Abstract
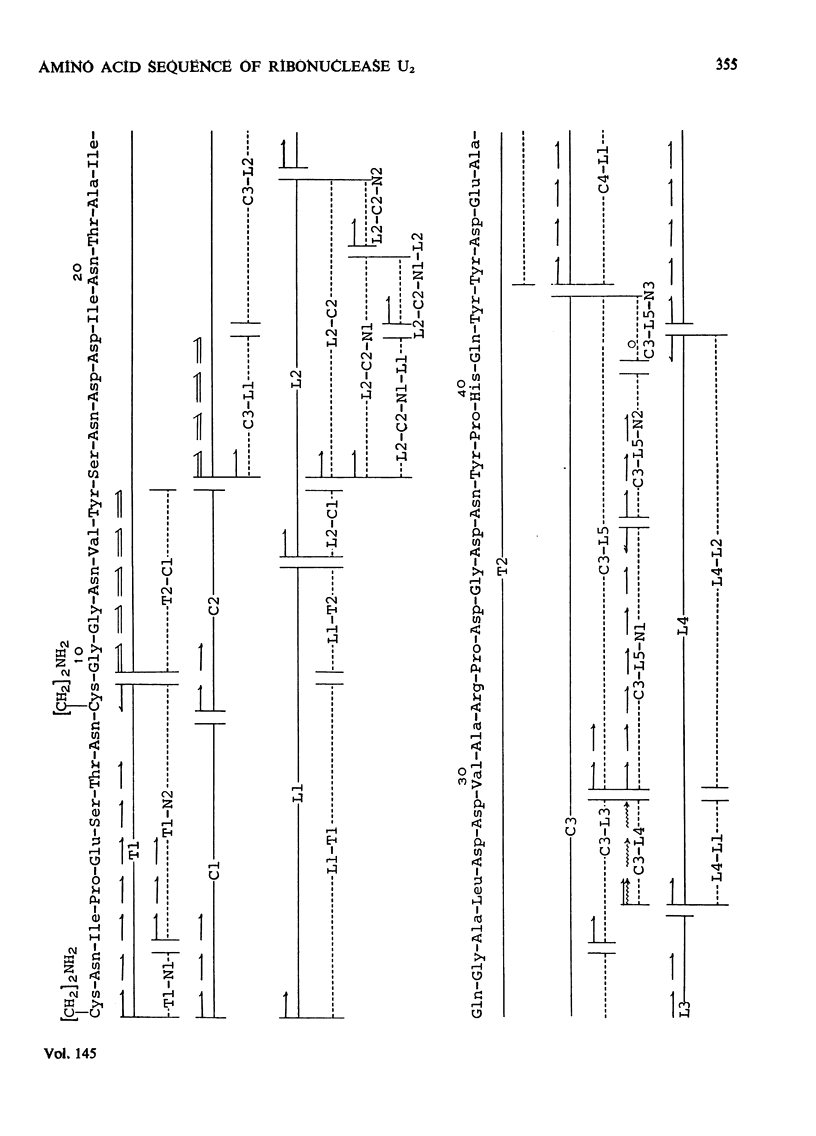
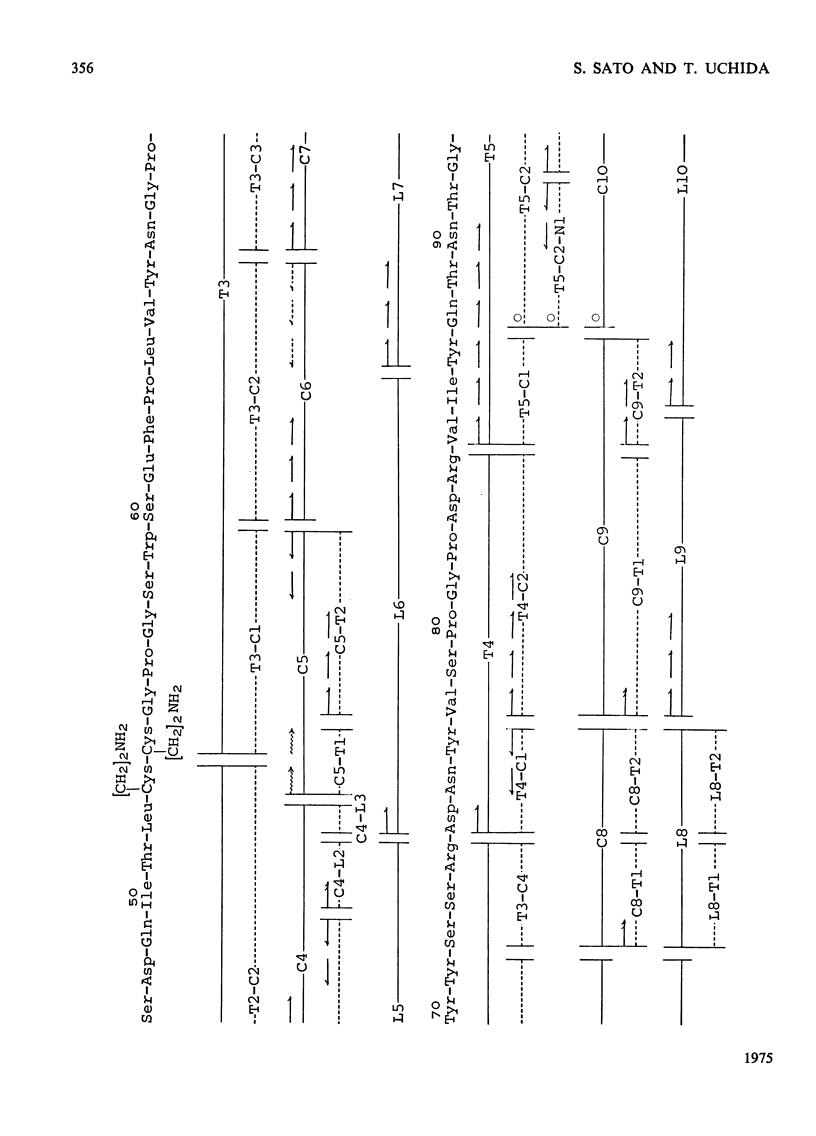
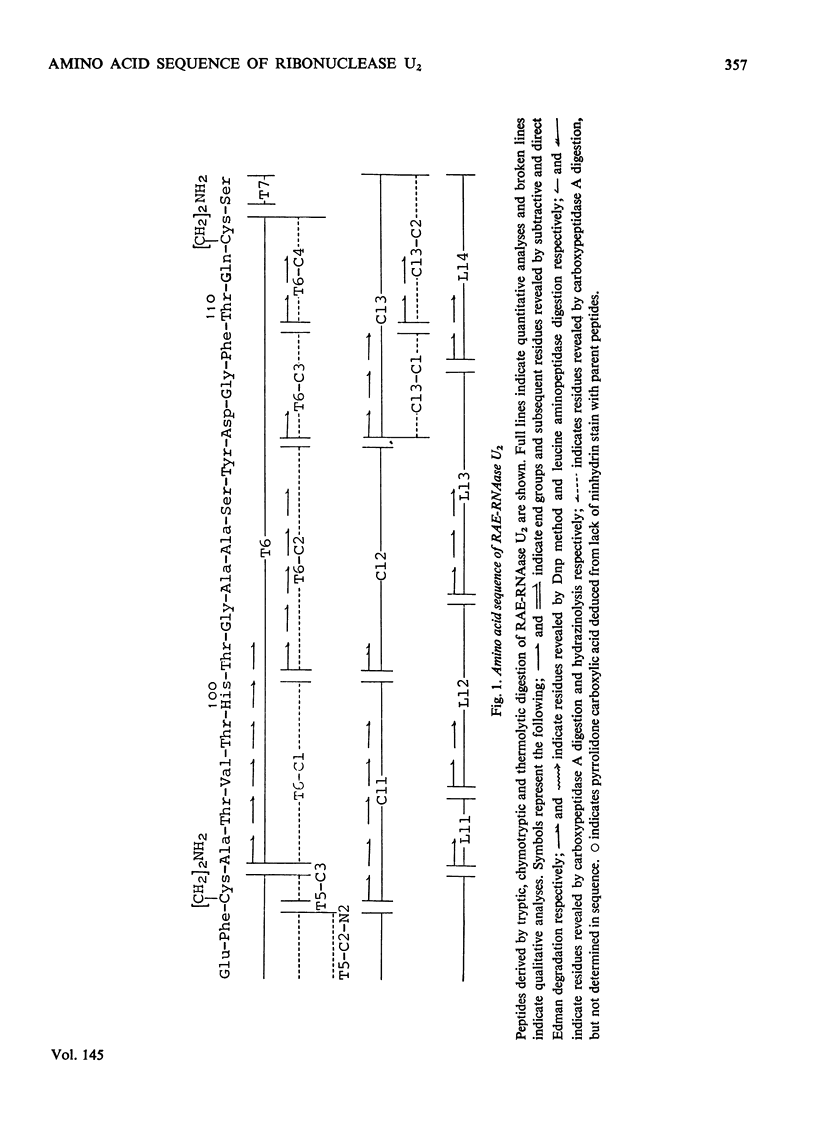
1. RNAase (ribonuclease) U2, a purine-specific RNAase, was reduced, aminoethylated and hydrolysed with trypsin, chymotrypsin and thermolysin. On the basis of the analyses of the resulting peptides, the complete amino acid sequence of RNAase U2 was determined, 2. When the sequence was compared with the amino acid sequence of RNAase T1 (EC 3.1.4.8), the following regions were found to be similar in the two enzymes; Tyr-Pro-His-Gln-Tyr (38-42) in RNAase U2 and Tyr-Pro-His-Lys-Tyr (38-42) in RNAase T1, Glu-Phe-Pro-Leu-Val (61-65) in RNAase U2 and Glu-Trp-Pro-Ile-Leu (58-62) in RNAase T1, Asp-Arg-Val-Ile-Tyr-Gln (83-88) in RNAase U2 and Asp-Arg-Val-Phe-Asn (76-81) in RNAase T1 and Val-Thr-His-Thr-Gly-Ala (98-103) in RNAase U2 and Ile-Thr-His-Thr-Gly-Ala (90-95) in RNAase T1. All of the amino acid residues, histidine-40, glutamate-58, arginine-77 and histidine-92, which were found to play a crucial role in the biological activity of RNAase T1, were included in the regions cited here. 3. Detailed evidence for the amino acid sequence of the sequence of the proteins has been deposited as Supplementary Publication SUP 50041 (33 PAGES) AT THE British Library (Lending Division)(formerly the National Lending Library for Science and Technology), Boston Spa, Yorks. LS23 7BQ, U.K., from whom copies can be obtained on the terms indicated in Biochem. J. (1975), 145, 5.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arima T., Uchida T., Egami F. Studies on extracellular ribonucleases of Ustilago sphaerogena. Characterization of substrate specificity with special reference to purine-specific ribonucleases. Biochem J. 1968 Feb;106(3):609–613. doi: 10.1042/bj1060609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blombäck B., Blombäck M., Edman P., Hessel B. Human fibrinopeptides. Isolation, characterization and structure. Biochim Biophys Acta. 1966 Feb 28;115(2):371–396. doi: 10.1016/0304-4165(66)90437-5. [DOI] [PubMed] [Google Scholar]
- FRAENKEL-CONRAT H., HARRIS J. I., LEVY A. L. Recent developments in techniques for terminal and sequence studies in peptides and proteins. Methods Biochem Anal. 1955;2:359–425. doi: 10.1002/9780470110188.ch12. [DOI] [PubMed] [Google Scholar]
- Offord R. E. Electrophoretic mobilities of peptides on paper and their use in the determination of amide groups. Nature. 1966 Aug 6;211(5049):591–593. doi: 10.1038/211591a0. [DOI] [PubMed] [Google Scholar]
- Raftery M. A., Cole R. D. On the aminoethylation of proteins. J Biol Chem. 1966 Aug 10;241(15):3457–3461. [PubMed] [Google Scholar]
- Takahashi K., Stein W. H., Moore S. The identification of a glutamic acid residue as part of the active site of ribonuclease T-1. J Biol Chem. 1967 Oct 25;242(20):4682–4690. [PubMed] [Google Scholar]
- Takahashi K. The structure and function of ribonuclease T1. XI. Modification of the single arginine residue in ribonuclease T1 by phenylglyoxal and glyoxal. J Biochem. 1970 Nov;68(5):659–664. doi: 10.1093/oxfordjournals.jbchem.a129399. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Uchida T., Egami F. Ribonuclease T1, Structure and function. Adv Biophys. 1970;1:53–98. [PubMed] [Google Scholar]
- Uchida T., Arima T., Egami F. Specificity of RNase U2. J Biochem. 1970 Jan;67(1):91–102. doi: 10.1093/oxfordjournals.jbchem.a129239. [DOI] [PubMed] [Google Scholar]


