Abstract
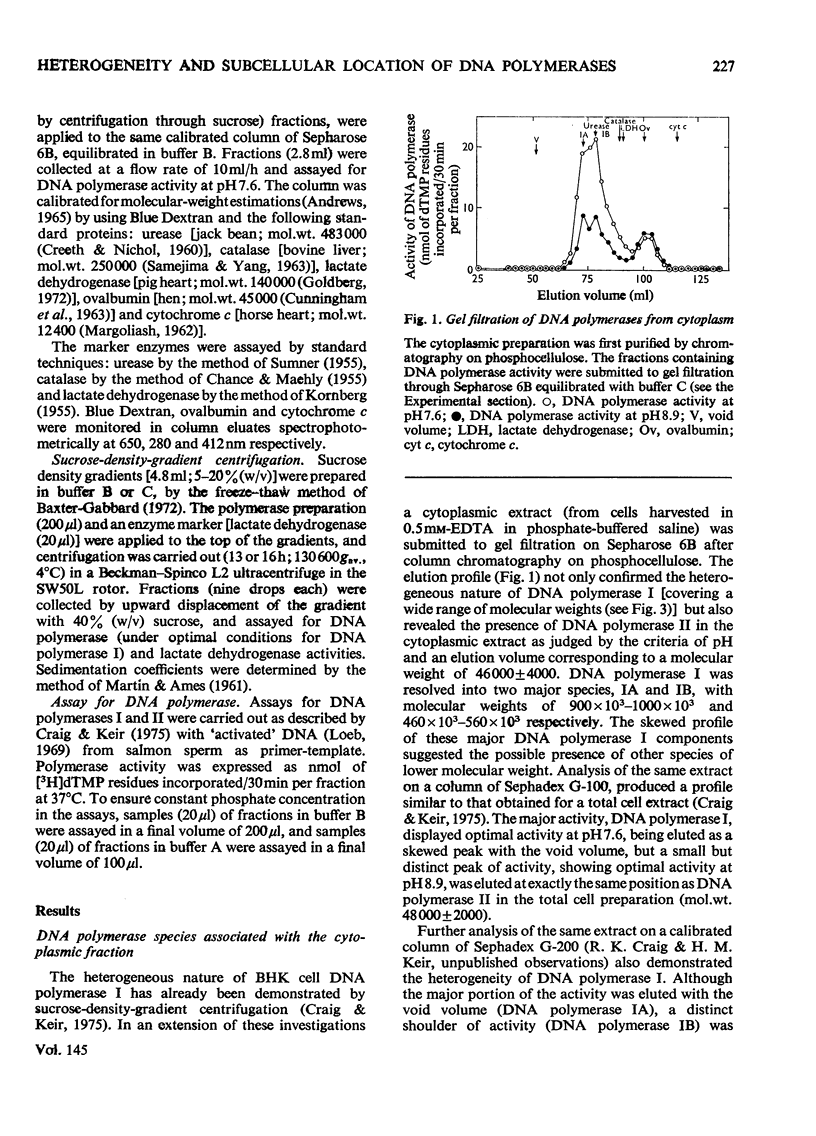
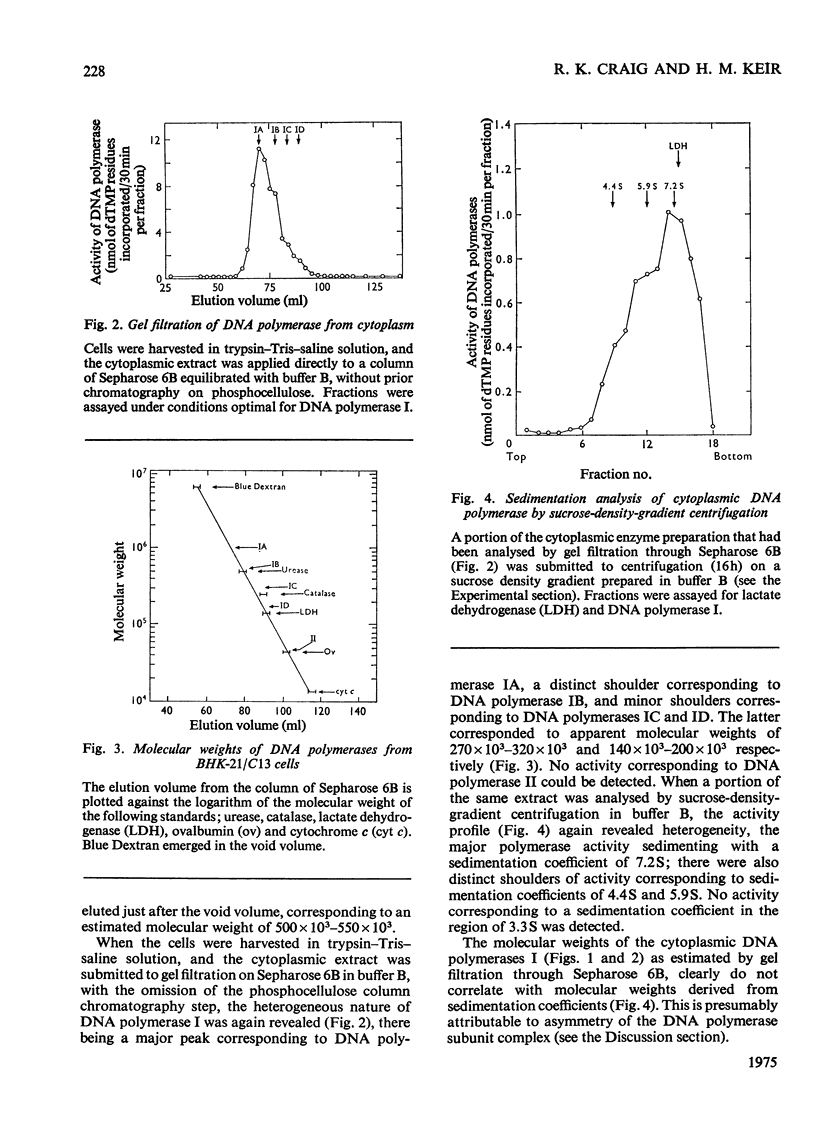
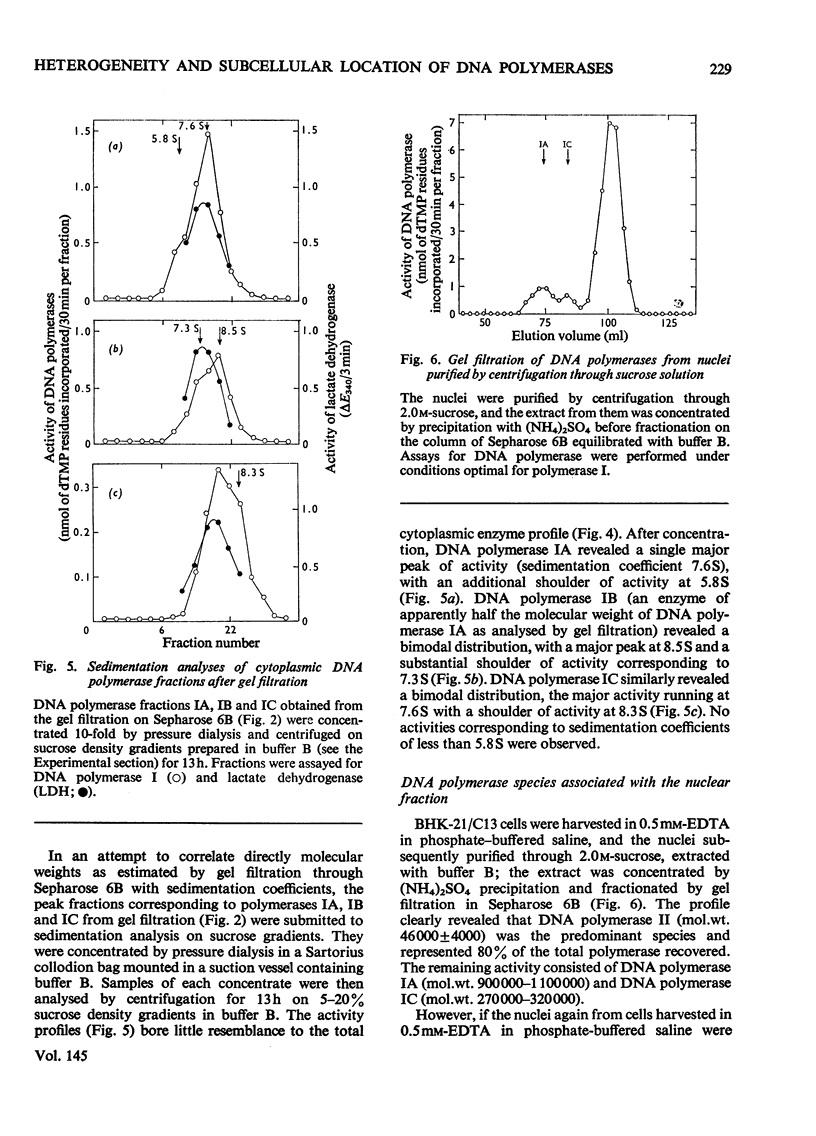
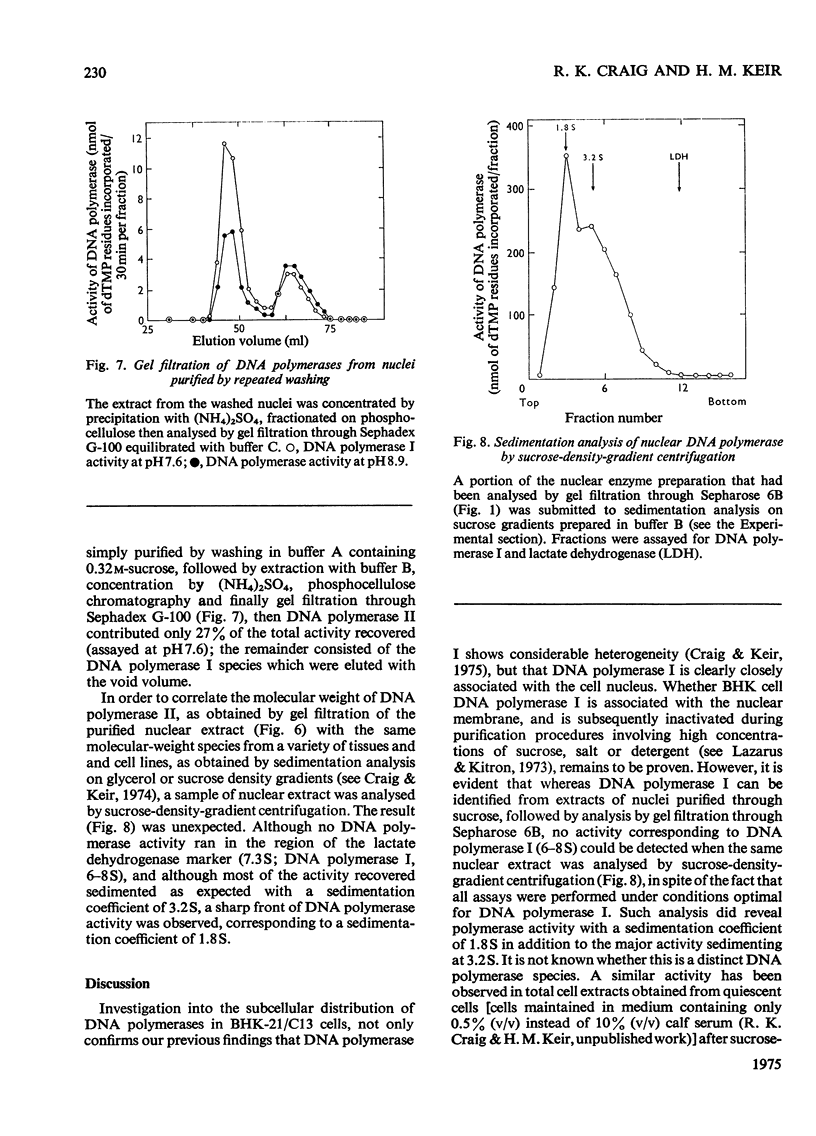
Nuclear and cytoplasmic fractions were prepared from exponentially-growing BHK-21/C13 cells; DNA polymerase was extracted from them and analysed by gel filtration and sucrose-density-gradient centrifugation. DNA polymerase I is heterogeneous comprising species covering a considerable range of molecular weights. These have been tentatively identified as four subspecies of apparent molecular weights 900000-1000000 (IA), 460000-560000 (IB), 270000-320000 (IC) and 140000-200000 (ID), as assessed by gel filtration through Sepharose 6B. DNA polymerase II has a mol.wt. of 46000 +/- 4000 as assessed by gel filtration on Sepharose 6B, and 48000 +/- 2000 as assessed by gel filtration on Sephadex G-100. Sedimentation analyses on sucrose density gradients showed that the DNA polymerase I species had sedimentation coefficients predominantly in the range 6-8 S. DNA polymerase II had predominantly a sedimentation coefficient of 3.2 S although a component with lower sedimentation coefficient was found. The lack of correlation between the molecular weights derived from gel filtration and the sedimentation coefficients is attributed to molecular asymmetry. DNA polymerase I was found to be associated predominantly with the cytoplasm although certain types of nuclear preparation contained large amounts of it. DNA polymerase II was found to be mostly if not exclusively in nuclear preparations.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams R. L., Henderson M. A., Wood W., Lindsay J. G. Multiple forms of nuclear deoxyribonucleic acid polymerases and their relationship with the soluble enzyme. Biochem J. 1973 Feb;131(2):237–246. doi: 10.1042/bj1310237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter-Gabbard K. L. A simple method for the large-scale preparation of sucrose gradients. FEBS Lett. 1972 Jan 15;20(1):117–119. doi: 10.1016/0014-5793(72)80031-0. [DOI] [PubMed] [Google Scholar]
- Brun G., Rougeon F., Lauber M., Chapeville F. Purification and properties of DNA polymerases from chick embryo. Eur J Biochem. 1974 Jan 16;41(2):241–251. doi: 10.1111/j.1432-1033.1974.tb03265.x. [DOI] [PubMed] [Google Scholar]
- CREETH J. M., NICHOL L. W. Evidence for the chemical interaction of urease in solution. Biochem J. 1960 Nov;77:230–239. doi: 10.1042/bj0770230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUNNINGHAM L. W., CLOUSE R. W., FORD J. D. HETEROGENEITY OF THE CARBOHYDRATE MOIETY OF CRYSTALLINE OVALBUMIN. Biochim Biophys Acta. 1963 Oct 29;78:379–381. doi: 10.1016/0006-3002(63)91652-4. [DOI] [PubMed] [Google Scholar]
- Chang L. M. Low molecular weight deoxyribonucleic acid polymerase from calf thymus chromatin. I. Preparation of homogeneous enzyme. J Biol Chem. 1973 Jun 10;248(11):3789–3795. [PubMed] [Google Scholar]
- Craig R. K., Costello P. A., Keir H. M. Dexyribonucleic acid polymerases of BHK-21/C13cells. Relationship to the physiological state of the cells, and to synchronous indution of synthesis of deoxyribonuleic acid. Biochem J. 1975 Feb;145(2):233–240. doi: 10.1042/bj1450233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig R. K., Keir H. M. Deoxyribonucleic acid polymerases of BHK-21/C13 cells. Partial purification and characterization of the enzymes. Biochem J. 1975 Feb;145(2):215–224. doi: 10.1042/bj1450215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg E. Amino acid composition and properties of crystalline lactate dehydrogenase X from mouse testes. J Biol Chem. 1972 Apr 10;247(7):2044–2048. [PubMed] [Google Scholar]
- Haines M. E., Wickremasinghe R. G., Johnston I. R. Purification and partial characterisation of rat-liver nuclear DNA polymerase. Eur J Biochem. 1972 Nov 21;31(1):119–129. doi: 10.1111/j.1432-1033.1972.tb02508.x. [DOI] [PubMed] [Google Scholar]
- Harm K., Hilz H. A DNA polymerase from Ehrlich ascites tumor cells with preference for native DNA. Hoppe Seylers Z Physiol Chem. 1971 Nov;352(11):1469–1479. doi: 10.1515/bchm2.1971.352.2.1469. [DOI] [PubMed] [Google Scholar]
- Hecht N. B., Davidson D. The presence of a common active subunit in low and high molecular weight murine DNA polymerases. Biochem Biophys Res Commun. 1973 Mar 17;51(2):299–305. doi: 10.1016/0006-291x(73)91256-4. [DOI] [PubMed] [Google Scholar]
- Hecht N. B. Interconvertibility of mouse DNA polymerase activities derived from the nucleus and cytoplasm. Biochim Biophys Acta. 1973 Jul 13;312(3):471–483. doi: 10.1016/0005-2787(73)90445-0. [DOI] [PubMed] [Google Scholar]
- Holmes A. M., Johnston I. R. Molecular asymmetry of rat liver cytoplasmic DNA polymerase. FEBS Lett. 1973 Jan 1;29(1):1–6. doi: 10.1016/0014-5793(73)80002-x. [DOI] [PubMed] [Google Scholar]
- Lazarus L. H., Kitron N. Letter: Cytoplasmic DNA polymerase: polymeric forms and their conversion into an active monomer resembling nuclear DNA polymerase. J Mol Biol. 1973 Dec 25;81(4):529–534. doi: 10.1016/0022-2836(73)90522-6. [DOI] [PubMed] [Google Scholar]
- Loeb L. A. Purification and properties of deoxyribonucleic acid polymerase from nuclei of sea urchin embryos. J Biol Chem. 1969 Apr 10;244(7):1672–1681. [PubMed] [Google Scholar]
- MARGOLIASH E. Amino acid sequence of chymotryptic peptides from horse heart cytochrome c. J Biol Chem. 1962 Jul;237:2161–2174. [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- SAMEJIMA T., YANG J. T. RECONSTITUTION OF ACID-DENATURED CATALASE. J Biol Chem. 1963 Oct;238:3256–3261. [PubMed] [Google Scholar]
- Sedwick W. D., Wang T. S., Korn D. Purification and properties of nuclear and cytoplasmic deoxyribonucleic acid polymerases from human KB cells. J Biol Chem. 1972 Aug 25;247(16):5026–5033. [PubMed] [Google Scholar]
- Tanabe K., Takahashi T. Conversion of DNA polymerase extracted from rat ascites hepatoma cells. Biochem Biophys Res Commun. 1973 Jul 2;53(1):295–301. doi: 10.1016/0006-291x(73)91433-2. [DOI] [PubMed] [Google Scholar]
- Wallace P. G., Hewish D. R., Venning M. M., Burgoyne L. A. Multiple forms of mammalian deoxyribonucleic acid polymerase. An attempt to relate their interactions with nuclei and free deoxyribonucleic acid in vitro with their possible functions in vivo. Biochem J. 1971 Nov;125(1):47–54. doi: 10.1042/bj1250047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T. S., Sedwick W. D., Korn D. Nuclear deoxyribonucleic acid polymerase. Purification and properties of the homogeneous enzyme from human KB cells. J Biol Chem. 1974 Feb 10;249(3):841–850. [PubMed] [Google Scholar]
- Wicha M., Stockdale F. E. DNA-dependent DNA polymerases in differentiating embryonic muscle cells. Biochem Biophys Res Commun. 1972 Sep 5;48(5):1079–1087. doi: 10.1016/0006-291x(72)90819-4. [DOI] [PubMed] [Google Scholar]


