Abstract
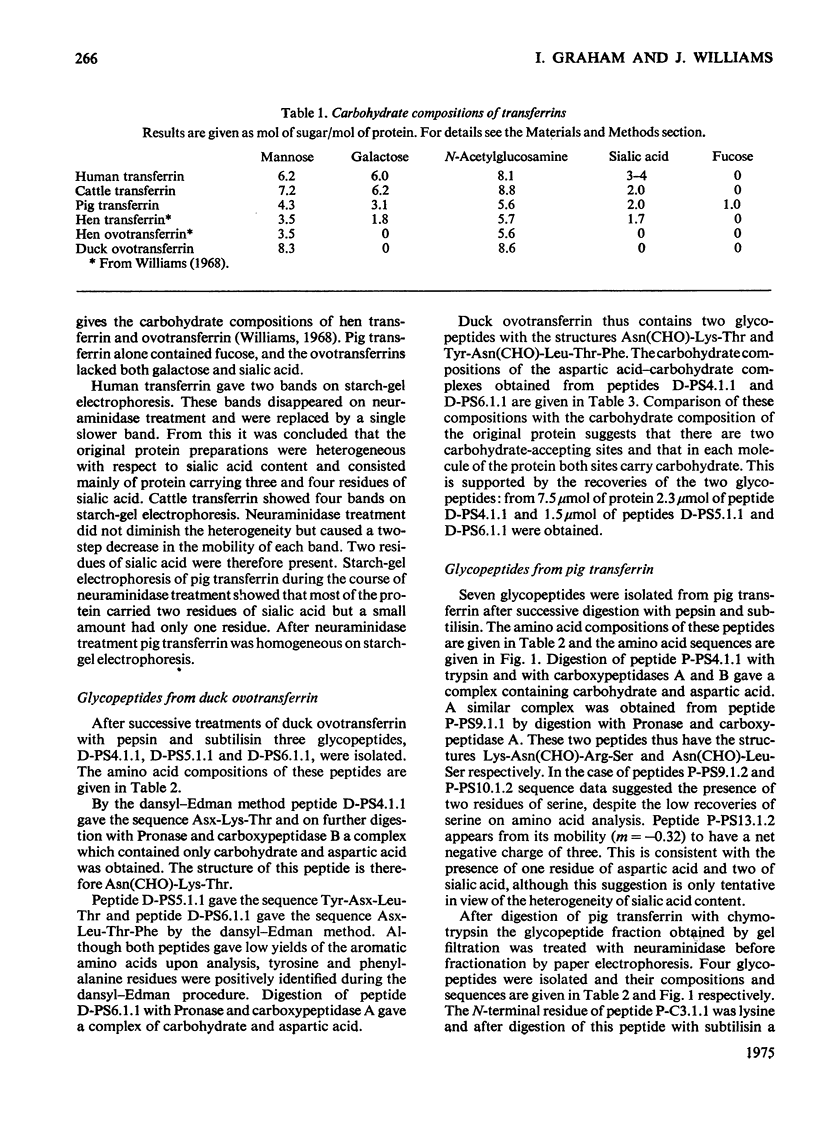
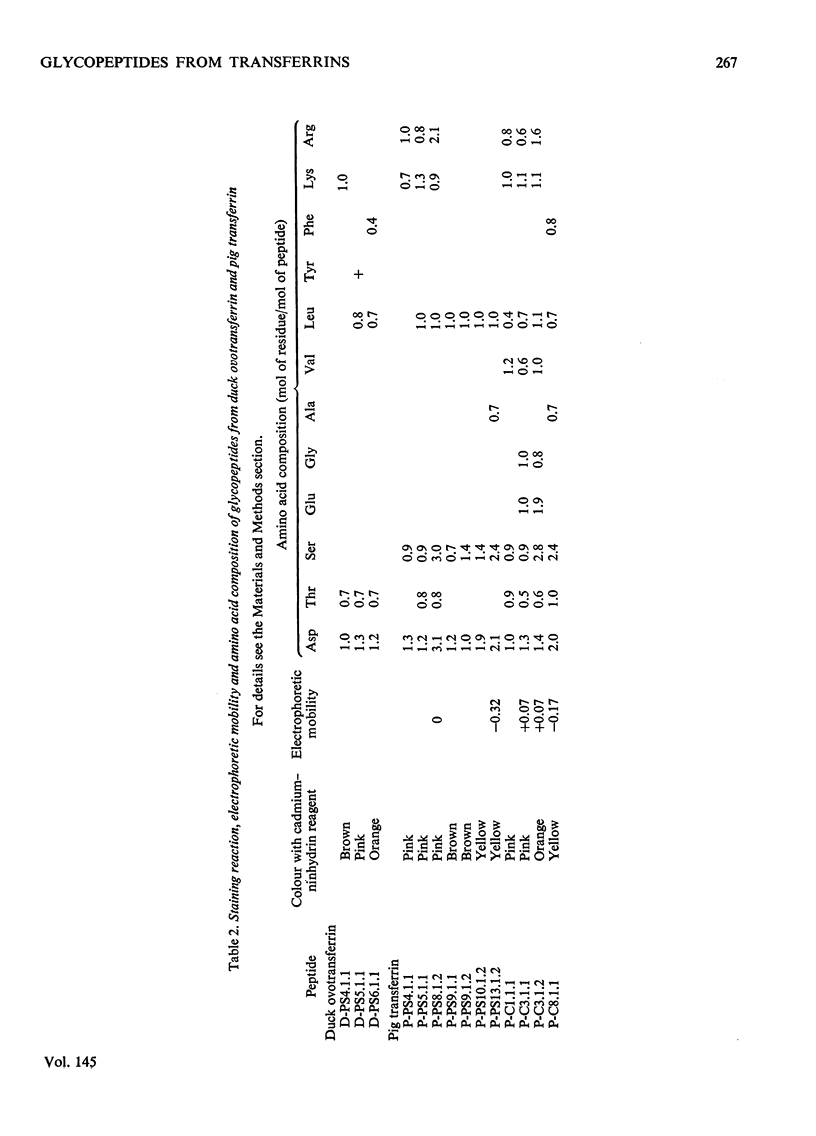
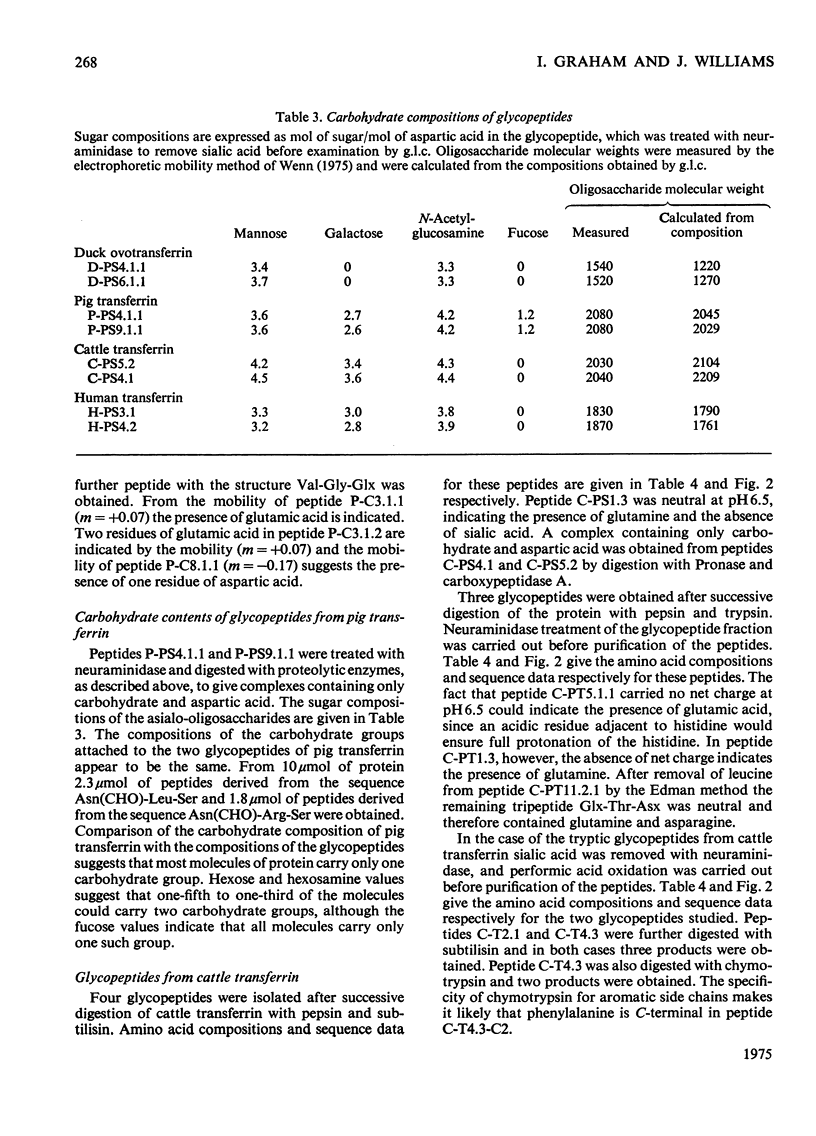
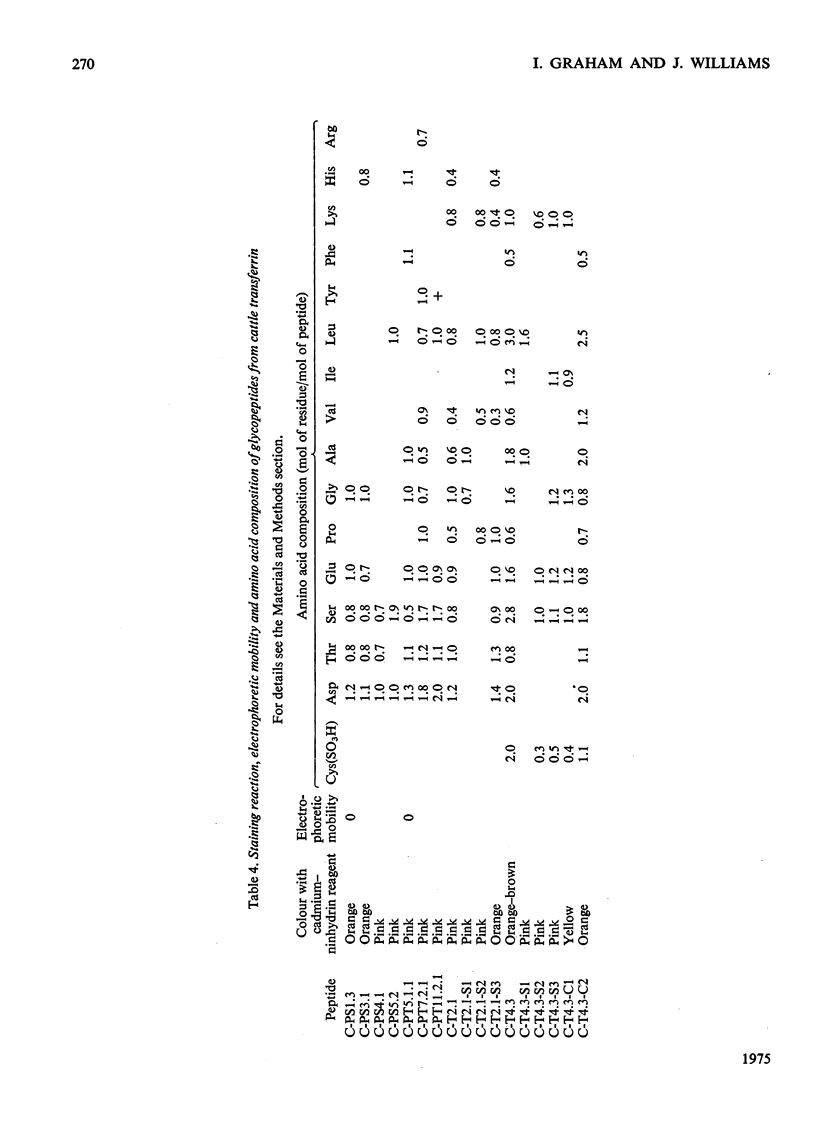
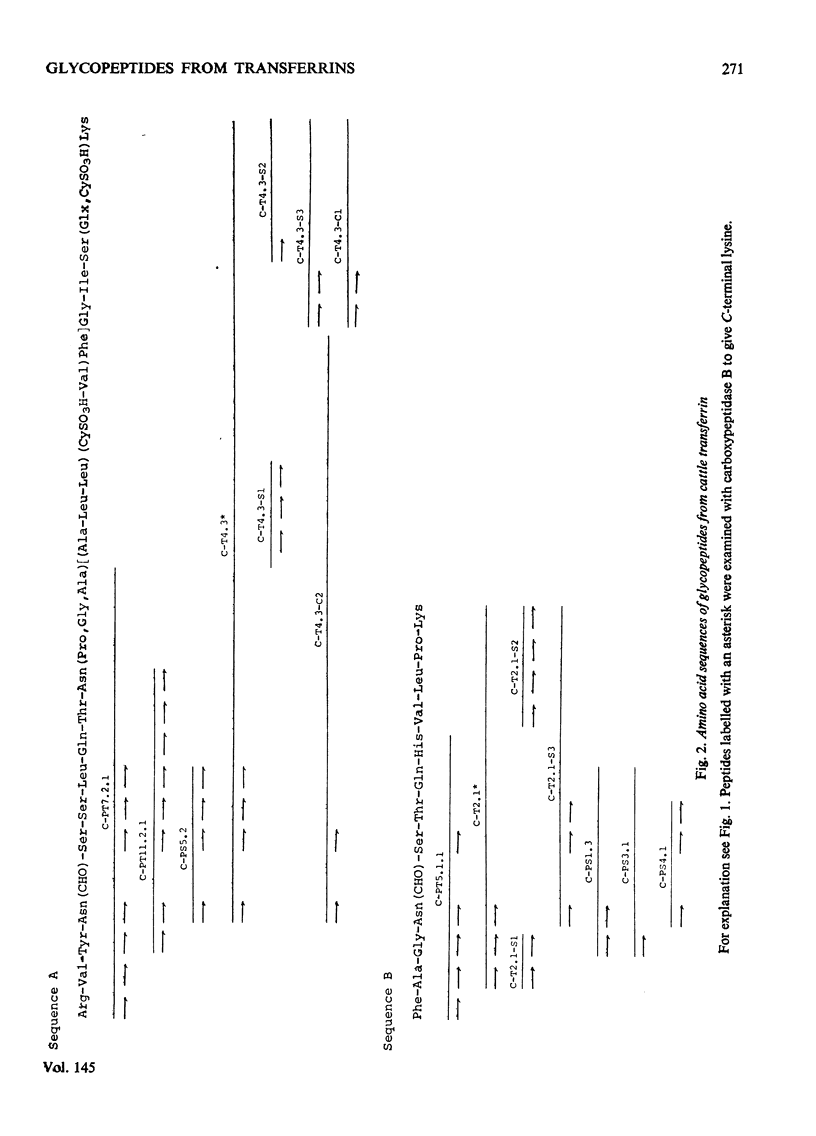
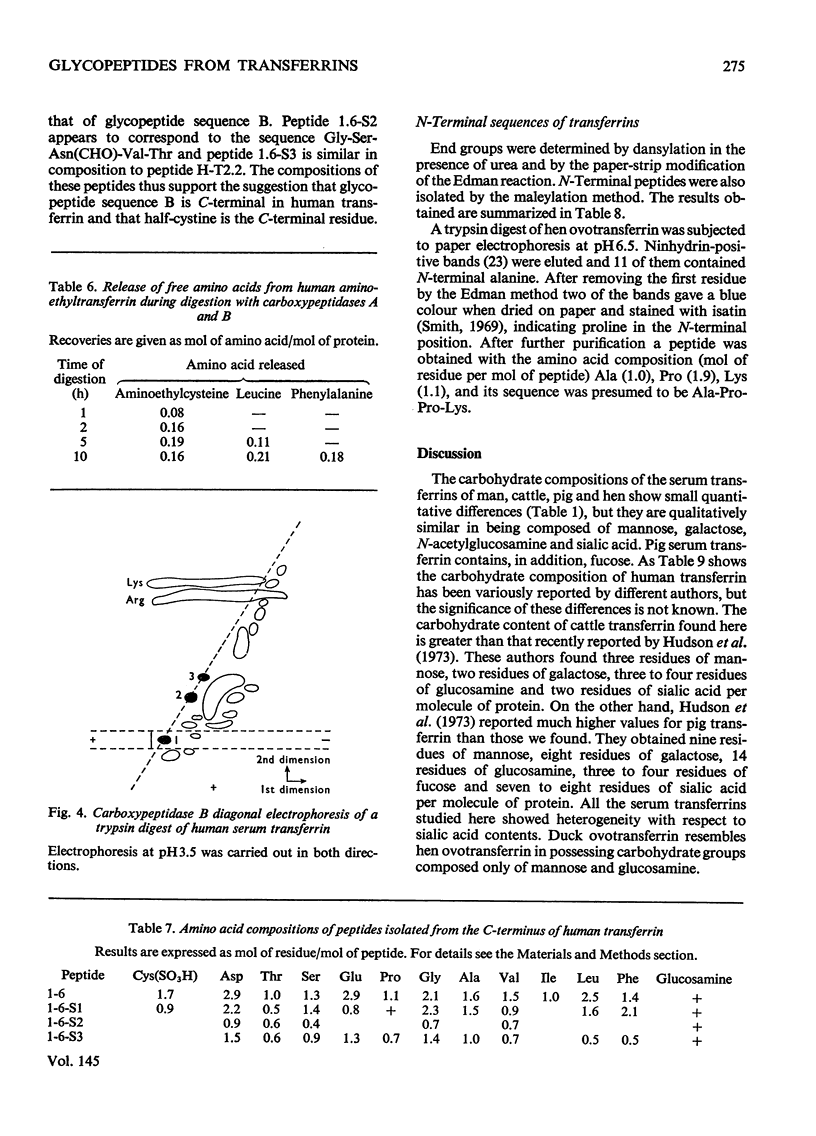
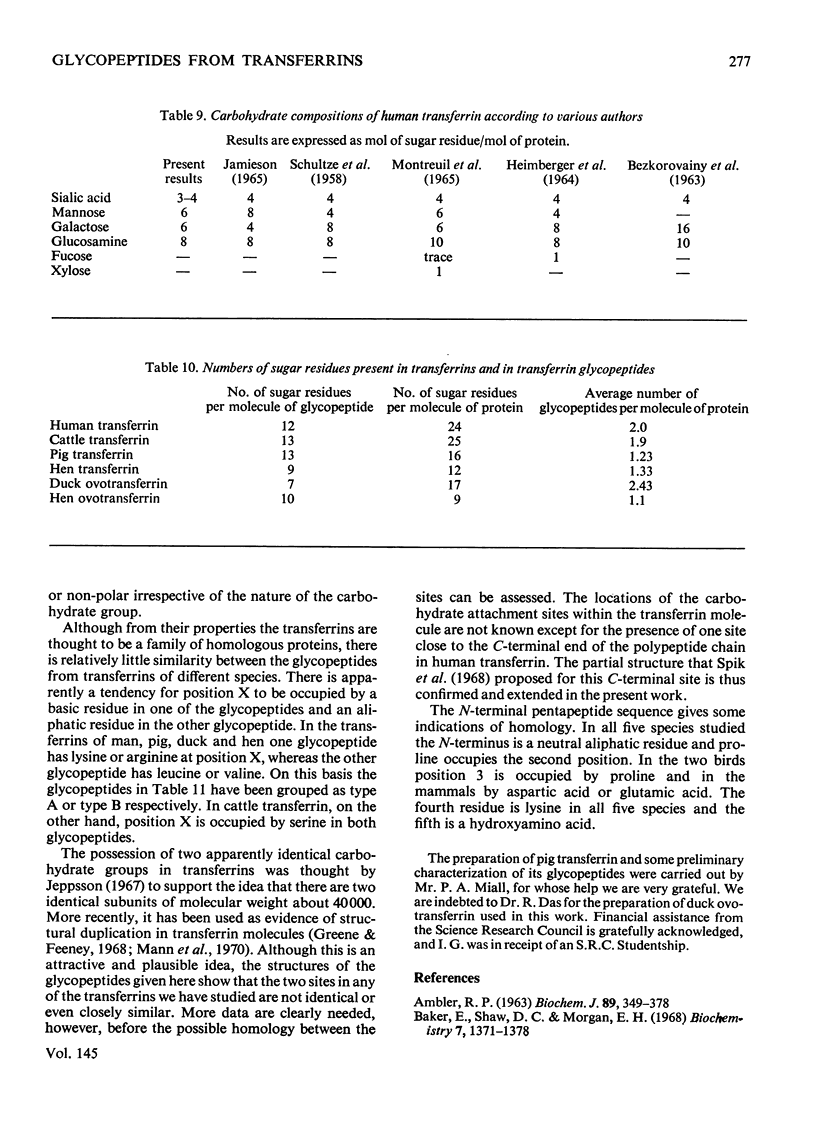
1. The carbohydrate compositions of human, pig and cattle transferrins and duck ovotransferrin have been determined. 2. Glycopeptides have been prepared from these transferrins and their carbohydrate compositions and amino acid sequences determined. One of the glycopeptides from human transferrin carries the C-terminal residue of the protein. 3. Each tranferrrin yielded two glycopeptides that appeared to be identical in carbohydrate composition but different in amino acid sequence. The two glycopeptides have been distinguished as type A, in which the residue following Asn(CHO)(where CHO represents a carbohydrate moiety) is a basic amino acid and type B in which Asn(CHO) is followed by a neutral aliphatic amino acid. Cattle transferrin is exceptional in having two glycopeptides in which this position is occupied by serine. 4. It is suggested that each molecule of human and cattle transferrin and duck ovotransferrin carries an average of two carbohydrate prosthetic groups. Hen and pig transferrins appear to carry only one carbohydrate group per mol of protein. 5. The N-terminal sequences of hen and duck ovotransferrins and of cattle, human and pig transferrins were also determined.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMBLER R. P. THE AMINO ACID SEQUENCE OF PSEUDOMONAS CYTOCHROME C-551. Biochem J. 1963 Nov;89:349–378. doi: 10.1042/bj0890349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEZKOROVAINY A., RAFELSON M. E., Jr, LIKHITE V. ISOLATION AND PARTIAL CHARACTERIZATION OF TRANSFERRIN FROM NORMAL HUMAN PLASMA. Arch Biochem Biophys. 1963 Dec;103:371–378. doi: 10.1016/0003-9861(63)90427-2. [DOI] [PubMed] [Google Scholar]
- Baker E., Shaw D. C., Morgan E. H. Isolation and characterization of rabbit serum and milk transferrins. Evidence for difference in sialic acid content only. Biochemistry. 1968 Apr;7(4):1371–1378. doi: 10.1021/bi00844a019. [DOI] [PubMed] [Google Scholar]
- Barnett D. R., Lee T. H., Bowman B. H. Amino-acid sequence of the carboxyl terminal octapeptide of human haptoglobin beta chain. Nature. 1970 Mar 7;225(5236):938–939. doi: 10.1038/225938a0. [DOI] [PubMed] [Google Scholar]
- Bruton C. J., Hartley B. S. Chemical studies on methionyl-tRNA synthetase from Escherichia coli. J Mol Biol. 1970 Sep 14;52(2):165–178. doi: 10.1016/0022-2836(70)90023-9. [DOI] [PubMed] [Google Scholar]
- Charet P., Monsigny M., Spik G., Montreuil J. Etudes sur les glycoprotéines. Etude des séquences peptidiques de deux glycopeptides isolés d'hydrolysats trypsiques de la transferrine humaine. C R Acad Sci Hebd Seances Acad Sci D. 1969 Sep 15;269(11):1019–1022. [PubMed] [Google Scholar]
- Crowshaw K., Jessup S. J., Ramwell P. W. Thin-layer chromatography of 1-dimethylaminonaphthalene-5-sulphonyl derivatives of amino acids present in superfusates of cat cerebral cortex. Biochem J. 1967 Apr;103(1):79–85. doi: 10.1042/bj1030079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eylar E. H. On the biological role of glycoproteins. J Theor Biol. 1966 Jan;10(1):89–113. doi: 10.1016/0022-5193(66)90179-2. [DOI] [PubMed] [Google Scholar]
- FRAENKEL-CONRAT H., HARRIS J. I., LEVY A. L. Recent developments in techniques for terminal and sequence studies in peptides and proteins. Methods Biochem Anal. 1955;2:359–425. doi: 10.1002/9780470110188.ch12. [DOI] [PubMed] [Google Scholar]
- Greene F. C., Feeney R. E. Physical evidence for transferrins as single polypeptide chains. Biochemistry. 1968 Apr;7(4):1366–1371. doi: 10.1021/bi00844a018. [DOI] [PubMed] [Google Scholar]
- HEIMBURGER N., HEIDE K., HAUPT H., SCHULTZE H. E. BAUSTEINANALYSEN VON HUMANSERUMPROTEINEN. Clin Chim Acta. 1964 Oct;10:293–307. doi: 10.1016/0009-8981(64)90059-2. [DOI] [PubMed] [Google Scholar]
- Hudson B. G., Ono M., Brockway W. J., Castellino F. J. Chemical and physical properties of serum transferrins from several species. Biochemistry. 1973 Mar 13;12(6):1047–1053. doi: 10.1021/bi00730a005. [DOI] [PubMed] [Google Scholar]
- JAMIESON G. A. STUDIES ON GLYCOPROTEINS. II. ISOLATION OF THE CARBOHYDRATE CHAINS OF HUMAN TRANSFERRIN. J Biol Chem. 1965 Jul;240:2914–2920. [PubMed] [Google Scholar]
- Jackson R. L., Hirs C. H. The primary structure of porcine pancreatic ribonuclease. I. The distribution and sites of carbohydrate attachment. J Biol Chem. 1970 Feb 10;245(3):624–636. [PubMed] [Google Scholar]
- Jeppsson J. O. Subunits of human transferrin. Acta Chem Scand. 1967;21(7):1686–1694. doi: 10.3891/acta.chem.scand.21-1686. [DOI] [PubMed] [Google Scholar]
- Jett M., Jamieson G. A., DeBernardo S. L. The carbohydrate sequence of the glycopeptide chains of human transferrin. J Biol Chem. 1971 Jun 10;246(11):3686–3693. [PubMed] [Google Scholar]
- Leibman A. J., Aisen P. Preparation of single crystals of transferrin. Arch Biochem Biophys. 1967 Sep;121(3):717–719. doi: 10.1016/0003-9861(67)90058-6. [DOI] [PubMed] [Google Scholar]
- Mann K. G., Fish W. W., Cox A. C., Tanford C. Single-chain nature of human serum transferrin. Biochemistry. 1970 Mar 17;9(6):1348–1354. doi: 10.1021/bi00808a008. [DOI] [PubMed] [Google Scholar]
- Montreuil J., Spik G., Monsigny M., Descamps J., Biserte G., Dautrevaux M. Etude comparée de la composition en oses et en amino-acides de la transferrine et de la lactotransferrine humaines. Experientia. 1965 May 15;21(5):254–256. doi: 10.1007/BF02297009. [DOI] [PubMed] [Google Scholar]
- NAUGHTON M. A., HAGOPIAN H. Some applications of two-dimensional ionophoresis. Anal Biochem. 1962 Apr;3:276–284. doi: 10.1016/0003-2697(62)90111-2. [DOI] [PubMed] [Google Scholar]
- Offord R. E. Electrophoretic mobilities of peptides on paper and their use in the determination of amide groups. Nature. 1966 Aug 6;211(5049):591–593. doi: 10.1038/211591a0. [DOI] [PubMed] [Google Scholar]
- PARKER W. C., BEARN A. G. Studies on the transferrins of adult serum, cord serum, and cerebrospinal fluid. The effect of neuraminidase. J Exp Med. 1962 Jan 1;115:83–105. doi: 10.1084/jem.115.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POULIK M. D. Starch gel electrophoresis in a discontinous system of buffers. Nature. 1957 Dec 28;180(4600):1477–1479. doi: 10.1038/1801477a0. [DOI] [PubMed] [Google Scholar]
- Richardson N. E., Buttress N., Feinstein A., Stratil A., Spooner R. L. Structural studies on individual components of bovine transferrin. Biochem J. 1973 Sep;135(1):87–92. doi: 10.1042/bj1350087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roop W. E., Putnam F. W. Purification and properties of human transferrin C and a slow moving genetic variant. J Biol Chem. 1967 May 25;242(10):2507–2513. [PubMed] [Google Scholar]
- SCHULTZE H. E., SCHMIDTBERGER R., HAUPT H. Untersuchungen über die gebundenen Kohlenhydrate in isolierten Plasmaproteiden. Biochem Z. 1958;329(6):490–507. [PubMed] [Google Scholar]
- Spik G., Monsigny M., Montreuil J. Etudes sur les glycoprotéines. XXV. Les acides aminés C-terminaux de la transferrine et de la lactotransferrine humaines. Bull Soc Chim Biol (Paris) 1968;50(11):2186–2187. [PubMed] [Google Scholar]
- Stratil A., Spooner R. L. Isolation and properties of individual components of cattle transferrin: the role of sialic acid. Biochem Genet. 1971 Aug;5(4):347–365. doi: 10.1007/BF00485861. [DOI] [PubMed] [Google Scholar]
- Van Orden H. O., Carpenter F. H. Hydrolysis of phenylthiohydantoins of amino acids. Biochem Biophys Res Commun. 1964;14:399–403. doi: 10.1016/0006-291x(64)90075-0. [DOI] [PubMed] [Google Scholar]
- WILLIAMS J. A comparison of conalbumin and transferrin in the domestic fowl. Biochem J. 1962 May;83:355–364. doi: 10.1042/bj0830355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenn R. V. The electrophoretic mobilities of 5-dimethylaminoaphthalene-1-suphonyl-glycopeptides and their relation molecular weight. Biochem J. 1975 Feb;145(2):281–285. doi: 10.1042/bj1450281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. A comparison of glycopeptides from the ovotransferrin and serum transferrin of the hen. Biochem J. 1968 Jun;108(1):57–67. doi: 10.1042/bj1080057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods K. R., Wang K. T. Separation of dansyl-amino acids by polyamide layer chromatography. Biochim Biophys Acta. 1967 Feb 21;133(2):369–370. doi: 10.1016/0005-2795(67)90078-5. [DOI] [PubMed] [Google Scholar]


