Introduction
Autosomal recessive polycystic kidney disease (ARPKD) is a rare, potentially fatal genetic disorder affecting approximately 1 in 20,000 children. ARPKD is characterized by diffuse renal microcysts that become more prominent over time resulting in early kidney failure in 50% of children with ARPKD.1 Unfortunately, there are currently no treatments to prevent ARPKD kidney disease progression. One of the key challenges to developing and evaluating potential therapies for ARPKD is the lack of effective biomarkers to identify patients at high risk of progression and to assess kidney disease progression during a clinical trial.1,2 Except for the most severely affected infants, the rates of glomerular filtration rate decline are highly variable in patients with ARPKD.1,3,4 Further, total kidney volume, which has been extensively validated as a clinical biomarker for autosomal dominant polycystic kidney disease, is unfortunately not a useful biomarker for ARPKD kidney disease, because the kidneys of patients with ARPKD stabilize in size despite progressive disease.5 Further, published studies demonstrate that there is no relationship between total kidney volume and the rate of estimated glomerular filtration rate (eGFR) decline in ARPKD.4 As such, improved biomarkers are still needed to sensitively and reliably detect and stratify ARPKD kidney disease.
Building on our previous work in rodent models of ARPKD,6,7 the overall goal of this first-in-patients-with-ARPKD pilot study was to determine the capability of 3 novel quantitative MRI kidney imaging biomarkers: T1 and T2 relaxation time maps of kidney cystic burden from magnetic resonance fingerprinting (MRF)8 and kidney cortical perfusion from arterial spin labeling (ASL)9 to assess kidney disease in patients with ARPKD across the spectrum of disease severity in comparison to healthy young adult volunteers.
Results
In this initial study, patients with ARPKD (n = 13, 5 males and 8 females, aged 6–22 years) were evaluated both as a single cohort as well as 2 subgroups based on kidney function: (i) early chronic kidney disease (CKD) (n = 7; median eGFR = 98 ml/min per 1.73 m2, range = 91–109 ml/min per 1.73 m2), and (ii) mild to moderate CKD (n = 6; median eGFR = 74 ml/min per 1.73 m2, range = 52–87 ml/min per 1.73 m2) (Table 1). Patients with ARPKD in the mild to moderate CKD group were slightly older as expected given the progressive nature of the disease (median age = 12 vs. 7 years). The majority of patients with ARPKD had hypertension (11/13) and were receiving angiotensin-converting enzyme inhibitor or angiotensin receptor blocker therapy as typical (9/13). Proteinuria was minimal for all ARPKD subjects (range = 0.09–0.2 mg/mg). Healthy young adult volunteers reported no known history of kidney disease (n = 8, aged 18–36 years). Additional information on the study methods is included in the Supplementary Materials.
Table 1.
ARPKD subject demographics and clinical data
| Metric | All ARPKD subjects | ARPKD subjects with early CKD | ARPKD subjects with mild to moderate CKD |
|---|---|---|---|
| Subjects (gender distribution) | 13 (5M/8F) | 7 (2M/5F) | 6 (3M/3F) |
| Age: median (range) | 10 (6–22) yr | 7 (6–22) yr | 12 (6–21) yr |
| eGFR: median (range) | 91 (52–109) ml/min per 1.73 m2 | 98 (91–109) ml/min per 1.73 m2 | 74 (52–87) ml/min per 1.73 m2 |
| Subjects with systemic hypertension | 11 | 5 | 6 |
| Subjects prescribed ACE/ARB | 9 | 4 | 5 |
| Urine protein-to-creatinine ratio: median (range) | 0.1 (0.09–0.2) mg/mg | 0.13 (0.1–0.2) mg/mg | 0.1 (0.09–0.2) mg/mg |
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; ARPKD, autosomal recessive polycystic disease; eGFR, estimated glomerular filtration rate; F, female; M, male.
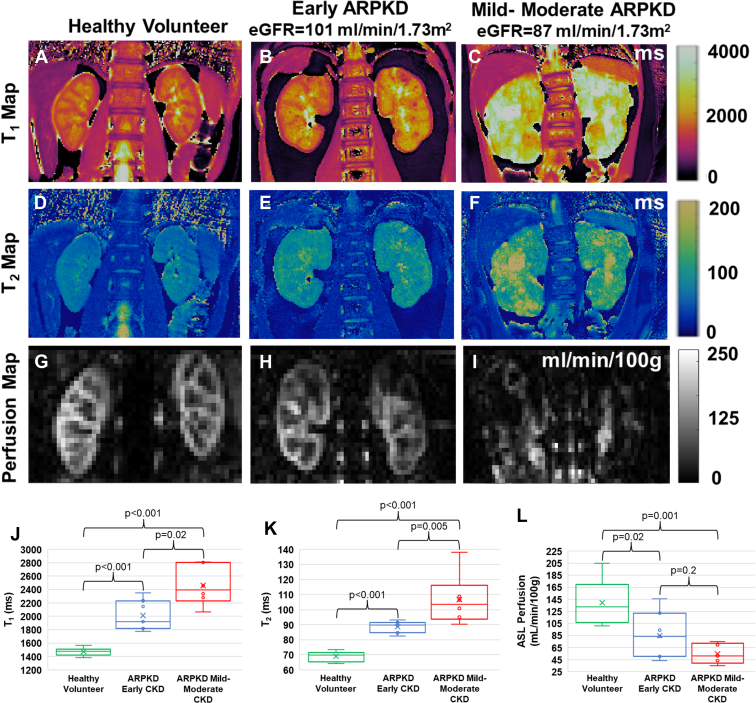
In Figure 1, we show representative kidney T1 (a–c) and T2 (d–f) maps (obtained from novel MRF8), and kidney perfusion maps (g–i) (obtained from kidney ASL9) for a healthy volunteer (left column) and 2 patients with ARPKD (middle and right columns). The patients with ARPKD kidneys show visible increases in T1 and T2, consistent with increased kidney cystic burden as well as decreases in kidney perfusion in comparison to each other as well as the healthy volunteer. Importantly, the ARPKD subject with mild to moderate CKD (right column) shows visibly increased kidney T1 and T2 values and decreased perfusion as compared to the ARPKD subject with early CKD (middle column) despite relatively similar eGFR (87 vs. 101 ml/min per 1.73 m2, respectively). These initial observations suggest that differences in T1, T2 and/or perfusion may be able to sensitively identify and measure early disease and differentiate early from more severe disease, even in patients with relatively intact kidney function.
Figure 1.
Multimodal imaging in patients with ARPKD and healthy volunteers. Representative kidney (a–c) T1, (d–f) T2, and (g–i) perfusion maps from a healthy volunteer (left column) and 2 pediatric patients with ARPKD (middle and right columns). (J–L) Box and Whisker plots for mean kidney (j) T1, (k) T2, and (l) perfusion from 8 healthy volunteers and 13 patients with ARPKD divided into 2 cohorts: early CKD (eGFR ≥ 90 ml/min per 1.73 m2, n = 7) and mild to moderate CKD (eGFR 52–89 ml/min per 1.73 m2, n = 6). MRI assessments of cystic burden (mean T1 and T2) detected significant differences between the cohorts of patients with ARPKD with early and mild to moderate CKD (P = 0.02 and P = 0.005, respectively) as well as between the patients with ARPKD with early CKD and the healthy volunteers (P < 0.001). The ASL-based perfusion assessments showed a significant difference between the patients with ARPKD with early CKD and healthy volunteers (P = 0.02) but no significant difference between the early versus mild to moderate CKD ARPKD cohorts (P = 0.20). All 3 MRI biomarkers showed significant differences between the volunteers and ARPKD subjects with mild to moderate CKD (P 0.001). ARPKD, autosomal recessive polycystic kidney disease; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; MRI, magnetic resonance imaging.
A statistical comparison of the kidney MRI assessments showed significant increases in both kidney T1 and T2 as well as significantly reduced perfusion for the entire cohort of patients with ARPKD in comparison to the healthy volunteers (mean T1: 2218 vs. 1473 ms; mean T2: 97 vs. 69 ms; mean cortical perfusion: 72 vs. 139 ml/min/100 g; P 0.001 for all 3 measures) (see Supplementary Statistical Analysis section). Even with these limited cohort sizes, these multimodal MRI assessments consistently detected significant differences in mean kidney T1 and T2 as follows: (i) between the cohorts of patients with ARPKD with early and mild to moderate CKD (P = 0.02 and P = 0.005, respectively), and (ii) between the patients with ARPKD with early CKD and the healthy volunteers (P < 0.001). The ASL-based perfusion assessment showed a significant difference between the patients with ARPKD with early CKD and healthy volunteers (P = 0.02). However, ASL differences detected between the early versus mild to moderate CKD ARPKD cohorts was not significant (P = 0.20) (Figure 1 j–l). Statistical associations between the MRI biomarkers with eGFR in the patients with ARPKD (correlation coefficients for T1: r = −0.47 (P = 0.10), T2: r = −0.41 (P = 0.17), perfusion: r = 0.48 (P = 0.09), data not shown) was not significant for this initial study.
In Supplementary Figure S1, we depict a visual 3-dimensional comparison for these 3 MRI biomarkers of cystic burden (T1, T2) and perfusion for the ARPKD patient cohorts and healthy volunteers. Importantly, the multimodal MRI biomarkers, in combination, were able to stratify all 3 cohorts (2 ARPKD subgroups and the cohort of healthy volunteers) with limited overlap, demonstrating the utility of a multimodal MRI approach. Scans on consecutive days for a subset of patients with ARPKD (n = 9) revealed mean variation of 2.1% for T1, 2.8% for T2, and 16.3% for perfusion (Supplementary Figure S2). The superior precision of the kidney MRF-based T1 and T2 assessments are consistent with previous MRF studies in children and adults.8
Discussion
This pilot cross-sectional MRI study in patients with ARPKD and healthy young adult volunteers suggests that these novel MRF assessments of kidney cystic burden (i.e., mean kidney T1 and T2) and ASL MRI assessment of kidney cortical perfusion have the potential to sensitively and reproducibly stage ARPKD kidney disease. Specifically, we showed that these 3 quantitative MRI biomarkers may be capable of distinguishing ARPKD subjects with differences in cystic burden and altered perfusion despite relatively similar renal function (Figure 1 and Supplementary Figure S1). Further, when used in combination, these MRI biomarkers can: (i) stratify ARPKD CKD subgroups; and (ii) differentiate early CKD from healthy volunteers despite relatively normal kidney function (Supplementary Figure S1).
The multimodal MRI methods used were obtained in ARPKD subjects aged as young as 6 years and completed within 15 minutes of scan time with no injectable MRI contrast agent or sedation. Despite the small cohort size of this pilot cross-sectional study, our findings suggest that these 3 MRI biomarkers (alone or in combination) have the potential to be used in a future ARPKD clinical trial design to: (i) identify patients with relatively intact renal function but at high risk for rapid disease progression, and/or (ii) noninvasively assess response to a new therapeutic intervention. Importantly, a larger, longitudinal study is already underway to validate these initial findings and determine the sensitivity of these MRI biomarkers to detect disease progression.
Disclosure
CJM, MEK, JP, SF, MM, DM, YC, and CAF report support from Siemens Healthineers. KMD serves as a site investigator for 2 clinical trials supported by Otsuka Pharmaceuticals. All the other authors declared no competing interests.
Acknowledgments
This research was supported by NIH / NIDDK R01 DK114425. In addition, this work utilized the imaging capabilities for the Imaging Research Core for the Case Comprehensive Cancer Center (NIH / NCI P30 CA043703), the Digestive Diseases Research Core Center (NIH / NIDDK P30 DK097948), and the Cystic Fibrosis Foundation Research Development Program. This work was also supported by T32 GM007250, LR1 TR002549, and TL1 DK132770 and 1UM1TR004528-01.
Footnotes
Supplementary File (PDF, annotated .docx, and TIFF)
Supplementary Methods.
Supplementary References.
Figure S1. 3-dimensional visualization of multimodal MRI assessments in patients with ARPKD and healthy adult volunteers.
Figure S2. Repeatability of MRI assessments.
Supplementary Material
Supplementary Methods. Supplementary References. Figure S1. 3-dimensional visualization of multimodal MRI assessments in patients with ARPKD and healthy adult volunteers. Figure S2. Repeatability of MRI assessments.
References
- 1.Dell K.M. The spectrum of polycystic kidney disease in children. Adv Chronic Kidney Dis. 2011;18:339–347. doi: 10.1053/j.ackd.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benz E.G., Hartung E.A. Predictors of progression in autosomal dominant and autosomal recessive polycystic kidney disease. Pediatr Nephrol. 2021;36:2639–2658. doi: 10.1007/s00467-020-04869-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burgmaier K., Kilian S., Bammens B., et al. Clinical courses and complications of young adults with autosomal recessive polycystic kidney disease (ARPKD) Sci Rep. 2019;9:7919. doi: 10.1038/s41598-019-43488-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abdul Majeed N., Font-Montgomery E., Lukose L., et al. Prospective evaluation of kidney and liver disease in autosomal recessive polycystic kidney disease-congenital hepatic fibrosis. Mol Genet Metab. 2020;131:267–276. doi: 10.1016/j.ymgme.2020.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avni F.E., Guissard G., Hall M., Janssen F., DeMaertelaer V., Rypens F. Hereditary polycystic kidney diseases in children: changing sonographic patterns through childhood. Pediatr Radiol. 2002;32:169–174. doi: 10.1007/s00247-001-0624-0. [DOI] [PubMed] [Google Scholar]
- 6.Erokwu B.O., Anderson C.E., Flask C.A., Dell K.M. Quantitative magnetic resonance imaging assessments of autosomal recessive polycystic kidney disease progression and response to therapy in an animal model. Pediatr Res. 2018;83:1067–1074. doi: 10.1038/pr.2018.24. [DOI] [PubMed] [Google Scholar]
- 7.MacAskill C.J., Erokwu B.O., Markley M., et al. Multi-parametric MRI of kidney disease progression for autosomal recessive polycystic kidney disease: mouse model and initial patient results. Pediatr Res. 2021;89:157–162. doi: 10.1038/s41390-020-0883-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacAskill C.J., Markley M., Farr S., et al. Rapid B1-insensitive MR fingerprinting for quantitative kidney imaging. Radiology. 2021;300:380–387. doi: 10.1148/radiol.2021202302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alhummiany B.A., Shelley D., Saysell M., et al. Bias and precision in magnetic resonance imaging-based estimates of renal blood flow: assessment by triangulation. J Magn Reson Imaging. 2022;55:1241–1250. doi: 10.1002/jmri.27888. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Methods. Supplementary References. Figure S1. 3-dimensional visualization of multimodal MRI assessments in patients with ARPKD and healthy adult volunteers. Figure S2. Repeatability of MRI assessments.