Abstract
Natural as well as experimental infections with pathogenic mycoplasmas lead to cellular responses characterized by early polymorphonuclear leukocyte influx, which in turn is followed by infiltration of macrophages. Since some of the most potent leukocyte chemoattractants are macrophage products, we investigated whether the 2-kDa macrophage-activating lipopeptide (MALP-2) from Mycoplasma fermentans was capable of inducing chemoattractant chemokines and initiating an in vivo inflammatory effect. MALP-2 was a potent in vitro inducer of the chemokines macrophage inflammatory protein 1α (MIP-1α), monocyte chemoattractant protein 1 (MCP-1), and MIP-2, yielding a maximal response at 0.1 ng/ml (5 × 10−11 M). Leukocyte infiltration was determined after intraperitoneal injection of MALP-2, liposome-encapsulated MALP-2, and heat-killed mycoplasmas. There was a steady increase in the number of peritoneal cells over 72 h in response to these agents. Polymorph counts were maximal by 24 to 48 h, decreasing thereafter. Monocytes/macrophages had significantly increased after 3 days. MIP-1α, MCP-1, and MIP-2 levels in serum or peritoneal lavage fluid were determined. MIP-1α and MCP-1 levels were elevated by 2 to 6 h after injection and were still above control values after 24 h. In contrast, MIP-2 levels reached their maximum at 2 h, dropping to control values after 24 h. We conclude that macrophage-stimulating mycoplasmal lipoproteins, exemplified by MALP-2, play an important role in the late phase of phagocyte recruitment at sites of infection and that this is affected by leukoattractive chemokines.
Mycoplasmas are pleiomorphic wall-less bacteria with a minimal genome which require host cells and their products for growth in their natural habitat. Depending on the mycoplasma species and respective hosts, these organisms can occur as harmless commensals or may cause inflammatory disease states, such as atypical pneumonia, nongonococcal urethritis, mastitis, salpingitis, or arthritis (reviewed in references 3, 38, and 40). Natural (33) as well as experimental infections with pathogenic mycoplasmas in several systems (6, 17, 21, 22, 39) lead to mycoplasma-associated cellular responses, characterized by early influx of polymorphonuclear leukocytes (PMN) which is followed by infiltration of macrophages and lymphocytes. Save for one report on a membrane protein preparation from Mycoplasma pulmonis with in vitro chemoattractant properties for B lymphocytes (34), little is known to date about mycoplasmal compounds with leukocyte chemotactic properties, and nothing is known about their way of action.
We have recently described the isolation and characterization of a class of macrophage-activating compounds from two mycoplasmas, Mycoplasma fermentans (31) and Mycoplasma hyorhinis (32), species which incidentally are both arthritogenic. These macrophage-activating agents are naturally occurring lipopeptides and are similar to the classic Escherichia coli murein lipoprotein in that they carry a fatty acid-substituted N-terminal S-(2,3 bisacyloxypropyl)cysteinyl group but lack the N-acyl long-chain fatty acid of the classical bacterial lipoproteins. This feature renders these mycoplasmal compounds exceptionally active in vitro macrophage stimulators (31, 32). Since some of the most potent leukocyte chemoattractants are macrophage products, we investigated whether the 2-kDa macrophage-activating lipopeptide (MALP-2) from M. fermentans as a model compound for a typical mycoplasmal membrane lipopeptide was capable of inducing the in vitro liberation of chemoattractant chemokines and could initiate an in vivo inflammatory effect similar to that effected by mycoplasmas. We used the synthetic MALP-2 S-[2,3-bispalmitoyloxypropyl]cysteinyl-GNNDESNISFKEK, which differs from the natural compound in two aspects: it is a mixture of two optical isomers with respect to the asymmetric carbon atom in the 2 position of the bisacyloxypropyl group, and it carries no unsaturated fatty acid (31). Intraperitoneal (i.p.) injection in the mouse was chosen as an experimental system that has been used to study the effects of several mediators, including cytokines (36) and mycobacteria (2), on PMN infiltration. To simulate the natural situation optimally, MALP-2 was also incorporated into liposomes, vehicles that have been previously used either to specifically eliminate (42) or to stimulate (41) macrophages in experimental animals. Our present studies show that free as well as liposome-encapsulated MALP-2 was capable of stimulating the in vitro as well as in vivo release of the chemokines macrophage inflammatory protein 1α (MIP-1α), monocyte chemoattractant protein 1 (MCP-1), and macrophage inflammatory protein 2 (MIP-2) with concomitant early influx of PMN followed by infiltration of primarily macrophages. In view of the fact that earlier studies with synthetic analogues of bacterial lipopeptides at doses of up to 1 mg per mouse showed no rise in circulating cytokines (15), our data may be the first example of powerful in vivo responses to microgram quantities of a prototype mycoplasmal lipopeptide.
MATERIALS AND METHODS
Mycoplasma cultures.
M. fermentans clone II-29/1 from M. fermentans D15-86 (31) and clone 39 from M. fermentans PG 18 (kindly supplied by K. Wise) were grown at 37°C in a 7.5% CO2 atmosphere in GBF-3 medium consisting of bicarbonate-buffered, modified Eagle’s medium alpha, 10% heat-inactivated newborn calf serum (Sigma, Deisenhofen, Germany), 0.5% (wt/vol) Bacto Tryptone with 5 mM fructose, and 10 mg of each of the following nucleosides per liter: guanosine, cytidine, uridine, 2′-deoxyadenosine, 2′-deoxyguanosine, 2′-deoxycytidine, and 2′-deoxythymidine (31). The medium is free of endotoxin, and no precipitates are formed upon incubation. Mycoplasmas were heat killed at 95°C for 10 min and kept frozen at −20°C until use. These mycoplasmas contained 38% chloroform-methanol extractable lipid and 60% protein according to Lowry et al. (23).
Extraction of macrophage-stimulating material.
A suspension of heat-killed mycoplasmas containing about 5 mg of protein/ml was diluted 1:1 with 25 mM n-octyl-beta-d-gluco-pyranoside (octyl glucoside) (Sigma) and heated for 2 min at 100°C. Insoluble material was sedimented by centrifugation for 10 min at 11,000 × g. The supernatant solution contains a mixture of lipophilic compounds, including macrophage-stimulating lipoproteins and lipopeptides (31, 32).
Macrophage activators.
MALP-2 was synthesized as described previously (31) and kept as a stock solution of 1 mg/ml in water–2-propanol (1:1) (vol/vol) at 4°C. The exact peptide content was determined by amino acid analysis. For in vivo use, MALP-2 was diluted with isotonic saline for injection (Fresenius, Bad Homburg, Germany). For in vitro use, stock solutions were first diluted with 25 mM octyl glucoside in saline to provide a carrier and optimal solubilization (31) and were then further diluted in several steps with culture medium. The detergent (maximum final concentration in these studies, 6 μM) had no effects on the cell cultures. The biological activity of MALP-2 was tested by the nitric oxide release assay as described below. The batch of MALP-2 used in this study was half-maximally active at 9 pg/ml (compare also references 31 and 32). Lipopolysaccharide (LPS) was prepared from smooth-form Salmonella typhimurium by the phenol/water method (44).
Phospholipids and preparation of liposomes.
1,2-dipalmitoyl-l-α-phosphatidyl-ethanolamine, 1,2-dipalmitoyl-l-α-phosphatidyl-dl-glycerol, 1,2-dipalmitoyl-dl-α-phosphatidyl-serine, and cholesterol were purchased from Sigma. N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)-1,2-dihexadecanoyl-sn-glycero-3-phospho-ethanolamine, triethylammonium salt was purchased from Molecular Probes (Leiden, The Netherlands). Liposomes were prepared from a mixture of phosphatidylglycerol, phosphatidylserine, cholesterol, and phosphatidylethanolamine or, in the case of fluorescent liposomes, N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)-1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine, respectively (molar ratios, 1.08:1:0.25:0.006). The mixture was dried by rotary evaporation. For the preparation of MALP-2-containing liposomes, MALP-2 was added to the lipid mixture before drying. The dried lipids were dissolved in 0.05 M Tris-buffered 100 mM octyl glucoside in isotonic saline (pH 7) for injection and dialyzed at room temperature against a 50-fold excess of 0.1 M Tris-buffered saline. The dialysis medium was changed three times at 24-h intervals. Finally, liposomes were centrifuged at 47,800 × g for 30 min to remove unincorporated material. The liposomes were washed twice in isotonic saline and resuspended in saline. Phospholipid yields were estimated by determination of inorganic phosphate (24). MALP-2 activity was assayed by the nitric oxide release test after optimal solubilization in 25 mM octyl glucoside. This test allows the determination of macrophage-stimulating activity (MSA) and is a convenient and inexpensive semiquantitative assay (29). By the final preparation, 88% of the MSA and 84% of the lipid phosphate were recovered in the final sediment, indicating a stable incorporation or encapsulation of MALP-2 into the liposomes. The liposomes were multilamellar and round with a median size of 200 to 400 nm, as determined by electron microscopy (data not shown).
Mice.
C3H/HeJ LPS low-responder mice were obtained from Bomholtgaard (Ry, Denmark), and outbred female NMRI mice were obtained from Harlan Winkelmann (Borchen, Germany). They were under 5 months of age when used. The animals were healthy and free of ectoparasites or microbial pathogens. Histological inspection of their organs showed no symptoms of disease.
In vitro release of chemokines and cytokines from stimulated peritoneal macrophages.
Resident peritoneal exudate cells (PEC) from NMRI outbred mice were used as a source of peritoneal macrophages. Mice were asphyxiated with CO2 immediately before i.p. injection of about 3 ml of ice-cold Hanks’ balanced salt solution (HBSS) with 1% fetal calf serum (FCS). PEC were withdrawn, centrifuged in the cold, and suspended in Dulbecco’s modified Eagle’s medium containing 5% heat-inactivated FCS, 2 mM glutamine, and 2.5 × 10−5 M 2-mercaptoethanol (culture medium). Cells were seeded in 96-well flat-bottom microtiter plates at a density of 105 cells in 100-μl volumes per well and incubated overnight at 37°C in humidified 7.5% CO2 in air. The microtiter plate was vibrated on a Wellmixx 4 orbital shaker (Denley Instruments Ltd., Billingshurst, United Kingdom) for 10 s at setting 8. Nonadherent cells were removed, and the plates were washed twice with 100 μl of HBSS containing 1% FCS/well. The remaining macrophages were then supplied with 100 μl of fresh culture medium containing the indicated concentrations of the stimulators per well. Levels of chemokines and cytokines were determined from parallel cultures after the indicated times.
Determination of levels of IL-6, MIP-1α, MCP-1, and MIP-2.
Interleukin 6 (IL-6) levels were determined in a capture enzyme-linked immunosorbent assay by using the IL-6-specific monoclonal antibody (MAb) MM600C (mouse immunoglobulin G1k [IgG1k]; Endogen, Cambridge, Mass.) as a capture antibody (Ab) and a biotinylated MAb from clone 6B4 (43) (a kind gift from J. van Snick) for determination. For calculation of IL-6 activity in the samples, an authentic standard preparation of mouse recombinant IL-6 (Boehringer, Mannheim, Germany) was used. Antigenic murine MIP-1α, MCP-1, and MIP-2 concentrations were measured by using the Quantikine M immunoassay kits from R & D Systems (Wiesbaden, Germany). The assays were performed according to the manufacturer’s instructions. The detection limits for IL-6, MIP-1α, MCP-1, and MIP-2 were 1.5 ng/ml, 2.5 pg/ml, 8 pg/ml, and 4 pg/ml, respectively.
TNF-α cytotoxicity assay.
Tumor necrosis factor alpha (TNF-α) was determined in a cytotoxicity assay by using a TNF-α-sensitive L929 cell clone (C5F6) (a generous gift of C. Galanos, Freiburg, Germany) as target cells (13). Cells were plated at a density of 5 × 104 cells/well in 96-well microtiter plates and incubated for 3 h at 37°C in humidified 7.5% CO2 in air. After exposure to TNF-α for 20 h in the presence of 4 μg of actinomycin D/ml, viability of C5F6 cells was determined by staining the surviving cells with crystal violet. The TNF-α activity was calibrated by using a standard preparation of mouse recombinant TNF-α (Boehringer). The detection limit was 5 pg/ml.
Determination of MSA by nitric oxide release assay and definition of activity units.
The in vitro MSA was determined with the nitric oxide release assay as described previously (29). Briefly, resident PEC from C3H/HeJ mice served as the macrophage source. They were seeded in 96-well microtiter plates and simultaneously stimulated with recombinant gamma interferon (rIFN-γ) (a generous gift of G. R. Adolf, Ernst Boehringer Institut für Arzneimittelforschung, Vienna, Austria) and a serial dilution of macrophage-activating material. After an incubation period of 45 to 48 h, nitrate was reduced with nitrate reductase (Boehringer), and the nitric oxide level was calculated from the sum of nitrate and nitrite after the staining reaction with Griess reagent. One unit of MSA/ml is defined by the dilution yielding half-maximal nitric oxide release. Where indicated, samples were solubilized in 25 mM octyl glucoside. When synthetic MALP-2 was solubilized in this way, 1 U of MSA corresponded to 9 pg of synthetic MALP-2.
The i.p. injection of agents and identification of accumulated leukocytes.
MALP-2, liposomes containing about 0.2 mg of lipid with or without MALP-2, or heat-killed M. fermentans cells (equivalent to 0.2 mg of protein or 0.33 mg dry wt) were dissolved or suspended, respectively, in up to 0.4-ml volumes of pyrogen-free isotonic saline and i.p. injected, using groups of six mice for each treatment. Controls were injected with physiological saline (groups of three to six mice). In earlier experiments, saline containing 2% 2-propanol was used as a control vehicle. Results obtained with these controls did not differ from those obtained with saline. After various time intervals, the mice were killed by asphyxiation in CO2 and their peritoneal cavities were lavaged with 1.2 ml of HBSS containing 1% FCS and 10 U of heparin/ml. The individual peritoneal exudates were centrifuged at 300 × g for 10 min at 4°C. The peritoneal lavage fluid and the serum of each mouse were collected and stored at −20°C until assayed. PEC were resuspended to 1.5 × 106 cells/ml and differentiated histologically or analyzed by fluorescence-activated cell sorter (FACS) analysis. For histological differentiation, cells were incubated with heat-killed, opsonized Staphylococcus aureus (approximately 100 bacteria/cell) for 10 min at 37°C. The cells were washed twice with PBS–2% FCS to remove nonphagocytosed bacteria and sedimented onto microscope slides with a cytocentrifuge. The preparations were stained with Wright solution. When analyzing monocytes/macrophages in PEC from treated animals, we preferred this method rather than that using specific MAb because the expression of these markers decreases after activation (11). For FACS analysis, Fc receptors were blocked with mouse IgG (Dianova, Hamburg, Germany) and then labeled with fluorescein isothiocyanate (FITC)-conjugated anti-Gr1 (PMN-specific rat IgG2b; ImmunoKontact, Frankfurt, Germany), FITC-conjugated anti-CD19 (rat IgG2; Dianova), anti-Thy 1.2 (rat IgG2b; Becton Dickinson, Heidelberg, Germany), or F4/80 (rat IgG2b) MAbs. Where appropriate, fluorescein-conjugated goat anti-rat IgG antiserum (MEDAC, Hamburg, Germany) was used as second Ab. The distribution of antigens was analyzed by flow cytometry (FACScan; Becton Dickinson). Erythrocytes and dead cells were gated out.
Statistical analysis.
Data are expressed as means ± standard deviations (SD). For calculation of statistical significance, the unpaired two-sample t test was used. When control values were below the limits of detection, the one-sample t test was used, assuming μ0 to be equal to the limit of detection. P values of <0.05 were considered significant.
RESULTS
Release of the leukocyte chemoattractant proteins MIP-1α, MCP-1, and MIP-2 by MALP-2- and LPS-stimulated peritoneal macrophages.
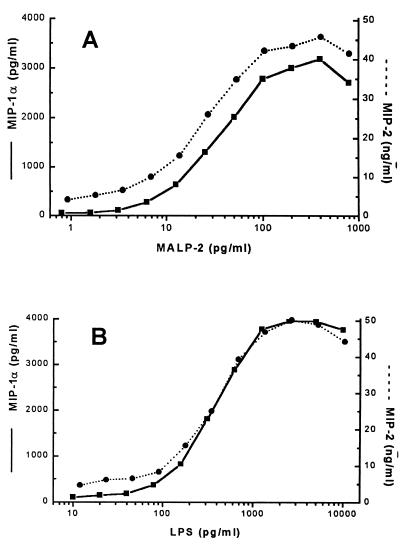
In previous work, we have shown that very low concentrations of mycoplasmal lipopeptides stimulate the release of proinflammatory cytokines from peritoneal macrophages (29). In view of the reported leukocyte infiltration in response to mycoplasma infections, we were now interested in studying the release of leukocyte chemoattractant chemokines in this system. A dose response experiment showed that the M. fermentans lipopeptide MALP-2 was a powerful inducer of the chemokines MIP-1α and MIP-2; in fact, it was more potent than LPS on a weight basis (Fig. 1A and B). Similar doses were required for the release of MCP-1 (not shown). Three separate experiments were done with cells from different animals. While the maximal release of the mediators varied between macrophage cultures from individual mice, similar doses of MALP-2 or LPS, respectively, were required to yield half-maximal release of the mediators in all experiments. To correlate cytokine release with that of the chemokines under identical conditions, the proinflammatory cytokines TNF-α and IL-6 were tested in parallel cultures and yielded similar dose dependencies (not shown).
FIG. 1.
MALP-2- and LPS-stimulated in vitro synthesis of chemokines. PEC from NMRI mice were stimulated with the indicated concentrations of MALP-2, optimally solubilized with octyl glucoside (see Materials and Methods) (A) or in parallel cultures with S. typhimurium S-form LPS (B). MIP-1α (■) and MIP-2 (●) levels were determined after 6 h. Values in unstimulated cultures were 65 pg of MIP-1α/ml and 4.2 ng of MIP-2/ml. The data are representative results from one of three separate experiments with cells from different animals.
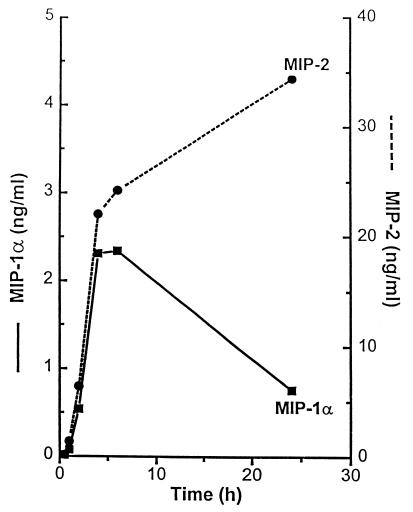
The concentrations of MIP-1α and MIP-2 in the medium of MALP-2-stimulated cultures showed a different time course. While both chemokines were rapidly liberated, the MIP-1α level formed a peak after 6 h and decreased thereafter, whereas the MIP-2 level was stable, still increasing up to 48 h. Again, three separate experiments were performed with macrophages from different animals, and typical data are shown in Fig. 2. The time course of MCP-1 liberation resembled that of MIP-2 (not shown), reaching levels of 770 ± 360 pg/ml after 24 h (mean of three cultures from different animals ± SD).
FIG. 2.
Time course of chemokine synthesis in MALP-2-stimulated macrophage cultures. PEC from NMRI mice were stimulated with 200 pg (22 U of MSA) of octyl glucoside-solubilized MALP-2/ml for the indicated times, and MIP-1α (■) and MIP-2 (●) levels in the culture medium were determined at the indicated time points. Chemokine concentrations in unstimulated control cultures after 6 h were 190 pg of MIP-1α/ml and 7 ng of MIP-2/ml, respectively.
Encapsulation of MALP-2 into liposomes.
MALP-2 is membrane bound in the natural state, as are other mycoplasmal lipoproteins, and does not occur as free lipopeptide. To provide a lipopeptide preparation which resembles the physicochemical microenvironment in the mycoplasma membrane optimally and which can be compared with the free lipopeptide or heat-killed mycoplasmas in various assay systems, MALP-2 was incorporated into liposomes. The liposomes were designed to contain, besides cholesterol, acidic phospholipids similar in their composition to those from mycoplasmas.
In vitro macrophage activation by mycoplasmas and liposome-encapsulated MALP-2.
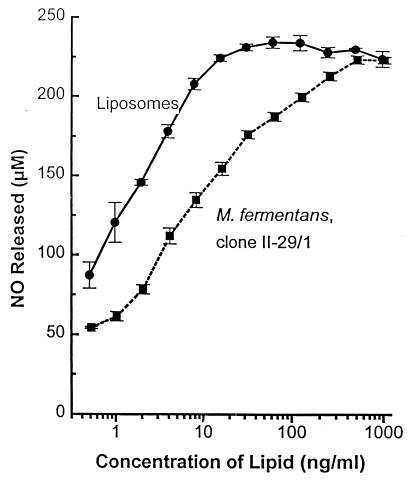
As previously shown and discussed above, the macrophage stimulatory activity of MALP-2 is highest when the lipopeptide is solubilized in octyl glucoside; in other words, MALP-2 is less potent either when naturally incorporated in the mycoplasmal membrane or artificially encapsulated into liposomes. We were interested to compare the macrophage stimulatory capacity of liposome-encapsulated MALP-2 in the nitric oxide release assay with that of natural MALP-2 present in heat-killed M. fermentans clone II-29/1, an experiment that ought to indicate whether MALP-2 is equally accessible to the macrophage in both types of vehicle. The MALP-2 content of both preparations is given in units per milligram of lipid and was 106 U per mg of lipid in the heat-killed mycoplasmas and 7 × 106 U per mg of lipid in the liposomes. As shown in Fig. 3, mycoplasmas, equivalent to 10 ng of lipid containing 10 U, stimulated half-maximal nitric oxide release, whereas about 2 ng of liposomes containing 14 U was required to achieve the same effect. Thus, MALP-2, when liposome encapsulated, is about as potent as in its natural environment, which is the mycoplasma membrane. As previously stated (31), solubilized MALP-2 is 10 to 14 times more active than when it is membrane incorporated.
FIG. 3.
Comparison of nitric oxide release by C3H/HeJ PEC in response to heat-killed M. fermentans and liposome-encapsulated MALP-2. A 1/2 serial dilution of liposomes in medium containing MALP-2 corresponding to 7 × 106 U of MSA per mg of lipid or of heat-killed M. fermentans clone II-29/1 containing 106 U of MSA per mg of lipid was added to the PEC in the presence of 60 U of mouse rIFN-γ/ml. The nitric oxide level was determined after 45 h as the sum of nitrite and nitrate. Control cultures with only IFN-γ produced <45 μM. Control liposomes without MALP-2 at 1 μg of lipid/ml had no effect above that in IFN-γ control cultures. Values are means ± SD of triplicate cultures.
Leukocyte infiltration in response to i.p. injected heat-killed M. fermentans clones with different MSA.
To test whether the capacity of mycoplasmas to induce leukocyte infiltration is in any way related to their ability to stimulate macrophages, a pilot experiment was performed by injecting two M. fermentans clones, clone II-29/1 and clone 39, which differ in their content of macrophage-stimulating material. Octyl glucoside extracts of both clones were assayed in the nitric oxide release test for MSA. This batch of clone II-29/1 contained 430 kU of MSA per mg of protein compared to 13 kU of MSA per mg of protein in clone 39. To exclude the influence of uncontrollable effects through viable mycoplasmas, such as different rates of survival or proliferation after infection, the mycoplasmas were heat killed. Groups of three mice were i.p. injected with the equivalent of 23 μg of mycoplasma protein. Twenty-four hours later, the percentage of PMN in the peritoneal lavage fluid was determined. Peritoneal cells from mice that had received clone II-29/1 (10 kU) contained 24.4% ± 5.4% PMN, whereas those from mice injected with clone 39 (0.3 kU) contained 4.4% ± 1.6% PMN (values are means ± SD; P value, <0.05). The percentage of PMN in PEC of mice having received saline was 2.4% ± 2.8%. These data indicate that, at least in this model, the capacity of mycoplasmas to induce leukocyte infiltration is positively correlated with their MSA.
Leukocyte infiltration in response to i.p. injected soluble and liposome-encapsulated MALP-2.
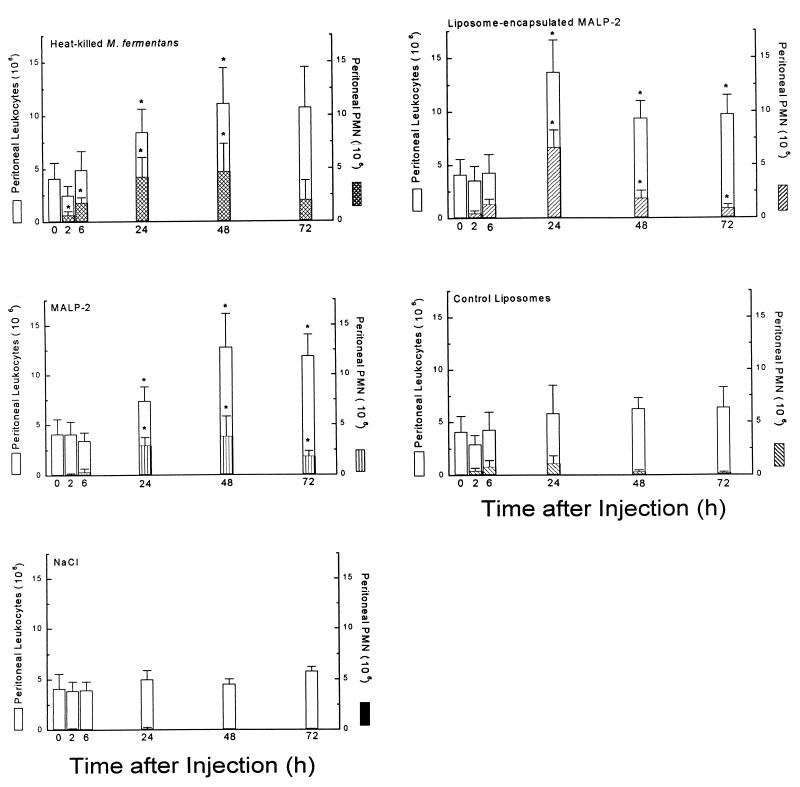
With particulate and soluble MALP-2 preparations available, and knowing that MALP-2 is a strong macrophage stimulator and causes the release of leukocyte chemoattractant proteins, we tested the capacity of such preparations to attract leukocytes into the peritoneal cavity. It was particularly interesting to investigate whether MALP-2, taken as an example for mycoplasmal lipoproteins, is a factor contributing to leukocyte accumulation at sites of mycoplasma infection. An amount of heat-killed clone II-29/1 mycoplasmas that gave maximal response served as positive control. The data are summarized in Fig. 4. After injection of heat-killed mycoplasmas, MALP-2, or liposome-encapsulated MALP-2, there was a steady increase in the number of total peritoneal cells over 72 h and longer (later times not shown). Most conspicuous in animals which were injected with mycoplasmas, MALP-2, or MALP-2 containing liposomes was a rapid PMN infiltration which peaked at 24 to 48 h and decreased thereafter but remained well above PMN counts in control animals for an additional 2 days. However, control liposomes also elicited a moderate PMN response.
FIG. 4.
Leukocyte infiltration in response to heat-killed mycoplasmas and soluble or liposome-encapsulated MALP-2. Groups of six NMRI mice were i.p. injected with M. fermentans clone II-29/1 (0.2 mg of mycoplasma protein containing 0.9 μg or 105 U of MALP-2), 9 μg (106 U) of MALP-2 in saline, 0.2 mg of liposomes containing 9 μg of MALP-2, control liposomes (0.2 mg), or saline, respectively. Animals were sacrificed after the indicated times, and total and differential cell counts in the peritoneal lavage fluid were determined. Lavage fluid from untreated animals contained PEC with 0.4% ± 0.5% PMN. Values are means from six animals ± SD or means from three animals ± SD for mice injected with saline. ∗, significantly higher (P < 0.05) than values of control groups injected with control liposomes or saline, respectively, as calculated by Student’s t test.
Since there was still an overall increase of total peritoneal cells at 72 h when the PMN counts had decreased (Fig. 4), it was of interest to determine if this late increase of peritoneal cells was due to infiltration of monocytes/macrophages, lymphocytes, or both. Again, groups of six animals were i.p. injected with heat-killed M. fermentans, MALP-2, or liposome-encapsulated MALP-2, and 72 h later, differential leukocyte counts in the peritoneal lavage fluid were determined. The data in Table 1 show that the absolute number of monocytes/macrophages had by this time significantly increased in animals treated with MALP-2 in either soluble or liposomal form, an effect which was even more pronounced in mice that had received heat-killed mycoplasmas. There was no significant increase in the number of lymphocytes nor in the percentage of thy 1.2-positive T cells above that in control animals (2 to 5%) at any time after any of the treatments.
TABLE 1.
Leukocyte infiltration in response to mycoplasmas and mycoplasmal lipopeptide MALP-2 after 72 h
| Treatment | No. (in millions) or % ± SD of the indicated cells
|
||||||
|---|---|---|---|---|---|---|---|
| PEC (n) | Monocytes/macrophages
|
Lymphocytes
|
PMN
|
||||
| n | % | n | % | n | % | ||
| M. fermentans (0.2 mg of protein)a | 14.8 ± 5.9 | 7.1 ± 3b | 48.7 ± 8.9 | 3.6 ± 2 | 25.4 ± 12b | 3.9 ± 2.9 | 24.2 ± 14.8b |
| MALP-2 (9 μg) | 11.2 ± 3.1b | 5 ± 1.2b | 47.4 ± 15.2 | 3.5 ± 1.2 | 32 ± 11b | 2.6 ± 2.7 | 20.2 ± 13.2b |
| Liposome-encapsulated MALP-2 (9 μg) | 8.5 ± 1.2c | 4.9 ± 0.9c | 57.6 ± 8.1c | 2.9 ± 1 | 33.4 ± 9c | 0.8 ± 0.2c | 9.7 ± 2.2c |
| Control liposomes | 6.4 ± 1.9 | 2.9 ± 1.2 | 44.5 ± 6.8 | 3.2 ± 0.9 | 51.8 ± 6.7 | 0.2 ± 0.08 | 2.9 ± 1.4 |
| NaCl (0.9%) | 5.8 ± 0.5 | 2.8 ± 0.4 | 47.9 ± 1.7 | 2.8 ± 0.2 | 48.8 ± 1 | 0.05 ± 0.03 | 0.9 ± 0.6 |
Estimated to contain about 0.9 μg of MALP-2 according to MSA in the nitric oxide release assay.
Significantly different (P < 0.05) from values for saline-treated animals as calculated by Student’s t test.
Significantly different (P < 0.05) from values for animals injected with control liposomes as calculated by Student’s t test.
Leukocyte chemoattractant chemokines and proinflammatory cytokines in serum and peritoneal fluid of MALP-2-treated mice.
As shown above, MALP-2 induced cultured macrophages to release the leukocyte chemoattractant chemokines MIP-1α, MCP-1, and MIP-2. It was therefore an attractive hypothesis to assume that MALP-2-dependent infiltration of leukocytes into the peritoneal cavity was caused by local release of these chemokines by resident peritoneal macrophages. To test this, serum and peritoneal lavage fluid were collected from the same animals that had been used to observe leukocyte infiltration at various times after injection of heat-killed mycoplasmas, MALP-2, and liposome-encapsulated MALP-2. It was first examined in pilot experiments whether higher titers of these mediators were found in lavage fluid or in serum. Accordingly, MIP-1α was assayed in the peritoneal lavage fluid where, in spite of the dilution of the peritoneal fluid with HBSS, higher titers than in the serum of the identical animals were found. It should be noted that the kinetics of appearance of MIP-1α in serum and peritoneal wash fluid were parallel, being maximal after 6 h. All other mediators were determined in the serum.
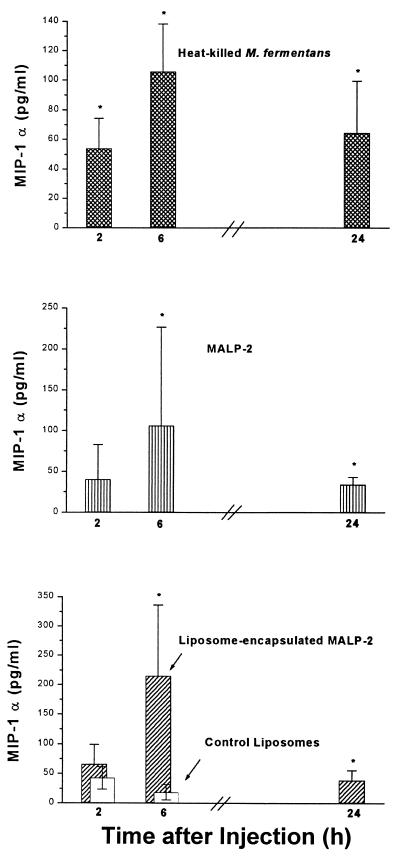
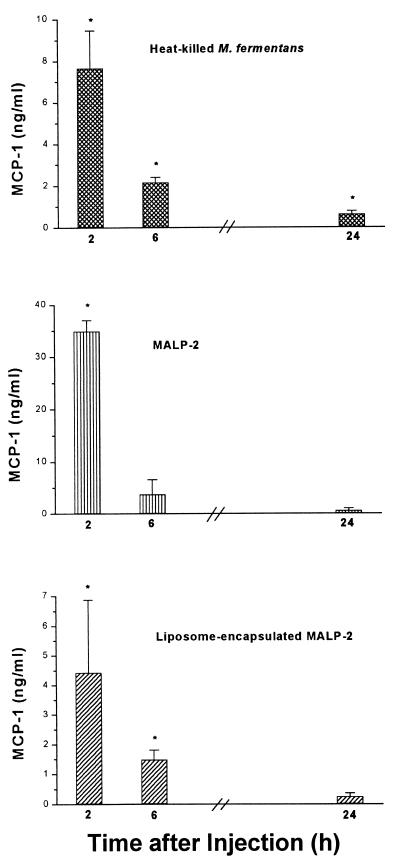
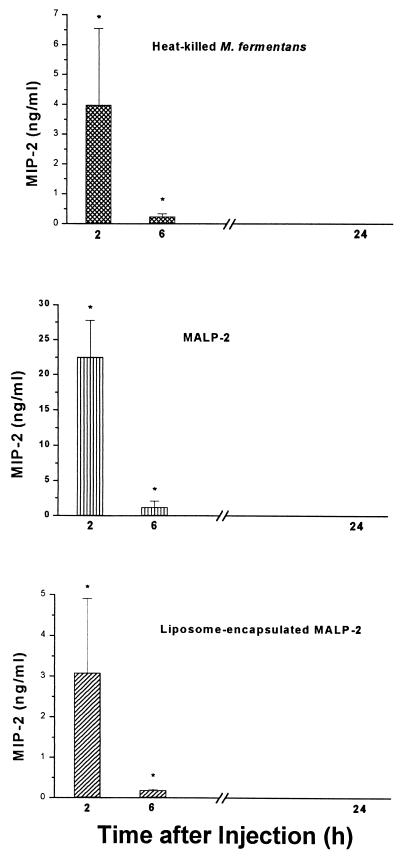
The time course of the in vivo concentration of MIP-1α (Fig. 5) was comparable to the in vitro time dependency (Fig. 2). Such a close correlation of the respective kinetics was not observed with the other two chemokines. The in vivo concentration of MCP-1 was highest after 2 h and dropped thereafter (Fig. 6), while in vitro levels of MCP-1 still increased up to 24 h (not shown). Similarly, in vivo MIP-2 reached a maximum level at the earliest time point, becoming undetectable after 24 h (Fig. 7), whereas the in vitro concentration was still rising at this time point (Fig. 2). Furthermore, the respective concentrations of the individual chemokines depended on whether MALP-2 was applied in solution or in liposome-encapsulated form: e.g., free MALP-2 was more efficient than liposome-encapsulated MALP-2 in eliciting MCP-1 or MIP-2 generation (Fig. 6 and 7), whereas liposome-encapsulated MALP-2 was equal to or more potent than free MALP-2 in inducing in vivo MIP-1α release (Fig. 5).
FIG. 5.
Concentration of MIP-1α in peritoneal lavage fluid of NMRI mice after i.p. injection with heat-killed M. fermentans (cross-hatched bars) or with soluble (vertically striped bars) or liposome-encapsulated MALP-2 (diagonally hatched bars) as described for Fig. 4. The level of MIP-1α in the peritoneal lavage fluid of the same animals that had served to measure leukocyte infiltration was determined. The MIP-1α concentrations in untreated animals or saline-treated mice were <5 pg/ml. Values are means from six animals ± SD or means from three to six animals ± SD for mice injected with saline. ∗, significantly different (P < 0.05) from values of control groups injected with control liposomes or saline, respectively, as calculated by Student’s t test.
FIG. 6.
Concentration of MCP-1 in serum of NMRI mice after i.p. injection with heat-killed M. fermentans or with soluble or liposome-encapsulated MALP-2. The level of MCP-1 in the serum of the same animals that had served to measure leukocyte infiltration was determined. Symbols in the bar graph are as defined for Fig. 5. The MCP-1 concentrations in the serum of untreated animals were <16 pg/ml, and those from mice injected with control liposomes or saline were <160 pg/ml. Values are means from three animals ± SD. ∗, significantly different (P < 0.05) from values of control groups injected with control liposomes or saline, respectively, as calculated by Student’s t test.
FIG. 7.
Concentration of MIP-2 in serum of NMRI mice after i.p. injection with heat-killed M. fermentans or with soluble or liposome-encapsulated MALP-2. The level of MIP-2 in the serum of the same animals that had served to measure leukocyte infiltration was determined. Symbols in the bar graph are as defined for Fig. 5. The MIP-2 concentrations in the serum of untreated animals were ≤14 pg/ml, and those from mice injected with control liposomes or saline were <200 pg/ml or <15 pg/ml, respectively. Values are means from six animals ± SD or means from three to six animals ± SD for mice injected with saline. ∗, significantly different (P < 0.05) from values of control groups injected with control liposomes or saline, respectively, as calculated by Student’s t test.
To assess further possible systemic effects of MALP-2 treatment, the levels of proinflammatory cytokines TNF-α and IL-6 were measured 2, 6, and 24 h after i.p. injection of MALP-2. In both the serum and the peritoneal lavage fluid of treated animals, TNF-α levels were not significantly elevated at any of the time points investigated above those in animals injected with saline controls. In contrast, levels of IL-6 in serum 2 h after application rose to 27.3 ± 3.8 ng/ml (six animals) as opposed to <1.5 ng/ml in control animals which had received saline (six animals; P value, <0.05). IL-6 levels were not significantly elevated at later times.
DISCUSSION
The migration of leukocytes to sites of infection is an important aspect of the early phase of the innate host defense. The effects of several microbial products on leukocyte chemotaxis have been reviewed (20). None of the agents mentioned was detected in mycoplasmas. Similarly, mycoplasmas are devoid of LPS, a further bacterial substance that induces PMN infiltration (19). As referred to in the introductory section above, upon infection, mycoplasmas are capable of causing accumulation of PMN, macrophages, and lymphocytes in the affected tissue. Apart from an in vitro active B lymphocyte chemoattractant restricted to membranes from M. pulmonis (34), to date, no well-defined mycoplasma-derived agents have been shown to cause in vivo leukocyte infiltration.
We have studied leukocyte infiltration into the peritoneal cavity in response to i.p. injected mycoplasmal lipopeptide MALP-2 in free or liposome-encapsulated form. As shown by others, leukocytes emigrate from the circulation into the peritoneal cavity via the omentum (9). We are aware of the fact that, as with most experimental in vivo systems, compromises have to be made between reality and feasibility. The natural habitats of mycoplasmas are the epithelia of the airways and the urogenital tract. Experimental mycoplasma infections in mice are not easy to control, and host responses, such as local release of chemokines or leukocyte infiltration at the sites of natural infection, are difficult to quantify. The present system was not primarily meant to be a model of mycoplasma infection but was chosen to allow us to assay the host reactions to MALP-2, a well-defined mycoplasmal component with a high macrophage-activating potential. Heat-killed mycoplasmas rather than live organisms served as a positive control in order to avoid complications brought on by uncontrolled spreading, clearance, or rates of survival of live mycoplasmas. Others have used i.p. injection to study leukocyte influx and other host reactions to Mycobacterium avium (2), bacterial lipopeptides (15), leukotrienes (14), or IL-1 (36).
MALP-2 was a likely candidate as a mycoplasma-derived leukotaxis-inducing agent, since it resembles LPS functionally in stimulating macrophages, which are a major source of chemokines and other mediators of leukotaxis. In fact, MALP-2 proved as potent in recruiting leukocytes (Fig. 4) as comparable amounts of S. typhimurium LPS in this system (our unpublished data).
Leukocyte infiltration is a multistep, multifactor process. There was a protracted influx of leukocytes in response to heat-killed mycoplasmas or MALP-2, with PMN infiltration beginning as early as 2 h after injection (Fig. 4). Early influx of PMN, being maximal 24 h after injection, was also caused by liposome-encapsulated MALP-2, and a rather moderate but distinct PMN influx was also noted in response to control liposomes (Fig. 4). By 72 h postinjection, there was also a significant influx of macrophages in response to mycoplasmas or MALP-2 in free or liposome-encapsulated form as compared to vehicle controls (Table 1).
In discussing our observations in the context of likely mechanisms, we would like to distinguish between an early phase of PMN influx, beginning around 2 to 6 h after injection, and a later phase of PMN infiltration, reaching maximum levels by 24 to 48 h, overlapping with and followed by macrophage influx. Principally, leukocytes could be directly attracted by mycoplasmal products, as, for example, by N-formyl methionine peptides, such as FMLP or analogues thereof. Mycoplasmas can theoretically produce N-formyl methionine, provided there is folic acid in the medium (26). As this is the case with GBF-3 medium, we cannot formally exclude N-formyl methionyl peptides contributing to the early phase of leukocyte infiltration caused by heat-killed mycoplasmas. However, FMLP was reported to be inactive in a mouse subcutaneous sponge implantation model (27).
Another mechanism that would explain mycoplasma-mediated leukocyte accumulation could be complement activation by the alternative pathway. Except for a recent report on C3 activation by an M. fermentans-derived lipoprotein (28), whose N-terminal portion is identical to MALP-2 and which is now called MALP-404 (4), little is known about complement activation by mycoplasmas. Earlier work is difficult to evaluate, since the classical mycoplasma growth media contain yeast components and thus zymosan-like contaminants. Since the mycoplasmas used in this study were grown in a well-defined medium, complement activation by such contaminants can be ruled out. In any case, a rapid response would be expected if complement C5a were involved (5). Preliminary experiments indicated that M. fermentans clone II-29/1, although devoid of MALP-404 (4, 31), activated human C5, while MALP-2 did not (6a). Complement activation would also explain the response to control liposomes, since it was reported that liposomes, particularly those carrying a surface charge such as those used in this study, can activate murine complement (7).
While an involvement of complement is likely in the early phase of mycoplasma-mediated leukocyte chemotaxis, MSA appears to be substantially involved in the later phases, beginning around 6 h after treatment. This was initially suggested by comparing leukocyte infiltration levels 24 h after injection of two M. fermentans clones, which differ in their MALP-2 content and thus in their macrophage-stimulating capacity. Their potential to attract leukocytes correlated with their MSA. The notion that leukocyte influx and MSA are correlated is further directly supported by the finding that the effects of heat-killed mycoplasmas could be simulated by soluble as well as liposome-encapsulated MALP-2.
Our experiments further show that MALP-2 was capable of inducing the chemokines MIP-1α, MCP-1, and MIP-2 in cell culture as well as in vivo. A comparison of the doses required to achieve half-maximal release of chemokines in culture showed the mycoplasma lipopeptide MALP-2 to be more potent than S. typhimurium S-form LPS on a weight basis. The in vivo production kinetics of the chemokines is in agreement with the early infiltration of PMN versus late accumulation of monocytes/macrophages (Table 1): MIP-2, primarily a PMN attractant (45), was formed early, reaching maximal levels by 2 h and having practically disappeared by 24 h (Fig. 7), whereas levels of the macrophage chemoattractants MIP-1α (1, 8) and MCP-1 (25) peaked after 2 to 6 h and were still measurable after 24 h (Fig. 5 and 6). The observed increase in peritoneal macrophages could be due to the chemoattractant properties of MIP-1α and MCP-1, the reported MIP-1α-mediated proliferation of mature macrophages (12), or to a combination of these effects. It is interesting to note that in vivo free MALP-2 was by far more potent in inducing MIP-2 and MCP-1 than the liposome-encapsulated substance (Fig. 6 and 7). This may be due to the systemic action of circulating soluble MALP-2 as opposed to more localized effects by the particulate liposomal preparation. The latter is evident from the higher efficiency of liposome-encapsulated MALP-2 in generating MIP-1α in peritoneal lavage fluid (Fig. 5).
When discussing possible temporal and causal relationships between MALP-2-mediated release of chemokines and leukocyte influx, one has to bear in mind that chemokines bind to heparin and other acidic glycosaminoglycans and that a concentration gradient is required for directional leukocyte migration (reviewed in reference 37). Circulating chemokines may therefore be regarded as being in excess and do in fact inhibit a reaction to local injections of these chemoattractants (16). The fact that injection of MALP-2 caused chemokine release at concentrations approaching (5, 35) or exceeding (46) those reported to mediate leukocyte influx at local sites of injection suggests strongly that MALP-2 injection, chemokine release, and leukocyte influx are causally related.
The chemokines MIP-1α, MCP-1, and MIP-2 may not be the only macrophage products acting as leukocyte attractants in this system, as other chemotactic factors, such as KC (18), leukotriene B4, or platelet activating factor, may well be formed upon MALP-2 stimulation of macrophages. It is also conceivable that the cytokines TNF-α and IL-1, though not chemoattractants per se, may play a role in MALP-2-mediated leukocyte infiltration. Both elicit PMN emigration (27, 36) and are capable of inducing chemokines in several cells other than macrophages (10). In neither the serum nor the peritoneal wash fluid were we able to detect any higher TNF-α levels in mycoplasma- or MALP-2-treated animals than in control animals. However, this may be due to the short in vivo half-life of TNF (47), since our earliest sample was taken 2 h after treatment. Also, our earlier work with a preparation called MDHM, which essentially consisted of mycoplasmal lipopeptides, induced primarily cell-associated IL-1 (30).
In conclusion, our data indicate that MALP-2 and presumably other macrophage-activating lipoproteins which are ubiquitously expressed in mycoplasma species play an important role in the late phase of phagocyte recruitment at sites of infection and that such mycoplasmal lipopeptides and lipoproteins act through macrophage stimulation, similar to the way LPS does in cases of infections with gram-negative bacteria (19).
ACKNOWLEDGMENTS
We thank T. Hirsch for excellent technical help and R. Süßmuth and G. Jung for a generous supply of synthetic MALP-2. We are also grateful to M. Rohde of the GBF for electron-microscopic characterization of the liposomes and J. Scriven for helpful editing.
This work was supported in part by the Deutsche Forschungsgemeinschaft (grant Mu 672/2-3).
REFERENCES
- 1.Alam R, Kumar D, Anderson-Walters D, Forsythe P A. Macrophage inflammatory protein-1α and monocyte chemoattractant peptide-1 elicit immediate and late cutaneous reactions and activate murine mast cells in vivo. J Immunol. 1994;152:1298–1303. [PubMed] [Google Scholar]
- 2.Appelberg R. Interferon-gamma (IFN-gamma) and macrophage inflammatory proteins (MIP)-1 and -2 are involved in the regulation of the T cell-dependent chronic peritoneal neutrophilia of mice infected with mycobacteria. Clin Exp Immunol. 1992;89:269–273. doi: 10.1111/j.1365-2249.1992.tb06943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baseman J B, Tully J G. Mycoplasmas: sophisticated, reemerging, and burdened by their notoriety. Emerg Infect Dis. 1997;3:21–32. doi: 10.3201/eid0301.970103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calcutt M J, Kim M F, Karpas A B, Mühlradt P F, Wise K S. Differential post-translational processing confers intraspecies variation of a major lipopeptide (MALP-2) of Mycoplasma fermentans. Infect Immun. 1999;67:760–771. doi: 10.1128/iai.67.2.760-771.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins P D, Jose P J, Williams T J. The sequential generation of neutrophil chemoattractant proteins in acute inflammation in the rabbit in vivo. J Immunol. 1991;146:677–684. [PubMed] [Google Scholar]
- 6.Dagnall G J R. Experimental infection of the conjunctival sac of lambs with Mycoplasma conjunctivae. Br Vet J. 1993;149:429–435. doi: 10.1016/S0007-1935(05)80109-5. [DOI] [PubMed] [Google Scholar]
- 6a.Deiters, U., G. Sonntag, O. Götze, and P. F. Mühlradt. Unpublished results.
- 7.Devine D V, Wong K, Serrano K, Chonn A, Cullis P R. Liposome-complement interactions in rat serum: implications for liposome survival studies. Biochim Biophys Acta. 1994;1191:43–51. doi: 10.1016/0005-2736(94)90231-3. [DOI] [PubMed] [Google Scholar]
- 8.DiPietro L A, Burdick M, Low Q E, Kunkel S L, Strieter R M. MIP-1α as a critical macrophage chemoattractant in murine wound repair. J Clin Investig. 1998;101:1693–1698. doi: 10.1172/JCI1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doherty N S, Griffiths R J, Hakkinen J P, Scampoli D N, Milici A J. Post-capillary venules in the “milky spots” of the greater omentum are the major site of plasma protein and leukocyte extravasation in rodent models of peritonitis. Inflamm Res. 1995;44:169–177. doi: 10.1007/BF01782815. [DOI] [PubMed] [Google Scholar]
- 10.Driscoll K E. Macrophage inflammatory proteins: biology and role in pulmonary inflammation. Exp Lung Res. 1994;20:473–490. doi: 10.3109/01902149409031733. [DOI] [PubMed] [Google Scholar]
- 11.Ezekowitz R A B, Austyn J, Stahl P D, Gordon S. Surface properties of Bacillus Calmette-Guérin-activated mouse macrophages. J Exp Med. 1981;154:60–76. doi: 10.1084/jem.154.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fahey T J, III, Tracey K J, Tekamp-Olson P, Cousens L S, Jones W G, Shires G T, Cerami A, Sherry B. Macrophage inflammatory protein 1 modulates macrophage function. J Immunol. 1992;148:2764–2769. [PubMed] [Google Scholar]
- 13.Freudenberg M A, Galanos C. Tumor necrosis factor alpha mediates lethal activity of killed gram-negative and gram-positive bacteria in d-galactosamine-treated mice. Infect Immun. 1991;59:2110–2115. doi: 10.1128/iai.59.6.2110-2115.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Griswold D E, Webb E F, Hillegass L M. Induction of plasma exudation and inflammatory cell infiltration by leukotriene C4 and leukotriene B4 in mouse peritonitis. Inflammation. 1991;15:251–258. doi: 10.1007/BF00917310. [DOI] [PubMed] [Google Scholar]
- 15.Hauschildt S, Beuscher H U, Jung G, Bessler W, Ulmer A. Intraperitoneal injection of synthetic bacterial lipopeptides does not cause a rise in circulating inflammatory cytokines. FEMS Immunol Med Microbiol. 1994;8:77–82. doi: 10.1111/j.1574-695X.1994.tb00428.x. [DOI] [PubMed] [Google Scholar]
- 16.Hechtman D H, Cybulsky M I, Fuchs H J, Baker J B, Grimbrone M A., Jr Intravascular IL-8. Inhibitor of polymorphonuclear leukocyte accumulation at sites of acute inflammation. J Immunol. 1991;147:883–892. [PubMed] [Google Scholar]
- 17.Howard C J, Anderson J C, Gourlay R N, Taylor-Robinson D. Production of mastitis in mice with human and bovine ureaplasmas (T-mycoplasmas) J Med Microbiol. 1975;8:523–529. doi: 10.1099/00222615-8-4-523. [DOI] [PubMed] [Google Scholar]
- 18.Introna M, Bast R, Tannenbaum C, Adams D. The effect of LPS on expression of the early “competence” genes JE and KC in murine peritoneal macrophages. J Immunol. 1987;138:3891–3896. [PubMed] [Google Scholar]
- 19.Issekutz A C, Bhimji S. Role for endotoxin in the leukocyte infiltration accompanying Escherichia coli inflammation. Infect Immun. 1982;36:558–566. doi: 10.1128/iai.36.2.558-566.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalmar J R, van Dyke T E. Effect of bacterial products on neutrophil chemotaxis. Methods Enzymol. 1994;236:58–87. doi: 10.1016/0076-6879(94)36009-x. [DOI] [PubMed] [Google Scholar]
- 21.Kennedy S, Ball H J. Pathology of experimental Ureaplasma mastitis in ewes. Vet Pathol. 1987;24:302–307. doi: 10.1177/030098588702400403. [DOI] [PubMed] [Google Scholar]
- 22.Lindsey J R, Cassell G H. Experimental Mycoplasma pulmonis infection in pathogen-free mice. Am J Pathol. 1973;72:63–90. [PMC free article] [PubMed] [Google Scholar]
- 23.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 24.Lowry O H, Roberts N R, Leiner K Y, Wu M-L, Farr A L. The quantitative histochemistry of brain. I. Chemical methods. J Biol Chem. 1954;207:1–17. [PubMed] [Google Scholar]
- 25.Luini W, Sozzani S, van Damme J, Mantovani A. Species-specificity of monocyte chemotactic protein-1 and -3. Cytokine. 1994;6:28–31. doi: 10.1016/1043-4666(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 26.Maniloff J. Evolution of wall-less procaryotes. Annu Rev Microbiol. 1983;37:477–499. doi: 10.1146/annurev.mi.37.100183.002401. [DOI] [PubMed] [Google Scholar]
- 27.Mason M J, van Epps D E. In vivo neutrophil emigration in response to interleukin-1 and tumor necrosis factor-alpha. J Leukocyte Biol. 1989;45:62–68. doi: 10.1002/jlb.45.1.62. [DOI] [PubMed] [Google Scholar]
- 28.Matsumoto M, Nishiguchi M, Kikkawa S, Nishimura H, Nagasawa S, Seya T. Structural and functional properties of complement-activating protein M161Ag, a Mycoplasma fermentans gene product that induces cytokine production by human monocytes. J Biol Chem. 1998;273:12407–12414. doi: 10.1074/jbc.273.20.12407. [DOI] [PubMed] [Google Scholar]
- 29.Mühlradt P F, Frisch M. Purification and partial biochemical characterization of a Mycoplasma fermentans-derived substance that activates macrophages to release nitric oxide, tumor necrosis factor, and interleukin-6. Infect Immun. 1994;62:3801–3807. doi: 10.1128/iai.62.9.3801-3807.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mühlradt P F, Schade U. MDHM, a macrophage-stimulatory product of Mycoplasma fermentans, leads to in vitro interleukin-1 (IL-1), IL-6, tumor necrosis factor, and prostaglandin production and is pyrogenic in rabbits. Infect Immun. 1991;59:3969–3974. doi: 10.1128/iai.59.11.3969-3974.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mühlradt P F, Kieß M, Meyer H, Süßmuth R, Jung G. Isolation, structure elucidation, and synthesis of a macrophage stimulatory lipopeptide from Mycoplasma fermentans acting at picomolar concentration. J Exp Med. 1997;185:1951–1958. doi: 10.1084/jem.185.11.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mühlradt P F, Kieß M, Meyer H, Süßmuth R, Jung G. Structure and specific activity of macrophage-stimulating lipopeptides from Mycoplasma hyorhinis. Infect Immun. 1998;66:4804–4810. doi: 10.1128/iai.66.10.4804-4810.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rollins S, Colby T, Clayton F. Open lung biopsy in Mycoplasma pneumoniae pneumonia. Arch Pathol Lab Med. 1986;110:34–41. [PubMed] [Google Scholar]
- 34.Ross S E, Simecka J W, Gambill G P, Davis J K, Cassell G H. Mycoplasma pulmonis possesses a novel chemoattractant for B lymphocytes. Infect Immun. 1992;60:669–674. doi: 10.1128/iai.60.2.669-674.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saukkonen K, Sande S, Cioffe C, Wolpe S, Sherry B, Cerami A, Tuomanen E. The role of cytokines in the generation of inflammation and tissue damage in experimental gram-positive meningitis. J Exp Med. 1990;171:439–448. doi: 10.1084/jem.171.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sayers T J, Wiltrout T A, Bull C A, Denn III A C, Pilaro A M, Lokesh B. Effect of cytokines on polymorphonuclear neutrophil infiltration in the mouse. J Immunol. 1988;141:1670–1677. [PubMed] [Google Scholar]
- 37.Taub D D. Chemokine-leukocyte interactions. The vodoo that they do so well. Cytokine Growth Factor Rev. 1996;7:355–376. doi: 10.1016/s1359-6101(97)89237-4. [DOI] [PubMed] [Google Scholar]
- 38.Taylor-Robinson D. Infections due to species of Mycoplasma and Ureaplasma: an update. Clin Infect Dis. 1996;23:671–684. doi: 10.1093/clinids/23.4.671. [DOI] [PubMed] [Google Scholar]
- 39.Tuffrey M A, Furr P M, Falder P, Taylor-Robinson D. The anti-Chlamydial effect of experimental Mycoplasma pulmonis infection in the murine genital tract. J Med Microbiol. 1984;17:357–362. doi: 10.1099/00222615-17-3-357. [DOI] [PubMed] [Google Scholar]
- 40.Tully J G. Current status of the mollicutes flora of humans. Clin Infect Dis. 1993;17(Suppl. 1):S2–S9. doi: 10.1093/clinids/17.supplement_1.s2. [DOI] [PubMed] [Google Scholar]
- 41.Utsugi T, Dinney C P N, Killion J J, Fidler I J. In situ activation of mouse macrophages and therapy of spontaneous renal cell cancer metastasis by liposomes containing the lipopeptide CGP 31362. Cancer Immunol Immunother. 1991;33:375–381. doi: 10.1007/BF01741597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Rooijen N, van Nieuwmegen R, Kamperdijk E W A. Elimination of phagocytic cells in the spleen after intravenous injection of liposome-encapsulated dichloromethylene diphosphonate. Ultrastructural aspects of elimination of marginal zone macrophages. Virchows Arch B Cell Pathol Incl Mol Pathol. 1985;49:375–383. doi: 10.1007/BF02912114. [DOI] [PubMed] [Google Scholar]
- 43.Vink A, Coulie P G, Wauters P, Nordan R P, van Snick J. B cell growth and differentiation activity of interleukin-HP1 and related murine plasmacytoma growth factors. Synergy with interleukin 1. Eur J Immunol. 1988;18:607–612. doi: 10.1002/eji.1830180418. [DOI] [PubMed] [Google Scholar]
- 44.Westphal O, Jann K. Bacterial lipopolysaccharides. Extraction with phenol-water and further applications of the procedure. Methods Carbohydr Chem. 1965;5:83–91. [Google Scholar]
- 45.Wolpe S D, Sherry B, Juers D, Davatelis G, Yurt R W, Cerami A. Identification and characterization of macrophage inflammatory protein 2. Proc Natl Acad Sci USA. 1989;86:612–616. doi: 10.1073/pnas.86.2.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zachariae C O C, Anderson A O, Thomson H L, Appella E, Mantovani A, Oppenheim J J, Katsushima K. Properties of monocyte chemotactic and activating factor (MCAF) purified from a human fibrosarcoma cell line. J Exp Med. 1990;171:2177–2182. doi: 10.1084/jem.171.6.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zuckerman S H, Evans G F, Butler L D. Endotoxin tolerance: independent regulation of interleukin-1 and tumor necrosis factor expression. Infect Immun. 1991;59:2774–2780. doi: 10.1128/iai.59.8.2774-2780.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]