.001a |
| Serum Angiotensin II (pmol/l) | 63.7 (11.3–217.3) | 45.5 (8.1–187.8) | 89.9 (28.0–245.0) | <0.001a |
| Serum Aldosterone (pmol/l) | 101.4 (15.7–505.8) | 68.5 (13.9–267.6) | 151.3 (28.0–684.3) | <0.001a |
| Dietary recall | ||||
| Total protein intake (g) | 67.0 (31.2–139.0) | 59.4 (28.1–118.0) | 75.5 (37.4–151.0) | <0.001a |
| Animal protein intake (g) | 35.2 (8.0–108.0) | 26.0 (3.2–84.2) | 47.6 (13.8–122.0) | <0.001a |
| Plant protein intake (g) | 18.4 (7.7–40.6) | 20.6 (8.6–46.2) | 16.4 (7.4–34.9) | <0.001a |
BMI, body mass index; CKD-EPI eGFR, Chronic Kidney Disease Epidemiology Collaboration no race formula estimated glomerular filtration rate; DBP, diastolic blood pressure; K+, potassium; Na+, sodium; SBP, systolic blood pressure, UACR urine albumin-to-creatinine ratio.
Mean ± SD; geometric mean (5th and 95th percentiles). Qualitative parameters are presented as fractions with percentages. χ2 tests for categorial variables and unpaired t test for quantitative parameters were used.
Equilibrium angiotensin levels were used to calculate combined parameters as surrogate markers (s) for the activity of circulating renin-angiotensin system enzyme renin-s (Angiotensin I and Angiotensin II).
Significant P values according to the Bonferroni correction.
Table 2.
Baseline characteristics of 1943 Black Adults in the PURE cohort
| Characteristics | N = 1943 |
|---|---|
| Male, n (%) | 723 (37.2) |
| Age, yrs | 49.7 ± 10.4 |
| HIV positive, n (%) | 307 (15.9) |
| Anthropometric measurements | |
| BMI (kg/m2) | 24.7 ± 7.0 |
| Waist circumference (cm) | 79.8 ± 13.0 |
| Waist to Height ratio | 0.50 ± 0.09 |
| Cardiovascular profile | |
| Clinic SBP (mm Hg) | 133.0 ± 24.1 |
| Clinic DBP (mm Hg) | 87.7 ± 14.5 |
| Hypertension, n (%) | 1017 (52.3) |
| Clinic BP ≥ 140 and/90 mm Hg and no BP medication | 695 (35.8) |
| Clinic BP ≥ 140 and/90 mm Hg and using BP medication | 223 (11.5) |
| Clinic BP < 140/90 mm Hg and using BP medication | 99 (5.0) |
| Kidney function | |
| eGFR (ml/min per 1.73 m2) (n = 1574) | 98.3 ± 18.9 |
| GFR <60 ml/min per 1.73 m2 | 58 (3.68) |
| UACR (mg/mmol) | 0.71 (0.14–7.95) |
| Urinary creatinine (mg/dl) | 86.7 (23.6–246.0) |
| Urinaryspot Na+ (mmol/l) | 114.0 ± 57.1 |
| Urinaryspot K+ (mmol/l) | 30.3 (7.2–86.8) |
| Urinaryspot Na+/K+ ratio (mmol/l) | 3.2 (0.9–9.5) |
| Uric acidspot (mg/dl) | 21.4 (7.1–62.1) |
| Osmolality (mOsm/kgH2O) | 529 ± 222 |
| Uromodulin (μg/ml) | 34.0 (7.4–154.0) |
| Male | 35.1 (7.3–154.7) |
| Female | 33.4 (7.5–153.5) |
| Uromodulin (mg/g creatinine) | 39.3 (10.8–99.0) |
| Male | 36.4 (9.8–98.3) |
| Female | 41.1 (11.3–99.8) |
| Renin-Angiotensin-Aldosterone Profile | |
| Plasma Renin (pg/ml) | 4.6 (1.2–23.9) |
BMI, body mass index; CKD-EPI eGFR, Chronic Kidney Disease Epidemiology Collaboration no race formula estimated glomerular filtration rate; DBP, diastolic blood pressure; K+, potassium; Na+, sodium; SBP, systolic blood pressure; UACR, urine albumin-to-creatinine ratio.
Mean ± SD; geometric mean (5th and 95th percentiles).
The distribution of uUMOD levels, expressed in concentration or indexed for urinary creatinine, in African-PREDICT (Panels A and B) and PURE (Panels C and D) is shown in Supplementary Figure S2, and Tables 1 and 2. In African-PREDICT, higher uUMOD/creatinine levels (42.0 [18.3–86.7] mg/g vs. 39.8 [19.4–77.0] mg/g; P = 0.045) (Supplementary Figure S2A) and uUMOD concentrations (59.9 [13.3–172.0] μg/ml vs. 50.5 [10.4–164.0] μg/ml; P < 0.001) (Supplementary Figure S2B) were detected in White compared to Black individuals.
Males had lower uUMOD/creatinine levels than females in the total population (36.9 mg/g vs. 45.0 mg/g), Black (36.5 mg/g vs. 43.2mg/g), and White (37.3 mg/g vs. 46.8 mg/g) (all P < 0.001) subgroups of African-PREDICT. There were no differences in absolute concentrations of uUMOD observed between the sexes. Similar results were observed in the PURE study (Supplementary Figures S2C and D, and Table 2).
Genetic Architecture of the UMOD-PDILT Locus in African Populations
Based on the previous associations of the UMOD-PDILT locus in Europeans,21,30,31 we analyzed the genotypes at rs4293393 and rs12917707 (UMOD) and rs12446492 (PDILT) in different African populations from the 1000 Genomes Project and the African-PREDICT and the PURE study cohorts (Table 3).
Table 3.
The distribution of UMOD-PDILT SNPs in African and European populations from the 1000 Genomes Project, the African-PREDICT and the PURE cohorts
| Locus | Allele frequency |
Genotype frequency |
|||||
|---|---|---|---|---|---|---|---|
| UMOD | PDILT | UMOD | PDILT | ||||
| Population | N | rs4293393 | rs12917707 | rs12446492 | rs4293393 | rs12917707 | rs12446492 |
| 1000 Genomes Project | |||||||
| Global | 2504 | T: 80.0%a C: 20.0% |
G: 90.2%a T: 9.8% |
T: 42.3%a A: 57.7% |
TT: 65.1% CT: 29.9% CC: 5.0% |
GG: 82.1% GT: 16.1% TT: 1.8% |
TT: 20.4% AT: 43.8% AA: 35.8% |
| African | 661 | T: 80.7%a C: 19.3% |
G: 97.7%a T: 2.3% |
T: 50.2%a A: 49.8% |
TT: 65.2% CT: 31.0% CC: 3.8% |
GG: 95.5% GT: 4.5% TT: 0% |
TT: 26.8% AT: 46.9% AA: 26.3% |
| European | 503 | T: 79.9%a C: 20.1% |
G: 79.9%a T: 20.1% |
T: 55.8%a A: 44.2% |
TT: 64.4% CT: 31.0% CC: 4.6% |
GG: 64.4% GT: 31.0% TT: 4.6% |
TT: 32.4% AT: 46.7% AA: 20.9% |
| African-PREDICT Study | |||||||
| Total population | 1202 | T: 74.5%a C: 25.5% |
G: 88.6%a T: 11.4% |
T: 45.5%a A: 53.5% |
TT: 55.7% CT: 37.6% CC: 6.7% |
GG: 78.6% TG: 20.0% TT: 1.4% |
TT: 21.8% TA: 49.4% AA: 28.8% |
| Black | 606 | T: 67.5%a C: 32.5% |
G: 93.8%a T: 6.2% |
T: 37.2%a A: 62.8% |
TT: 45.0% CT: 45.0% CC: 10.0% |
GG: 87.8% TG: 12.0% TT: 0.2% |
TT: 13.0% TA: 48.2% AA: 38.7% |
| White | 596 | T: 81.6%a C:18.4% |
G: 83.3%a T: 16.7% |
T: 55.9%a A: 44.1% |
TT: 66.5% CT: 30.1% CC: 3.4% |
GG: 69.3% TG: 28.1% TT: 2.6% |
TT: 30.5% TA: 50.7% AA: 18.8% |
| PURE study | |||||||
| Black | 1943 | T: 66.0%a C: 34.0% |
G: 94.4%a T: 5.6% |
T: 40.5%a A: 59.5% |
TT: 43.6% CT: 44.8% CC: 11.6% |
GG: 88.9% TG: 11.0 % TT: 0.1% |
TT: 15.1% TA: 50.8% AA: 34.1% |
CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate.
For rs4293393, alleles T/C on the reverse strand are reported.
rs4293393 (UMOD), rs12917707 (UMOD), and rs12446492 (PDILT) have been strongly associated with urinary levels of uromodulin, eGFR, and risk of CKD in European cohorts.8,9,20,21 In these studies, kidney-protective alleles and/or UMOD expression reducing alleles are the European minor alleles rs4293393(C), rs12917707(T), and rs12446492(A).
Refers to the European “CKD risk” SNP.
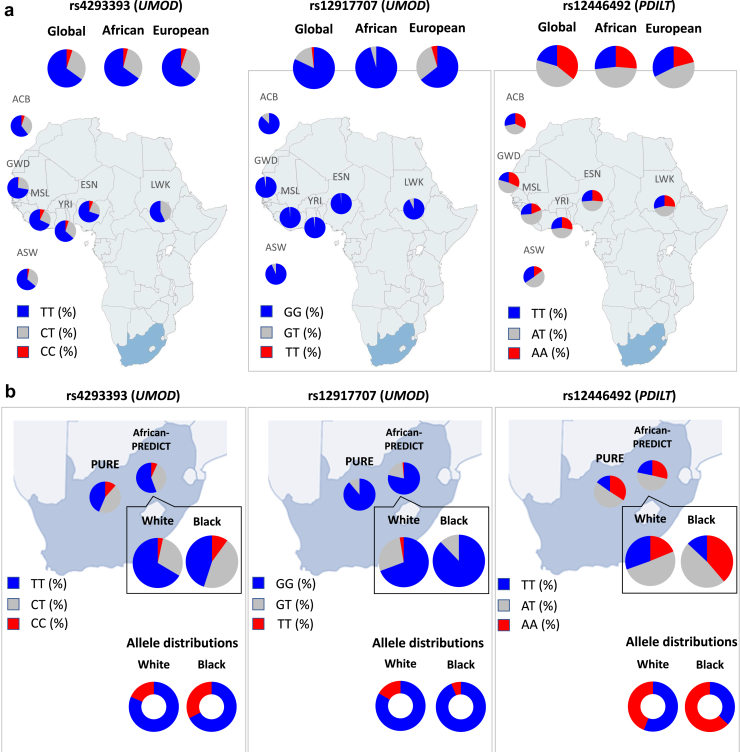
Analysis of the 1000 Genomes Project summary revealed a strikingly higher prevalence of the “CKD risk” allele G at rs12917707 in Africans. Consequently, the prevalence of homozygotes for the “CKD risk” alleles at rs12917707 was higher in Africans compared to Europeans (95.5% vs. 64.4%, respectively) with 0% protective allele homozygotes among Africans (vs. 4.6% in Europeans) (Figure 1a and Table 3). In African-PREDICT, we confirmed the higher prevalence of the risk allele at rs12917707 in Black individuals, with homozygotes for the protective allele virtually absent among Black individuals (0.2% vs. 2.6%). Black participants from African-PREDICT and PURE exhibited similar allele frequencies and genotype distributions for the 3 SNPs (Figure 1b and Table 3). When comparing Black versus White individuals in African-PREDICT, the overall fixation index for the 3 SNPs (FST = 0.03) was similar to the value calculated when using gnomAD data for the 3 SNPs for Europeans and Africans/African Americans (FST = 0.012) and higher than the value for Black participants recruited in African-PREDICT vs. PURE (FST = 0.0004), substantiating the genetic differentiation between Black and White individuals in the PREDICT study for this locus.
Figure 1.
Distribution of the UMOD/PDILT SNPs in African and European populations from the 1000 Genomes Project, the African-PREDICT and the PURE cohorts. (a) Genotype distribution of rs4293393 (UMOD), rs12917707 (UMOD), and rs12446492 (PDILT) in the African samples from the 1000 Genomes Project, compared to the global and European sample prevalence. (b) Genotype distribution of rs4293393, rs12917707, and rs12446492 in Black and White adults from the African-PREDICT and PURE studies. Blue sections represent the frequencies of “CKD risk” alleles and red sections the frequencies of “CKD protective” alleles. Populations represented in the European ancestry (n = 503) group of the 1000 Genomes Project included: Utah residents from North and West Europe CEU (n = 99), Toscani in Italia TSI (n = 107), Finnish in Finland FIN (n = 99), British in England and Scotland GBR (n = 91), and Iberian population in Spain IBS (n = 107). The African superpopulation is composed of the following populations: African Caribbean in Barbados ACB (n = 96), Gambian in Western Divisions GWD in the Gambia (n = 113), Luhya in Webuye, Kenya LWK (n = 99), Esan in Nigeria ESN (n = 99), Yoruba in Ibadan, Nigeria YRI (n = 108), Mende in Sierra Leone MSL (n = 85), and Americans with African ancestry in Southwest United States ASW (n = 61). The South African population is represented by the North-West Province PURE cohort (n = 1943) and the total of African-PREDICT cohort (n = 1202).
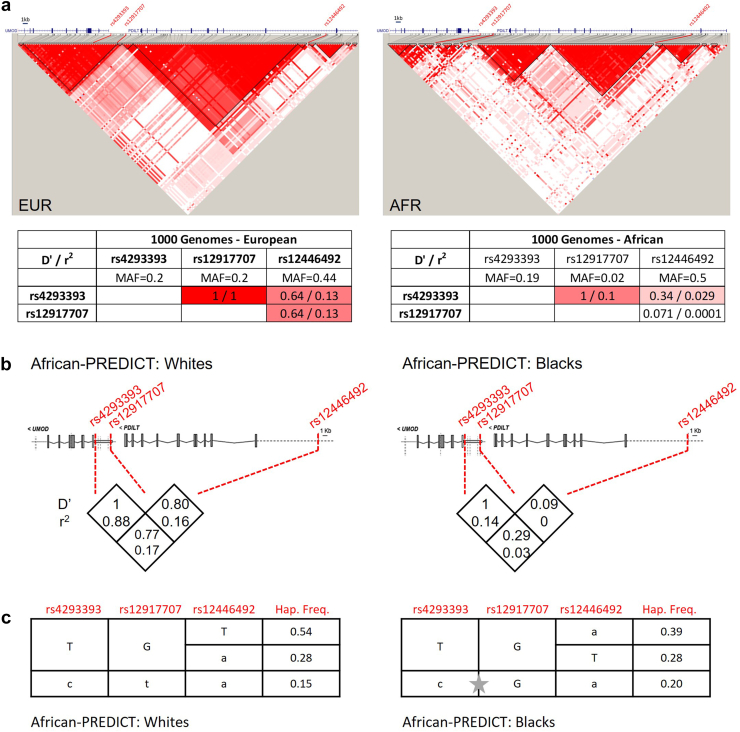
We next built LD plots based on summary data from the 1000 Genomes Project.32 In Europeans, 2 LD blocks span nearly the entirety of the UMOD and PDILT genes. The LD blocks were smaller in African populations, with fragmentation of the LD over the UMOD gene and the large European PDILT block being divided into 2 separate blocks in Africans (Figure 2a). In Europeans, the UMOD SNPs rs4293393 and rs12917707 are in perfect LD (r2 and D’ = 1), with evidence for substantial LD between UMOD SNPs and PDILT SNP rs12446492 (D’ = 0.64). In Africans, the D’ between the UMOD SNPs is 1 (likely inflated by the rarity of the alternative allele at rs12917707), with a reduced correlation coefficient (r2 = 0.1). The LD between the UMOD and PDILT SNPs is considerably weaker compared to Europeans (Supplementary Figure S3, Supplementary Table S1, and Figure 2a).
Figure 2.
Linkage disequilibrium (LD) and haplotype analysis of the UMOD-PDILT locus. (a) LD map of the UMOD-PDILT locus based on 1000 Genomes Project summary data for African (n = 661) and European (n = 502) populations. D’ values are indicated, and the 3 SNPs of interest highlighted on the graph with their LD statistics (D’ and r2) shown below. Graph generated using Haploview 4.2. (b) LD estimates (D’ and r2) for 3 SNPs of interest in Whites (n = 535) and Blacks (n = 543) from the African-PREDICT cohort. (c) Haplotype frequency estimates in Whites and Blacks from the African-PREDICT cohort. Only the 3 most common haplotypes are shown. The grey star shows a recombination event between rs4293393 and rs12917707 that is frequent in blacks and virtually absent in whites. MAF, minor allele frequencies.
Based on the SNP genotyping data in African-PREDICT, we estimated LD statistics and reconstructed haplotypes for White and Black participants. Consistent with the observations from the 1000 Genomes Project, LD was observed between the UMOD rs4293393 and rs12917707 in Whites: D’ = 1 and no recombination among the 3 most common haplotypes. In contrast, haplotype analysis predicted a recombination event between rs4293393 and rs12917707 in Black participants reflected by lower LD metrics (Figure 2b and 2c). Of note, the “all protective” haplotype “c-t-a” (0.15 in White participants) was very rare in Black participants (0.04) (Figure 2c).
Genetic Determinants of uUMOD Excretion in the African-PREDICT and PURE Cohorts
In the whole African-PREDICT cohort, we observed significant, positive correlations between the risk allele genotypes at the 3 investigated SNPs and absolute or normalized uUMOD levels (Supplementary Table S2). Importantly, these relationships were driven by the White participants and no significant (after correction for multiple testing) correlation was seen in young Black participants (Supplementary Table S2). In the PURE cohort of older Black individuals, we detected a significant but much weaker positive correlation between uUMOD levels and risk allele genotype at rs12917707 only (Supplementary Table S3).
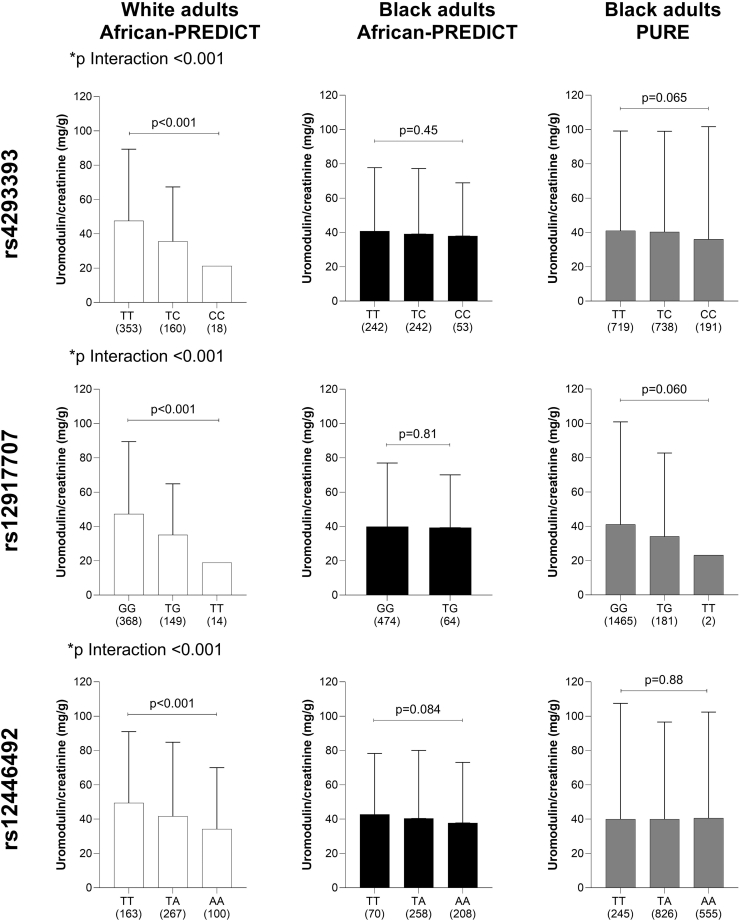
The effects of the UMOD-PDILT genotype on the uUMOD levels in the 2 cohorts are shown in Figure 3. In African-PREDICT, the uUMOD levels (absolute concentration (Supplementary Figure S4) or normalized to creatinine (Figure 3) significantly increased with each copy of the risk alleles for each of the 3 SNPS investigated in White participants (all P trend < 0.001), whereas these SNPs did not affect uUMOD levels in the Black participants. When introducing SNP ethnicity interaction terms in the linear regression analyses, we confirmed a significant interaction of ethnicity on the relationship between all 3 SNPs and uUMOD/creatinine (P < 0.001). Analyzing individuals homozygous for the 3 most common haplotypes in the respective population (Figure 2c), we noticed a striking reduction of uUMOD/creatinine only in Whites with the protective “c-t-a” haplotype (Supplementary Figure S5).
Figure 3.
Effects of the UMOD-PDILT genotype on urinary uromodulin levels. Comparison of urinary uromodulin/creatinine levels according to genotypes at rs4293393 (UMOD), rs12917707 (UMOD) and rs12446492 (PDILT) in ● Black and ○ White adults from the African-PREDICT study; and ● Black adults from the PURE study. Data presented as geometric mean and 95th percentile. Bonferroni corrected P values shown for Welch’s analysis of variance comparing uromodulin and uromodulin/creatinine across groups. An interaction term SNP∗ethnicity is reported as p interaction for all 3 SNPs. SNP, single-nucleotide polymorphism.
Bivariate and Multivariate Models for Determinants of uUMOD Excretion
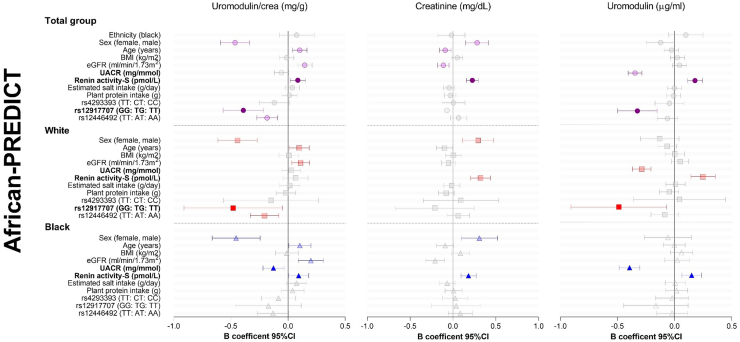
We performed Pearson correlations to determine bivariate relationships of uUMOD/creatinine with several anthropometric, kidney function, and blood pressure measures as well as dietary intakes in the African-PREDICT (Supplementary Table S2) and PURE (Supplementary Table S3) cohorts. We also included anthropometric, kidney function, renin-angiotensin-aldosterone system, dietary, and genetic variables into multivariate regression models to investigate the independent determinants of uUMOD/creatinine levels in the cohorts (Figures 4 and Supplementary Figure S6; Supplementary Tables S4 and S5).
Figure 4.
Determinants of urinary uromodulin in the African-PREDICT study. Multiple regression analyses in the total African-PREDICT study population and split according to ethnicity. Continuous variables were standardized by creating z-variables, which were included into multiple regression models for this forest plot. BMI, body mass index; UACR, urinary albumin-creatinine ratio.
In the total population of African-PREDICT (Figure 4 and Supplementary Table S4), female sex (Std. β = −0.232; P < 0.001), older age (Std. β = 0.102; P = 0.002), higher eGFR (Std. β = 0.145; P < 0.001), higher renin-s (Std. β = 0.085; P = 0.013) as well as the risk “GG” genotype of rs12917707 (UMOD) and the risk “TT” genotype of rs12446492 (PDILT) were independently associated with higher levels of uUMOD/creatinine. The model explained 14% of the variance in uUMOD/creatinine in the cohort, with the rs12917707 and rs12446492 SNP variants accounting for 1.7% and 1.4% of the variance of the trait, respectively. Sex, age, eGFR, rs12917707, and rs12446492 remained significant determinants of uUMOD/creatinine after correcting for multiple testing (Supplementary Table S4). Stratified according to ethnicity, the multiple regression model explained a greater percentage of the variance of uUMOD/creatinine in White than in Black adults (23% vs. 8%, respectively). The effects of sex, age, and eGFR were observed in White and Black adults. The risk “GG” genotype of rs12917707 and “TT” genotype of rs12446492 was associated with higher uUMOD/creatinine in White but not in Black adults. Lower urine albumin-to-creatinine ratio (Std. β = −0.131; P = 0.006) and higher renin-s (Std. β = 0.100; P = 0.039) contributed to higher levels of uUMOD/creatinine in Black but not White adults. The relationship with renin-s lost significance when correcting for multiple testing.
In older Black adults from PURE, female sex (Std. β = −0.189; P < 0.001), older age (Std. β = 0.092; P = 0.002), lower body mass index (Std. β= −0.109; p<0.001), higher eGFR (Std. β = 0.123; P < 0.001), as well as the risk “GG” genotype of rs12917707 (Std. β = −0.096; P < 0.001) were associated with higher uUMOD/creatinine levels. Overall, only 4% of the variance of uUMOD/creatinine for older Black adults was explained by the variables included in the multiple regression models (Supplementary Figure S6 and Supplementary Tables S5 and S6).
Sensitivity Analyses for Determinants of Urinary Creatinine Levels
We performed sensitivity analyses to determine whether any of the variables associated with uUMOD/creatinine were independently related to creatinine excretion (Figure 4, Supplementary Figure S6, and Supplementary Tables S4 and S5). In African-PREDICT and in PURE, increased spot urine creatinine was related to male sex, younger age, lower eGFR, and higher renin. Accordingly, higher uUMOD/creatinine levels associated with female sex, older age, and higher eGFR is likely confounded by lower urinary creatinine levels. In PURE, higher creatinine was also associated with a greater body mass index, which may explain the inverse association of uUMOD/creatinine with body mass index. In African-PREDICT, the relationship of uUMOD/creatinine with renin, rs12917707 (UMOD) and rs12446492 (PDILT) in the total group is likely not confounded by spot urine creatinine levels. Accordingly, higher absolute uUMOD concentration levels were shown to be associated with higher renin (Std. β = 0.178; P < 0.001) and the risk “GG” genotype of rs12917707 (P < 0.001). In PURE, we similarly found that higher uUMOD concentration levels were associated with higher renin (Std. β = 0.069; P = 0.007) and the risk “GG” genotype of rs12917707 (P = 0.042), in addition to being associated with older age (Std. β = 0.057; P = 0.043).
In sum, in White participants, higher uUMOD/creatinine levels appear to be associated primarily with the risk alleles at rs12917707 and rs12446492; whereas in young Black individuals from African-PREDICT, increased uUMOD/creatinine is associated with lower urine albumin-to-creatinine ratio but not UMOD-PDILT genotypes. In older Blacks from PURE, the risk allele at rs12917707 appears to be associated with higher levels of uUMOD/creatinine (smaller effect size than in White participants in African-PREDICT).
Discussion
We analyzed 2 South African cohorts of young Black and White (African-PREDICT) and older Black individuals (PURE-NWP-SA), in which we genotyped 3 selected SNPs at the UMOD-PDILT locus, to demonstrate ethnic differences in uUMOD levels and the determinants thereof. The main findings include the following: that (i) uUMOD levels are lower in Black than in White participants, (ii) allele frequencies for CKD risk-associated UMOD and PDILT SNPs and the UMOD-PDILT locus haplotype structure are different in Black and White participants, and (iii) determinants of uUMOD differ between White and Black individuals.
The relevance of these data reflects the emergence of the UMOD-PDILT locus as the main genetic locus influencing the risk for CKD in Europeans by driving UMOD expression.8,12 In young White adults, we observed that uUMOD is significantly attenuated with each copy of the “protective” alleles for each of the 3 SNPs investigated, and that the genotype at rs12917707 is an important determinant of uUMOD. In contrast, only SNP rs12917707 was weakly associated with uUMOD in older Black individuals from the PURE study (but not in younger Black individuals from the PREDICT study). In addition, the “protective” haplotype at rs12917707 is much rarer in Black participants.
Whereas in White participants, higher uUMOD levels were associated primarily with the risk allele at rs12917707, in young Black participants, higher uUMOD was associated with higher renin and lower urine albumin-to-creatinine ratio. Although female sex, older age, and higher eGFR were associated with higher uUMOD/creatinine in both White and Black adults, these associations were confounded by urinary creatinine. In older Black adults from the PURE study, uUMOD levels were weakly associated with the risk allele at rs12917707. In bivariate analysis, additional correlations of uUMOD with blood pressure, urinary uric acid, Na+, K+, and nitrites were observed. Based on the previous literature describing an effect of UMOD on these parameters,9,12,23,33 we did not include them in the multivariate models to avoid reverse causation.
LD describes the degree of correlation of nearby alleles within a given population. The LD architecture is influenced by the population bottleneck that is older in Africans compared to Europeans with generally shorter LD blocks and more LD units.34,35 Consistent with this, we observed shorter and more LD units over the UMOD-PDILT locus. Studying African populations can thus help to fine-map genetic association signals: for example, rs12917707 was the only signal in our study that remained significantly associated with uUMOD levels in (older) Black individuals.
The missing diversity of genomic studies prevents a better understanding of the genetic architecture of diseases.36 A small study including 166 Black South Africans found no association between the UMOD promoter SNP rs13333226 (in LD with rs4293393) and uUMOD levels.25 A UK Biobank GWAS study including approximately 6000 individuals of African ancestry reported the association of rs12917707 with metrics of kidney function, urate, and urea levels but no population sub analysis was shown. Derived polygenic risk scores for eGFR and creatinine explained significantly less variance in Africans compared to other populations.37 A phenome-wide association study of UMOD variants among 648,593 US veterans (19% Black African Americans, AAs)26 showed that in AAs, each copy of the risk allele at rs77924615 (PDILT) was associated with increased odds of CKD stage 3 and hypertensive CKD. These associations were however attenuated compared to White participants: in AAs, no phenome-wide significant associations were observed between the rs4293392 UMOD promoter variant and any of the clinical phenotypes for which associations were observed in White participants. In a GWAS of kidney traits conducted in >8000 AAs from the CARe Renal Consortium, the rs4293393 UMOD promoter variant did not reach significant association with CKD.24 The European lead SNP rs12917707 was not present in the AA dataset, reflecting our findings of shifted allelic prevalence. Finally, a GWAS meta-analysis for CKD progression found the strongest genome-wide association for longitudinal eGFR decline with the UMOD-PDILT locus. Importantly, this association was only significant for White participants and not for Black participants.38
These examples clearly highlight the fact that GWAS findings are not automatically replicable between populations or ancestries. The LD blocks over the UMOD-PDILT locus are smaller in Black individuals, with predicted recombinations between UMOD promoter SNPs that were not present in White individuals. The fragmented Black genome over UMOD-PDILT may explain why we see a selective and attenuated association between the SNPs previously reported in White individuals and uUMOD. Well-powered GWAS in African populations are needed to uncover potential associations between different SNPs at the UMOD-PDILT locus and uUMOD. At least in European populations, the CKD risk mediated by UMOD-PDILT SNPs is causally linked with the increased expression of uromodulin.13 Given that the UMOD-PDILT locus association with CKD appears much attenuated in individuals with African ancestry, one explanation for this may be the attenuated effect on uUMOD levels as shown in this study. Other potential consequences include the value of UMOD SNPs as predictor to response to loop diuretics.39
The strengths of our study include the relatively large sample of Black and White participants in 2 established cohorts of continental African origin and the detailed clinical/phenotypical and genotyping data, including centralized determination of uUMOD levels. Among the limitations are the fact that ethnic, demographic, and dietary characteristics are self-reported. Given the fact that only the 3 SNPs have been genotyped, association testing could not be adjusted for principal components of ancestry. Several other SNPs (e.g., PDILT rs77924615) were not investigated, and serum uromodulin levels were not available at the time of data analyses.
In conclusion, we evidenced important ethnic differences in the clinical and genetic determinants of uUMOD levels involving the 3 UMOD/PDILT SNPs that we genotyped in this study. Ethnic differences should be considered when analyzing the role of uromodulin in hypertension and kidney function as well as precision medicine recommendations in different ethnic groups.
Disclosure
All the authors declared no conflicting interest.
Acknowledgments
We acknowledge Anne Kipp and Larissa Govers for their participation in the early phase of this study and thank Nadine Nägele and Huguette Debaix for expert technical assistance.
Funding
EO is supported by Postdoc Mobility-Stipendien of the Swiss National Science Foundation Grants P2ZHP3_195181 and P500PB_206851, Kidney Research UK Grant Paed_RP_001_20180925. OD is supported by the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant (agreement N° 860977), the European Reference Network for Rare Kidney Diseases (project N° 739532), the Swiss National Science Foundation (grant 310030-189044), and the University Research Priority Program (URPP) ITINERARE at the University of Zurich. MS-K is supported by the National Research Foundation of South African (Postdoctoral Innovation grant; Grant Number: UID: 138499). JAS is funded by LifeArc, Medical Research Council (MR/Y007808/1), Kidney Research UK (Paed_RP_001_20180925) and the Northern Counties Kidney Research Fund (20/01). EO and OD are members of the European Reference Network for Rare Kidney Diseases (ERKNet).
The research funded in this manuscript is part of an ongoing larger research project financially supported by the South African Medical Research Council (SAMRC) with funds from National Treasury under its Economic Competitiveness and Support Package; the South African Research Chairs Initiative (SARChI) of the Department of Science and Innovation and the National Research Foundation (NRF) of South Africa (GUN 86895); SAMRC with funds received from the South African National Department of Health, GlaxoSmithKline R&D (Africa Non-Communicable Disease Open Lab grant), the UK Medical Research Council, and with funds from the UK Government’s Newton Fund; and corporate social investment grants from Pfizer (South Africa), Boehringer-Ingelheim (South Africa), Novartis (South Africa), the Medi Clinic Hospital Group (South Africa), and in-kind contributions of Roche Diagnostics (South Africa). Any opinion, findings, and conclusions or recommendations expressed in this material are those of the authors, and therefore, the NRF does not accept any liability in this regard.
Footnotes
Study Design and Methodology.
Supplementary References.
Figure S1. Position of the genotyped variants rs12917707, rs4293393, and rs12446492 along the adjacent UMOD and PDILT genes on chromosome 16 and reported GWAS associated as found in the GWAS catalogue (https://www.ebi.ac.uk/gwas/home).
Figure S2. Distribution of urinary UMOD levels. Top row: African-PREDICT (A) uromodulin/creatinine and (B) absolute UMOD concentrations; bottom row: PURE (C) UMOD/creatinine and (D) absolute UMOD concentrations.
Figure S3. Linkage disequilibrium map of the UMOD-PDILT locus based on 1000 Genomes Project summary data for African (n = 661) and European (n = 502) populations. r2 values are indicated and the 3 SNPs of interest are highlighted on the graph. Graph generated using Haploview 4.2.
Figure S4. Comparison of absolute urinary uromodulin concentrations levels according to genotypes at rs4293393 (UMOD), rs12917707 (UMOD), and rs12446492 (PDILT) in ● black and ○ white adults from the African-PREDICT study; and ● black adults from the PURE study. Data presented as geometric mean and 95th percentile. P values shown for Welch’s analysis of variance comparing uromodulin and uromodulin/creatinine across groups.
Figure S5. Comparison of urinary uromodulin/creatinine levels in individuals homozygous for haplotypes defined by rs4293393 (UMOD), rs12917707 (UMOD), and rs12446492 (PDILT) in ● black and ○ white adults from the African-PREDICT study. Data presented as geometric mean and 95th percentile. P values shown for Welch’s analysis of variance comparing uUMOD/creatinine across groups and for Bonferroni multiple comparison test.
Figure S6. Determinants of urinary uromodulin in the PURE-NWP-SA study. Multiple regression analysis in the PURE study population. Continuous variables were standardized by creating z-variables, which were included into multiple regression models for this Forest plot.
Table S1. Pairwise linkage disequilibrium data between UMOD/PDILT SNPs in African and European populations from the 1000 Genomes Project and the African-PREDICT cohort.
Table S2. Pearson correlations of possible determinants of uromodulin in the African-PREDICT cohort.
Table S3. Pearson correlations of possible determinants of uromodulin in the PURE cohort.
Table S4. Multiple regression analyses with urinary uromodulin/creatinine as the main dependent variable in the African-PREDICT cohort.
Table S5. Multiple regression analyses with urinary uromodulin/creatinine as the main dependent variable in the PURE cohort.
Table S6. Multiple regression with SNPs separately entered into models in the PURE cohort.
Strega Checklist.
Supplementary Material
Study Design and Methodology. Supplementary References. Figure S1. Position of the genotyped variants rs12917707, rs4293393, and rs12446492 along the adjacent UMOD and PDILT genes on chromosome 16 and reported GWAS associated as found in the GWAS catalogue (https://www.ebi.ac.uk/gwas/home). Figure S2. Distribution of urinary UMOD levels. Figure S3. Linkage disequilibrium map of the UMOD-PDILT locus based on 1000 Genomes Project summary data for African (n = 661) and European (n = 502) populations. Figure S4. Comparison of absolute urinary uromodulin concentrations levels according to genotypes at rs4293393 (UMOD), rs12917707 (UMOD), and rs12446492 (PDILT) in ● Black and ○ White adults from the African-PREDICT study; and ● Black adults from the PURE study. Figure S5. Comparison of urinary uromodulin/creatinine levels in individuals homozygous for haplotypes defined by rs4293393 (UMOD), rs12917707 (UMOD), and rs12446492 (PDILT) in ● Black and ○ White adults from the African-PREDICT study. Figure S6. Determinants of urinary uromodulin in the PURE-NWP-SA study. Table S1. Pairwise linkage disequilibrium data between UMOD/PDILT SNPs in African and European populations from the 1000 Genomes Project and the African-PREDICT cohort. Table S2. Pearson correlations of possible determinants of uromodulin in the African-PREDICT cohort. Table S3. Pearson correlations of possible determinants of uromodulin in the PURE cohort. Table S4. Multiple regression analyses with urinary uromodulin/creatinine as the main dependent variable in the African-PREDICT cohort. Table S5. Multiple regression analyses with urinary uromodulin/creatinine as the main dependent variable in the PURE cohort. Table S6. Multiple regression with SNPs separately entered into models in the PURE cohort. Strega Checklist.
References
- 1.Eckardt K.U., Coresh J., Devuyst O., et al. Evolving importance of kidney disease: from subspecialty to global health burden. Lancet. 2013;382:158–169. doi: 10.1016/S0140-673660439-0. [DOI] [PubMed] [Google Scholar]
- 2.GBD Chronic Kidney Disease Collaboration Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Lond Engl. 2020;395:709–733. doi: 10.1016/S0140-6736(20)30045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hariparshad S., Bhimma R., Nandlal L., Jembere E., Naicker S., Assounga A. The prevalence of chronic kidney disease in South Africa - limitations of studies comparing prevalence with sub-Saharan Africa, Africa, and globally. BMC Nephrol. 2023;24:62. doi: 10.1186/s12882-023-03109-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mortality and Cause of Death in South Africa: Findings from Death Notification 2019. Statistics South Africa. Statistical release P0309.3. https://www.statssa.gov.za/publications/P03093/P030932019.pdf
- 5.Moulin F., Ponte B., Pruijm M., et al. A population-based approach to assess the heritability and distribution of renal handling of electrolytes. Kidney Int. 2017;92:1536–1543. doi: 10.1016/j.kint.2017.06.020. [DOI] [PubMed] [Google Scholar]
- 6.Devuyst O., Pattaro C. The UMOD locus: insights into the pathogenesis and prognosis of kidney disease. J Am Soc Nephrol. 2018;29:713–726. doi: 10.1681/ASN.2017070716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stanzick K.J., Li Y., Schlosser P., et al. Discovery and prioritization of variants and genes for kidney function in >1.2 million individuals. Nat Commun. 2021;12:4350. doi: 10.1038/s41467-021-24491-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wuttke M., Li Y., Li M., et al. A catalog of genetic loci associated with kidney function from analyses of a million individuals. Nat Genet. 2019;51:957–972. doi: 10.1038/s41588-019-0407-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trudu M., Janas S., Lanzani C., et al. Common noncoding UMOD gene variants induce salt-sensitive hypertension and kidney damage by increasing uromodulin expression. Nat Med. 2013;19:1655–1660. doi: 10.1038/nm.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gorski M., Jung B., Li Y., et al. Meta-analysis uncovers genome-wide significant variants for rapid kidney function decline. Kidney Int. 2021;99:926–939. doi: 10.1016/j.kint.2020.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okada Y., Sim X., Go M.J., et al. Meta-analysis identifies multiple loci associated with kidney function-related traits in east Asian populations. Nat Genet. 2012;44:904–909. doi: 10.1038/ng.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Devuyst O., Olinger E., Rampoldi L. Uromodulin: from physiology to rare and complex kidney disorders. Nat Rev Nephrol. 2017;13:525–544. doi: 10.1038/nrneph.2017.101. [DOI] [PubMed] [Google Scholar]
- 13.Ponte B., Sadler M.C., Olinger E., et al. Mendelian randomization to assess causality between uromodulin, blood pressure and chronic kidney disease. Kidney Int. 2021;100:1282–1291. doi: 10.1016/j.kint.2021.08.032. [DOI] [PubMed] [Google Scholar]
- 14.Devuyst O., Olinger E., Weber S., et al. Autosomal dominant tubulointerstitial kidney disease. Nat Rev Dis Primers. 2019;5:60. doi: 10.1038/s41572-019-0109-9. [DOI] [PubMed] [Google Scholar]
- 15.Schaeffer C., Devuyst O., Rampoldi L. Uromodulin: roles in health and disease. Annu Rev Physiol. 2021;83:477–501. doi: 10.1146/annurev-physiol-031620-092817. [DOI] [PubMed] [Google Scholar]
- 16.Oh H.S.H., Rutledge J., Nachun D., et al. Organ aging signatures in the plasma proteome track health and disease. Nature. 2023;624:164–172. doi: 10.1038/s41586-023-06802-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pruijm M., Ponte B., Ackermann D., et al. Associations of urinary uromodulin with clinical characteristics and markers of tubular function in the general population. Clin J Am Soc Nephrol CJASN. 2016;11:70–80. doi: 10.2215/CJN.04230415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Troyanov S., Delmas-Frenette C., Bollée G., et al. Clinical, genetic, and urinary factors associated with uromodulin excretion. Clin J Am Soc Nephrol CJASN. 2016;11:62–69. doi: 10.2215/CJN.04770415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olinger E., Schaeffer C., Kidd K., et al. An intermediate-effect size variant in UMOD confers risk for chronic kidney disease. Proc Natl Acad Sci U S A. 2022;119 doi: 10.1073/pnas.2114734119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joseph C.B., Mariniello M., Yoshifuji A., et al. Meta-GWAS reveals novel genetic variants associated with urinary excretion of uromodulin. J Am Soc Nephrol. 2022;33:511–529. doi: 10.1681/ASN.2021040491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olden M., Corre T., Hayward C., et al. Common variants in UMOD associate with urinary uromodulin levels: a meta-analysis. J Am Soc Nephrol. 2014;25:1869–1882. doi: 10.1681/ASN.2013070781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghirotto S., Tassi F., Barbujani G., et al. The uromodulin gene locus shows evidence of pathogen adaptation through human evolution. J Am Soc Nephrol. 2016;27:2983–2996. doi: 10.1681/ASN.2015070830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weiss G.L., Stanisich J.J., Sauer M.M., et al. Architecture and function of human uromodulin filaments in urinary tract infections. Science. 2020;369:1005–1010. doi: 10.1126/science.aaz9866. [DOI] [PubMed] [Google Scholar]
- 24.Liu C.T., Garnaas M.K., Tin A., et al. Genetic association for renal traits among participants of African ancestry reveals new loci for renal function. PLoS Genet. 2011;7 doi: 10.1371/journal.pgen.1002264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nqebelele N.U., Dickens C., Dix-Peek T., Duarte R., Naicker S. Urinary uromodulin levels and UMOD variants in black South Africans with hypertension-attributed chronic kidney disease. Int J Nephrol. 2019;2019 doi: 10.1155/2019/8094049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akwo E.A., Chen H.C., Liu G., et al. Phenome-wide association study of UMOD gene variants and differential associations with clinical outcomes across populations in the Million Veteran Program a multiethnic biobank. Kidney Int Rep. 2022;7:1802–1818. doi: 10.1016/j.ekir.2022.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schutte A.E., Gona P.N., Delles C., et al. The African Prospective study on the Early Detection and Identification of cardiovascular disease and hypertension (African-PREDICT): design, recruitment and initial examination. Eur J Prev Cardiol. 2019;26:458–470. doi: 10.1177/2047487318822354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schutte A.E., Schutte R., Huisman H.W., et al. Are behavioural risk factors to be blamed for the conversion from optimal blood pressure to hypertensive status in Black South Africans? A 5-year prospective study. Int J Epidemiol. 2012;41:1114–1123. doi: 10.1093/ije/dys106. [DOI] [PubMed] [Google Scholar]
- 29.Teo K., Chow C.K., Vaz M., Rangarajan S., Yusuf S., PURE Investigators-Writing Group The Prospective Urban Rural Epidemiology (PURE) study: examining the impact of societal influences on chronic noncommunicable diseases in low-, middle-, and high-income countries. Am Heart J. 2009;158:1–7.e1. doi: 10.1016/j.ahj.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 30.Gudbjartsson D.F., Holm H., Indridason O.S., et al. Association of variants at UMOD with chronic kidney disease and kidney stones-role of age and comorbid diseases. PLoS Genet. 2010;6 doi: 10.1371/journal.pgen.1001039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Köttgen A., Pattaro C., Böger C.A., et al. New loci associated with kidney function and chronic kidney disease. Nat Genet. 2010;42:376–384. doi: 10.1038/ng.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.1000 Genomes Project Consortium, Auton A., Brooks L.D., et al. A global reference for human genetic variation. Nature. 2015;526:68–74. doi: 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mutig K., Kahl T., Saritas T., et al. Activation of the bumetanide-sensitive Na+,K+,2Cl- cotransporter (NKCC2) is facilitated by tamm-Horsfall protein in a chloride-sensitive manner. J Biol Chem. 2011;286:30200–30210. doi: 10.1074/jbc.M111.222968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu X., Yan D., Cooper R.S., et al. Linkage disequilibrium and haplotype diversity in the genes of the renin-angiotensin system: findings from the family blood pressure program. Genome Res. 2003;13:173–181. doi: 10.1101/gr.302003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vergara-Lope A., Jabalameli M.R., Horscroft C., Ennis S., Collins A., Pengelly R.J. Linkage disequilibrium maps for European and African populations constructed from whole genome sequence data. Sci Data. 2019;6:208. doi: 10.1038/s41597-019-0227-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sirugo G., Williams S.M., Tishkoff S.A. The missing diversity in human genetic studies. Cell. 2019;177:1080. doi: 10.1016/j.cell.2019.04.032. [DOI] [PubMed] [Google Scholar]
- 37.Sinnott-Armstrong N., Tanigawa Y., Amar D., et al. Genetics of 35 blood and urine biomarkers in the UK Biobank. Nat Genet. 2021;53:185–194. doi: 10.1038/s41588-020-00757-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robinson-Cohen C., Triozzi J.L., Rowan B., et al. Genome-wide association study of CKD progression. J Am Soc Nephrol. 2023;34:1547–1559. doi: 10.1681/ASN.0000000000000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCallum L, Lip S, McConnachie A, et al. UMOD genotype-blinded trial of ambulatory blood pressure response to torasemide. Hypertension. 2024;81:2049–2059. doi: 10.1161/HYPERTENSIONAHA.124.23122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study Design and Methodology. Supplementary References. Figure S1. Position of the genotyped variants rs12917707, rs4293393, and rs12446492 along the adjacent UMOD and PDILT genes on chromosome 16 and reported GWAS associated as found in the GWAS catalogue (https://www.ebi.ac.uk/gwas/home). Figure S2. Distribution of urinary UMOD levels. Figure S3. Linkage disequilibrium map of the UMOD-PDILT locus based on 1000 Genomes Project summary data for African (n = 661) and European (n = 502) populations. Figure S4. Comparison of absolute urinary uromodulin concentrations levels according to genotypes at rs4293393 (UMOD), rs12917707 (UMOD), and rs12446492 (PDILT) in ● Black and ○ White adults from the African-PREDICT study; and ● Black adults from the PURE study. Figure S5. Comparison of urinary uromodulin/creatinine levels in individuals homozygous for haplotypes defined by rs4293393 (UMOD), rs12917707 (UMOD), and rs12446492 (PDILT) in ● Black and ○ White adults from the African-PREDICT study. Figure S6. Determinants of urinary uromodulin in the PURE-NWP-SA study. Table S1. Pairwise linkage disequilibrium data between UMOD/PDILT SNPs in African and European populations from the 1000 Genomes Project and the African-PREDICT cohort. Table S2. Pearson correlations of possible determinants of uromodulin in the African-PREDICT cohort. Table S3. Pearson correlations of possible determinants of uromodulin in the PURE cohort. Table S4. Multiple regression analyses with urinary uromodulin/creatinine as the main dependent variable in the African-PREDICT cohort. Table S5. Multiple regression analyses with urinary uromodulin/creatinine as the main dependent variable in the PURE cohort. Table S6. Multiple regression with SNPs separately entered into models in the PURE cohort. Strega Checklist.