Abstract
Background
Lymphovascular invasion (LVI) plays an important role in determining the risk of lymph node metastasis (LNM) in T1 colorectal cancer (CRC) patients and influencing treatment decisions and patient outcomes.
Objective
This study evaluated how the detection of LVI varies between Dutch laboratories and investigated its impact on the treatment and oncological outcomes of T1 CRC patients.
Methods
Pathology reports and clinical data of T1 CRC patients who underwent local resection between 2015 and 2019 were obtained from the Dutch nationwide pathology databank (Palga cohort, n = 5513). Data on the standard of LVI diagnosis (H&E/Immunohistochemistry) were not available. We categorized laboratories as low, average, or high detectors and evaluated the impact of LVI detection practice on the surgical resection rate and the proportion of LNM‐negative (LNM‐) surgeries. In the second part of the study, we used the Dutch T1 CRC Working Group cohort (n = 1268) to evaluate the impact of LVI detection practice on cancer recurrences during follow‐up. Multivariable logistic regression analyses and Cox proportional hazard regression were used to study the association between LVI detection practice and the outcomes.
Results
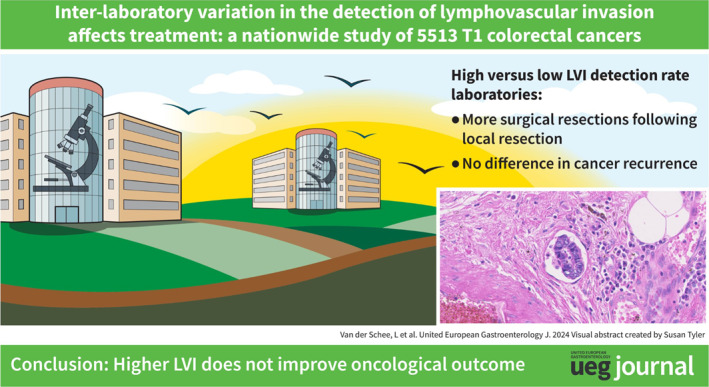
In the PALGA cohort, the proportion of surgical resections after local resection of a T1 CRC was significantly higher among patients diagnosed by laboratories with a high LVI detection rate (high vs. low: adjusted OR [aOR] 1.87; 95% confidence interval [CI] 1.52–2.31) as was the proportion of LNM‐surgeries (aOR 1.73; 95% CI 1.39–2.15). In the second cohort, no significant difference was observed in cancer recurrences among patients diagnosed in laboratories with high detection rates compared with low detection rates (aHR 2.23; 95% CI 0.94–5.23).
Conclusion
These findings suggest that a high detection rate of LVI does not improve oncological outcomes and may expose more patients to unnecessary oncological surgery, emphasizing the need for standardization of LVI diagnosis.
Keywords: bowel, CRC, histology, intestine, lymph node, metastasis, neoplasia, outcomes, stadiation, therapy
A high detection rate of LVI does not improve oncological outcomes and may expose more patients to unnecessary oncological surgery, emphasizing the need for standardization of LVI diagnosis.
Key summary.
Summarize the established knowledge on this subject
Lymphovascular invasion (LVI) is a risk factor for lymph node metastasis (LNM) in T1 colorectal cancer (CRC) and plays a crucial role in guiding treatment decisions.
Inter‐observer studies have revealed substantial variability among pathologists in the assessment of LVI.
The extent to which detection levels of LVI vary in everyday clinical practice and how this impacts treatment patterns and oncological outcomes is unknown.
What are the significant and/or new findings of this study?
LVI detection rates in locally resected T1 CRCs vary substantially between laboratories.
Patients diagnosed by laboratories with high LVI detection rates (>22.4%) have a significantly higher proportion of surgical resections and LNM‐negative surgeries following local resection of T1 CRC compared with those diagnosed by laboratories with low or average detection rates.
The proportion of cancer recurrences after local resection of T1 CRC does not differ significantly among patients diagnosed by laboratories with low, average, or high LVI detection rates.
These results show that a higher LVI detection rate in T1 CRCs does not result in improved oncological outcomes, and that more patients are unnecessarily exposed to the side effects of oncological surgery.
INTRODUCTION
Histopathological risk factors for lymph node metastasis (LNM) are the cornerstones in the treatment decision‐making process for T1 colorectal cancer (CRC). International guidelines recommend completion surgery when one or more of these risk factors are identified in a locally resected T1 CRC. 1 , 2 Although this approach of risk stratification is sensitive, specificity is known to be poor, with only 10%–16% of patients classified as ‘high‐risk’ actually having LNM. 3 , 4 , 5 , 6 , 7 Consequently, many patients receiving surgical intervention do so without any clinical benefit. Nevertheless, given the absence of validated, more precise prediction tools, the current risk stratification of T1 CRCs still depends on conventional histopathological risk factors.
It has been established that the assessment of histopathological features is subject to inter‐observer variability. 8 , 9 , 10 The extent of the variability may be influenced by many factors, including lack of clear definitions within guidelines, the type of staining applied, the number of slides and levels assessed, and the experience of the pathologists. 11 , 12 For lymphovascular invasion (LVI), one of the most important and extensively studied histological features in T1 CRC, inter‐observer agreement is reported to be poor. 13 This may partly explain the high variation in the reported detection rates of LVI in T1 CRCs within the available literature, ranging from 6% to 42%. 3 , 6 , 14 , 15 , 16 , 17
It is unknown if and how the variation in the detection of LVI within T1 CRCs affects patients' treatment and subsequent oncological outcomes. It could be hypothesized that high detection rates of LVI lead to higher rates of LNM‐negative completion surgery as it may overestimate the risk of LNM. Conversely, low detection rates of LVI may be associated with increased risk of cancer recurrence as it fails to accurately identify patients who are at risk of LNM and consequently may withhold them from receiving necessary surgical intervention. However, these hypotheses have not yet been evaluated in routine clinical practice. In the present study, we therefore aimed to study the impact of variation in LVI detection among locally resected T1 CRCs on patients' treatment strategies and oncological outcomes on a national level.
MATERIALS AND METHODS
Study populations
Two separate retrospective study populations were used to address the objectives of this study. The first cohort (hereafter referred to as the Palga cohort) was used to study the impact of the inter‐laboratory variation in the detection of LVI on the surgical resection rate and on the proportion of LNM‐negative surgeries. This cohort included all synoptically reported locally resected T1 CRCs diagnosed between January 1, 2015 and December 31, 2019 from the Dutch nationwide pathology databank (Palga). Primary surgical resections were excluded upfront since histopathological evaluation of risk factors in these specimens would not impact the choice of treatment and are potentially subject to underreporting of histopathological risk factors. Pathology reports of additional surgical resections, when performed, were also extracted for each patient. Exclusion criteria were: (1) non‐adenocarcinomas, (2) fragmented T1 CRC specimens, (3) neo‐adjuvant radiotherapy, (4) synchronous second primary CRC, and (5) reports from laboratories with less than five synoptically reported T1 CRCs per year. When patients had multiple T1 CRCs during the study period, the tumour with the first incidence date was included in the analyses. Fragmented T1 CRC specimens were excluded because of the complexity and inaccuracy of the assessment of histological risk factors in these specimens. Further explanations of variables and definitions included in this study are presented in Supporting Information S1. All data within Palga are pseudonymised by a trusted third party (ZorgTTP). The study protocol was approved by the scientific and privacy committee of Palga, and all data were handled in accordance with the General Data Protection Regulation Act.
To investigate the association between the LVI detection rate and cancer recurrences in patients not referred for surgery, we used our Dutch T1 CRC Working Group database (hereafter referred to as the T1 CRC Working Group cohort). This choice was made because of the insufficient coverage of cancer recurrence data in the Palga database. The T1 CRC Working Group cohort includes detailed data on follow‐up and cancer recurrences, which were previously obtained by reviewing the individual patient records (Medical Ethical Review Committee reference number 15‐487/C). From this multicenter database, including all consecutive T1 CRCs diagnosed between 1 January 2014 and 31 December 2017 in 12 hospitals, we extracted all locally resected T1 CRCs for the present study, with and without additional surgical resection. Exclusion criteria in this database were: (1) hereditary predisposition for CRC, (2) inflammatory bowel disease, (3) non‐adenocarcinomas, (4) neo‐adjuvant radiotherapy, (5) missing endoscopy or pathology reports, (6) synchronous second primary CRC, and (7) diagnosis of CRC within the previous 5 years. As for the Palga cohort, further explanations of variables and definitions used for the T1 CRC Working Group cohort are presented in Supporting Information S1.
Outcome measures
To assess the impact of variation in the detection of LVI on our outcome measures, we categorized pathology laboratories within the Palga cohort into three distinct groups based on their LVI detection rates. The categories were low detectors, average detectors and high detectors. The categorization was done by using the median proportion of LVI detection across all participating laboratories, along with the interquartile range (IQR) denoted by the first quartile (Q1) and the third quartile (Q3). Low detectors were defined as laboratories with an LVI detection rate lower than Q1, average detectors were considered laboratories with detection rates between the first (Q1) and the third quartile (Q3), and high detectors were defined as laboratories in which the LVI detection rate exceeded Q3.
We then evaluated the impact of variation in LVI detection rates on three outcome measures:
Surgical resection rate, defined as the proportion of patients who underwent completion surgery after local resection of a T1 CRC.
LNM‐negative surgeries are defined as the proportion of patients who underwent completion surgery after local resection of a T1 CRC and did not have tumour‐positive lymph nodes in the resection specimen (i.e., surgery that was unnecessary in hindsight).
Cancer recurrences are defined as the proportion of locoregional and/or distant cancer recurrences after local resection of a T1 CRC where no completion surgery was performed.
The first two outcomes were evaluated with the data from the Palga cohort and the final outcome was studied based on data from the T1 CRC Working Group cohort.
Statistical analyses
Baseline characteristics for patients with and without LVI were reported using standard descriptive statistics. Differences in categorical variables were analyzed by means of a chi‐square test, and for continuous data that were not normally distributed the Mann–Whitney‐U test was used.
Of each laboratory within the Palga cohort, the absolute proportion of T1 CRCs with LVI was determined. As a benchmark, the overall national proportion of LVI‐positive T1 CRCs of all laboratories was used. Since case‐mix factors might influence the detection of LVI and could differ per laboratory, we corrected for these factors through multivariable logistic regression to calculate case‐mix adjusted proportions per laboratory. 18 , 19 The variables included were age at diagnosis, year of diagnosis, size of the polyp, grade of differentiation and invasion depth. To visualize the inter‐laboratory variation in LVI detection, a funnel plot was used.
To evaluate the impact of LVI detection rate practice (low detectors, average detectors, and high detectors) on the surgical resection rate and the proportion of LNM‐negative surgical resections in the Palga cohort, we performed multivariable logistic regression analysis. Cox proportional hazard regression was used to study the association between LVI detection rate practice and cancer recurrence during follow‐up within the T1 CRC Working Group cohort. In these analyses, low detectors were used as the reference category and we corrected for age, polyp location, year of diagnosis, grade of differentiation, and resection margin status.
To study what the impact of the variation in LVI detection would be in the group of patients where LVI would have been the decisive factor in histological risk stratification, we performed sensitivity analyses in a subgroup of patients without other histological high‐risk factors included in the Dutch guideline at the time of the study period (high‐grade differentiation and/or positive [R1] or unassessable [Rx] resection margin). This subgroup had no other (histopathological) indication for completion surgery other than LVI (i.e., if LVI was absent, a T1 CRC would be classified as low‐risk and if LVI was present, a T1 CRC would be classified high‐risk).
For the Palga cohort, missing date were Missing Completely At Random (MCAR). Therefore, listwise deletion was performed when performing the analyses. For the T1 CRC Working Group cohort, missing data were not MCAR; thus, we performed multiple imputation before data analysis (using multivariate imputation by chained equations with 22 variables, 10 imputation data sets and 21 iterations). Rubin's rules were used to pool results across imputed datasets. All statistical analyses were performed using R version 4.0.3 (RStudio Inc.). A two‐sided p value <0.05 was considered significant.
RESULTS
Patient and tumour characteristics
Of all 6135 local CRC excisions from 6050 patients within the Palga cohort, 5513 individual patients were included in the final analyses (Figure 1). The final 5513 T1 CRCs were diagnosed within 36 of all 41 Dutch pathology laboratories between 2015 and 2019, ranging from 52 to 363 (median 133) locally excised T1 CRCs per laboratory. Patient and tumour characteristics of the 5513 included patients are shown in Table 1.
FIGURE 1.
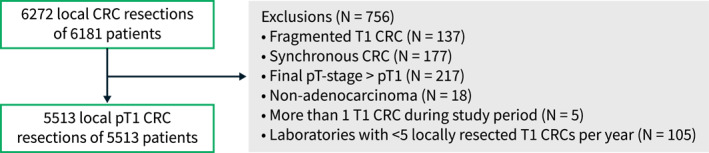
Flowchart of included and excluded patients of the Palga cohort. CRC, colorectal cancer.
TABLE 1.
Characteristics of the 5513 included patients with locally resected T1 CRCs from the Palga cohort.
| Total | LVI absent | LVI present | p Value | |
|---|---|---|---|---|
| n = 5513 | n = 4499 | n = 1014 | ||
| Age, in years, median (IQR) | 67 (12) | 67 (12) | 67 (12) | 0.212 |
| Gender, n (%) | 0.477 | |||
| Male | 3453 (62.6) | 2808 (62.5) | 645 (63.6) | |
| Female | 2060 (37.4) | 1691 (37.5) | 369 (36.4) | |
| Type of laboratory, n (%) | <0.001 | |||
| Non‐academic | 4908 (89.0) | 4078 (90.6) | 830 (81.9) | |
| Academic | 605 (11.0) | 421 (9.4) | 184 (18.1) | |
| Year of diagnosis, n (%) | <0.001 | |||
| 2015 | 1074 (19.5) | 910 (20.2) | 164 (16.2) | |
| 2016 | 1206 (21.9) | 1004 (22.3) | 202 (19.9) | |
| 2017 | 1102 (20.0) | 905 (20.1) | 197 (19.4) | |
| 2018 | 1177 (21.3) | 942 (20.9) | 235 (23.2) | |
| 2019 | 954 (17.3) | 738 (16.5) | 216 (21.3) | |
| Screen‐detected, n (%) | 0.104 | |||
| No | 2505 (45.4) | 2021 (44.9) | 484 (47.7) | |
| Yes | 3008 (54.6) | 2478 (55.1) | 530 (52.3) | |
| Location, n (%) | 0.825 | |||
| Colon | 3513 (64.2) | 2863 (64.2) | 650 (64.5) | |
| Rectum | 1955 (35.8) | 1598 (35.8) | 357 (35.5) | |
| Missing | 45 | 38 | 7 | |
| Diameter polyp, in mm, median (IQR) | 15 (10) | 15 (10) | 16 (12) | <0.05 |
| Differentiation, n (%) | <0.001 | |||
| Low‐grade | 5246 (98.3) | 4304 (98.8) | 942 (95.9) | |
| High‐grade | 91 (1.7) | 51 (1.2) | 40 (4.1) | |
| Missing | 176 | 144 | 32 | |
| Invasion depth, n (%) | <0.001 | |||
| sm1/Haggitt 1–2/<1 mm | 2413 (48.3) | 2029 (50.0) | 384 (50.0) | |
| sm2/Haggitt 3/1–2 mm | 1135 (22.7) | 859 (21.2) | 276 (29.5) | |
| sm3/Haggitt 4/>2 mm | 541 (10.8) | 416 (10.2) | 125 (13.3) | |
| Could not be assessed | 906 (18.1) | 754 (18.6) | 152 (16.2) | |
| Missing | 518 | 441 | 77 | |
| Resection margin, n (%) | 0.555 | |||
| R0 | 4185 (76.0) | 3408 (75.8) | 777 (76.7) | |
| R1 | 782 (14.2) | 636 (14.2) | 146 (14.4) | |
| Rx | 540 (9.8) | 450 (10.0) | 90 (8.9) | |
| Missing | 6 | 5 | 1 | |
| Treatment strategy, n (%) | <0.001 | |||
| Local resection only | 4176 (75.7) | 3750 (83.4) | 426 (42.0) | |
| Completion surgery | 1337 (24.3) | 749 (16.6) | 588 (58.0) |
Note: Significance for italic value is ‘These data were missing in the original pathology report’.
Abbreviations: CRC, colorectal cancer; IQR, inter quartile range; LVI, lymphovascular invasion; R0, free resection margin; R1, positive resection margin; Rx, unassessable resection margin.
Inter‐laboratory variation in detection of LVI
The overall national proportion of T1 CRC cases with LVI was 18.4%. Laboratory‐specific detection rates ranged from 8.0% to 43.9%. Fourteen laboratories (38.9%) reported proportions of LVI outside the 95% confidence interval (CI) of the national proportion.
After correction for case‐mix, still 12/36 laboratories (33.3%) fell outside the 95% CI of the national proportion and thus had significantly higher or lower detection rates than expected. Of the 12 laboratories outside the 95% CI, 6 (16.7%) reported a significantly, but only a slightly, lower proportion of LVI than the overall national proportion, while in 6 laboratories (16.7%) the proportion of LVI detection in T1 CRCs was significantly higher and more prominent (Figure 2).
FIGURE 2.
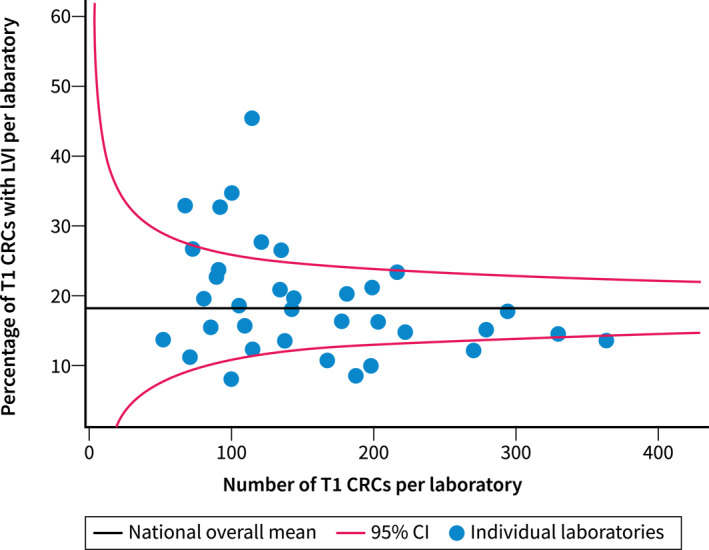
Funnel plot showing the percentage of T1 CRCs with positive LVI per laboratory from the Palga cohort adjusted for case‐mix factors, plotted against the total number of T1 CRCs from the corresponding laboratory. CI, confidence interval; CRC, colorectal cancer; LVI, lymphovascular invasion.
Impact of variation on patients' treatment and oncological outcomes
We categorized the laboratories into three groups based on their LVI detection rates: low detectors, average detectors, and high detectors. Low detectors included laboratories with less than 13.7% of T1 CRCs with positive LVI, average detectors had LVI detection rates between 13.7% and 22.4%, and high detectors had LVI detection rates of more than 22.4%.
Surgical resection rate
Of all 5513 patients, 1337 (24.3%) underwent completion surgery after local resection. Through multivariable logistic regression, we found that patients whose T1 CRCs were analyzed in laboratories with high LVI detection rates were treated significantly more often with completion surgery compared to patients whose T1 CRCs were analyzed in laboratories with low LVI detection rates (30.9% vs. 22.2%; adjusted OR [aOR] 1.87, 95% CI 1.52–2.31). No significant difference in treatment was observed between low and average detectors (22.2% vs. 23.0%; aOR average vs. low 1.05, 95% CI 0.89–1.25) (Table 2) (Figure 3).
TABLE 2.
Multivariable logistic regression analysis for the association between LVI detection practice and surgical resection rate or LNM‐negative surgeries in a nationwide Palga cohort of 5513 locally resected T1 CRCs.
| Multivariable OR (95% CI) | |
|---|---|
| Surgical resection rate | |
| LVI detection practice a | |
| Low detectors | Ref |
| Average detectors | 1.05 (0.89–1.25) |
| High detectors | 1.87 (1.52–2.31) |
| Differentiation | |
| Low‐grade | Ref |
| High‐grade | 5.57 (3.52–8.90) |
| Resection margin | |
| R0 | Ref |
| R1 | 6.66 (5.57–7.95) |
| Rx | 3.01 (2.44–3.70) |
| Age | 0.96 (0.95–0.97) |
| Tumour location | |
| Colon | Ref |
| Rectum | 0.46 (0.39–0.53) |
| Year of diagnosis | |
| 2015 | Ref |
| 2016 | 0.92 (0.75–1.13) |
| 2017 | 0.82 (0.66–1.02) |
| 2018 | 0.81 (0.66–1.01) |
| 2019 | 0.69 (0.55–0.87) |
| LNM‐negative surgeries | |
| LVI detection practice a | |
| Low detectors | Ref |
| Average detectors | 1.00 (0.84–1.20) |
| High detectors | 1.73 (1.39–2.15) |
| Differentiation | |
| Low‐grade | Ref |
| High‐grade | 3.91 (2.50–6.13) |
| Resection margin | |
| R0 | Ref |
| R1 | 5.39 (4.50–6.44) |
| Rx | 2.92 (2.35–3.60) |
| Age | 0.97 (0.96–0.98) |
| Tumour location | |
| Colon | Ref |
| Rectum | 0.47 (0.40–0.55) |
| Year of diagnosis | |
| 2015 | Ref |
| 2016 | 0.90 (0.73–1.11) |
| 2017 | 0.76 (0.60–0.95) |
| 2018 | 0.77 (0.62–0.97) |
| 2019 | 0.64 (0.50–0.81) |
Abbreviations: CI, confidence interval; CRC, colorectal cancer; LNM, lymph node metastasis; LVI, lymphovascular invasion; OR, odds ratio; Ref, reference category; R0, microscopically free resection margin; R1, microscopically positive resection margin; Rx, resection margin could not be assessed.
Low detectors: proportion of LVI positive T1 CRCs is lower than Q1 of the national median proportion, average detectors: proportion of LVI positive T1 CRCs is between Q1 and Q3 of the national median proportion, high detectors: proportion of LVI positive T1 CRCs is higher than Q3 of the national median proportion.
FIGURE 3.
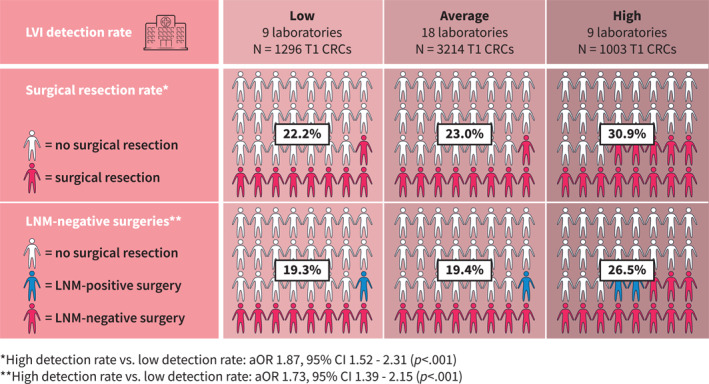
Percentage of T1 CRC patients treated with surgical resection after local excision and the percentage of patients who had LNM‐negative surgery in laboratories with low, average and high LVI detection rates (Palga cohort). aOR, adjusted odds ratio; CI, confidence interval; CRC, colorectal cancer; LNM, lymph node metastasis; LVI, lymphovascular invasion.
In the sensitivity analysis of a subset of 4026 patients without other known histological risk factors other than LVI (i.e., high‐grade differentiation or an R1 or Rx resection margin), results were consistent with the findings in the complete cohort (Supporting Information S1: Supplementary Results).
LNM‐negative surgeries
In 1139/5513 (20.7%) T1 CRC patients, completion surgery was performed without LNM being detected in the resection specimen. No difference in the percentage of—what afterwards turned out to be—unnecessary surgery was found between average detectors and low detectors (19.4% vs. 19.3%; aOR 1.00; 95% CI 0.84–1.20). However, LNM‐negative surgeries were significantly more common in patients who were diagnosed by laboratories with high LVI detection rates (26.5% vs. 19.3%; aOR 1.73; 95% CI 1.39–2.15) (Table 2) (Figure 3).
These results were confirmed in the sensitivity analyses, which included 4026 patients in whom LVI would have been the decisive factor in histological risk stratification (Supporting Information S1: Supplementary Results).
Cancer recurrences
To evaluate if low LVI detection practice was associated with an increased risk of cancer recurrence after local resection of T1 CRCs without additional surgery, we used data from the T1 CRC Working Group cohort. In total, 1268 locally resected T1 CRCs were included in this study (Figure S1). The overall proportion of T1 CRCs with LVI was 18.6% and laboratory‐specific proportions ranged from 8.2% to 34.7%.
The median follow‐up of this cohort was 51 months (IQR 32) and did not differ between patients from laboratories with low, average, and high LVI detection rates (52, 49, and 51 months, respectively).
Local resection without completion surgery was the final treatment in 847/1268 patients (66.8%). Recurrences after local resection only occurred in 35/847 (4.1%) T1 CRC patients. In both univariable and multivariable Cox proportional hazards regression analyses, no significant difference in the proportion of recurrences was observed between low (3.2%), average (4.0%), and high (5.2%) LVI detectors (high vs. low: unadjusted hazard ratio [HR] 1.78; 95% CI 0.80–3.97; adjusted HR 2.23; 95% CI 0.94–5.23) (Table 3). Stratification according to histopathological risk group and the type of recurrence did not reveal any significant differences between the low, average and high detectors (Table 4).
TABLE 3.
Multivariable Cox regression analysis for the association between LVI detection practice and cancer recurrences in the T1 CRC Working Group cohort of 847 locally resected T1 CRCs.
| Multivariable HR (95% CI) | |
|---|---|
| LVI detection practice a | |
| Low detectors | Ref |
| Average detectors | 1.36 (0.54–3.45) |
| High detectors | 2.23 (0.95–5.23) |
| Differentiation | |
| Low‐grade | Ref |
| High‐grade | 0.00 (0.00 ‐ ∞) |
| Resection margin | |
| R0 | Ref |
| R1 | 1.03 (0.30–3.58) |
| Rx | 1.78 (0.77–4.12) |
| Age | 1.04 (0.99–1.09) |
| Location | |
| Colon | Ref |
| Rectum | 3.91 (1.83–8.34) |
| Year diagnosis | |
| 2014 | Ref |
| 2015 | 1.41 (0.47–4.21) |
| 2016 | 2.00 (0.70–5.73) |
| 2017 | 1.64 (0.51–5.25) |
Abbreviations: CI, confidence interval; CRC, colorectal cancer; HR, hazard ratio; LVI, lymphovascular invasion; Ref, reference category; R0, microscopically free resection margin; R1, microscopically positive resection margin; Rx, resection margin could not be assessed.
Low detectors: proportion of LVI positive T1 CRCs is lower than Q1 of the national median proportion, average detectors: proportion of LVI positive T1 CRCs is between Q1 and Q3 of the national median percentage, high detectors: proportion of LVI positive T1 CRCs is higher than Q3 of the national median proportion.
TABLE 4.
Recurrences within the group of 847 T1 CRCs from the T1 CRC Working Group cohort that were treated with local resection only were stratified by LVI detection group.
| Low LVI detectors | Average LVI detectors | High LVI detectors | p Value | |
|---|---|---|---|---|
| N = 312 patients | N = 248 patients | N = 287 patients | ||
| Recurrences, total | 10/312 (3.2%) | 10 (4.0%) | 15 (5.2%) | 0.46 |
| Recurrences per histopathological risk group a | ||||
| Low‐risk | 5/217 (2.3%) | 2/151 (1.3%) | 8/186 (4.3%) | 0.24 |
| High‐risk | 5/90 (5.6%) | 8/86 (9.3%) | 7/89 (7.9%) | 0.62 |
| Unknown | 0/5 (0.0%) | 0/11 (0.0%) | 0/12 (0.0%) | – |
| Type of recurrence | ||||
| Locoregional only | 4/312 (1.3%) | 2/248 (0.8%) | 5/287 (1.7%) | 0.64 |
| Distant metastasis only | 2/312 (0.6%) | 4/248 (1.6%) | 5/287 (1.7%) | 0.44 |
| Both | 4/312 (1.3%) | 4/248 (1.6%) | 5/287 (1.7%) | 0.88 |
High‐risk was defined as T1 colorectal cancers (CRCs) with the presence of one or more of the following risk‐factors: lymphovascular invasion (LVI), high‐grade differentiation, positive resection margin (R1), or unassessable (Rx) resection margin. Low‐risk was defined as the absence of all these risk factors. Unknown risk was applied to T1 CRCs for which data on one or more risk factors were missing.
DISCUSSION
With this nationwide data of 5513 locally resected T1 CRCs, we studied the inter‐laboratory variability in the detection of LVI and examined its impact on patient treatment. Our findings indicate that the detection of LVI varies substantially between laboratories, and that patients treated at hospitals working with laboratories with high LVI detection rates have significantly higher rates of completion surgery without clinical benefit, as compared to patients in hospitals working with laboratories with low or average LVI detection rates. In contrast, the patients in the hospitals working with laboratories with low LVI detection rates were not at higher risk of developing local and distant recurrences after only local excision. This shows that a higher LVI detection rate does not result in improved oncological outcomes, and that more patients are unnecessarily exposed to the side effects of oncological surgery.
The underlying causes of this variability in LVI detection are likely diverse, existing both at the level of individual pathologists and at the laboratory level. One of the problems is the absence of standardized criteria for the assessment of LVI, for example, regarding the evaluation of deeper levels and the utilization of additional (immuno‐)histochemical staining (IHC). While several guidelines recommend the use of additional staining, none of these guidelines provide explicit instructions on when to apply them, what type of staining discriminates best and how to interpret the results of the staining. 2 , 20 , 21 , 22 , 23 Studies have demonstrated that the application of additional staining leads to an increase in the detection rate of LVI. 12 , 24 And although we acknowledge that staining techniques can facilitate the differentiation between genuine LVI and LVI‐mimicking artefacts, concerns arise regarding the reflex application of additional staining for LVI detection in every T1 CRC case. Such a strategy might identify more insignificant foci of LVI and lead to an increase in false‐positive staining results, potentially leading to an increase in overtreatment and significant costs. Considering this, we endorse the recommendation specified in some guidelines, which advocates for diagnosing LVI based on the H&E and reserving additional staining for equivocal cases where LVI is suspected but lacks conclusive affirmation on the H&E. 25 , 26 Although the dataset used in our study unfortunately lacks specific data on the utilization of additional staining, other cohort studies have demonstrated lower LVI detection rates with higher positive predictive values for LNM when LVI was solely assessed on H&E, as compared to studies using standard IHC alongside H&E. 5 , 6 , 27 , 28 Given the low positive predictive value of histological risk stratification in T1 CRCs in general, these findings might support the recommendation to refrain from routine application of additional staining techniques for the detection of LVI. Other factors likely to contribute to the inter‐laboratory variation include the interchangeable use of different terminologies for LVI (e.g., small vessel invasion or large vessel invasion, lymphatic or venous invasion, vascular invasion, LVI), and differences in pathologists' expertise, experience, caseload, and personal beliefs. However, investigating the causal relationships between these factors and the observed variation was beyond the scope of our current study, warranting future research to address these important aspects. Efforts to enhance inter‐observer agreement and decrease inter‐laboratory variation in the reporting of histopathological features have shown promising results in other fields and should be encouraged for the assessment of T1 CRCs. 29 , 30 , 31 , 32 , 33 We believe that creating awareness is a crucial first step. Findings from a study by Dooijeweert et al. suggest that reduction of variation can be effectively achieved through simply providing laboratory‐ and pathologist‐specific feedback regarding detection rates. 30 Furthermore, it is imperative to refine and standardize the definitions and criteria for detecting LVI, as the current guidelines leave room for different interpretations, which in turn likely contributes to the observed variation in clinical practice.
Under the assumption that the true prevalence of LVI in locally resected T1 CRCs aligns with the national percentage of 18.4%, one can hypothesize that LVI was underdetected in a subset of patients within the group of low detectors. If this hypothesis holds true, one might expect that patients classified as low‐risk within this subgroup, who solely underwent local resection as a treatment, may face a higher risk of cancer recurrence owing to an unrecognized risk of LNM. However, our results show that after a median follow‐up of 51 months, no significant differences were found in cancer recurrences between patients from low‐, average‐, and high‐detector laboratories. A potential explanation for this finding is the recent evidence that metastases of CRC do not solely arise from spread via locoregional lymph nodes, but that in fact most distant metastases follow a direct route via the venous system, independent of LNM. 34 , 35 Together with our observation that oncological long‐term outcomes did not differ between patients whose polyps were analyzed in low‐, average‐, and high‐detector laboratories, despite potential differences in classification of the risk of LNM, this challenge the current role of LNM in being the most important prognostic factor for T1 CRC. As opposed to the similar oncological outcomes independent of LVI detection rate, we did observe a significantly higher surgical resection rate in the patients treated at hospitals with high LVI detection. Most importantly, more than one‐fourth of the patients whose polyps were analyzed in high detector laboratories were unnecessarily put at the risk of surgery since no LNM was found in the resection specimen of these patients. For the low and average detectors, although still substantial, this was less than one‐fifth and significantly lower. The issue of surgical overtreatment in T1 CRC is widely recognized, and despite advancements in surgical techniques and perioperative care, the risks of surgical mortality and morbidity cannot be ignored. 36 Another interesting observation beyond the scope of our initial research objectives was that a considerable proportion of patients (42.0%) with a T1 CRC in which LVI was detected, did not undergo completion surgery, suggesting that clinicians often accept the risk of leaving LNM behind when weighing it against the risk of surgery associated morbidity and mortality. Location of the primary tumour seems to play a role in this decision, which is demonstrated by our multivariable analysis showing that rectal location was independently negatively associated with completion of surgery.
The substantial variation in the detection of LVI observed in our study underscores the challenges and subjectivity inherent in pathological assessment. Clinicians working together with pathologists must acknowledge the complexity of histopathological evaluation and recognize that it is a nuanced process that requires a comprehensive understanding of the pathologist's rationale behind their conclusions. We strongly advocate for the discussion of each T1 CRC during multidisciplinary meetings. This facilitates a careful consideration of the different treatment options, enabling a more precise evaluation of the risks and benefits associated with additional surgical resection.
A few limitations of our study need to be considered when interpreting the results. First, although we adjusted for most clinical and histopathological variables, we were not able to include tumour budding and polyp morphology as case‐mix variables. Tumour budding was introduced as a non‐mandatory factor in the synoptic report only in mid‐2016 in the Netherlands; therefore, this variable was missing in 72% of cases. Polyp morphology could only be extracted from the pathology reports, while it is known that morphology can alter during histological processing and was therefore considered unreliable. Still, all other variables important in the variation of case‐mix were included and adjusted for. Second, reports from fragmented resection specimens were excluded from this study because assessment of histopathological features is hampered in these specimens. It is conceivable that exclusion of piecemeal resected T1 CRCs in our study led to exclusion of the ones that were less amenable for en bloc resection, and thus may have introduced a selection bias towards smaller tumours with potentially a different LVI detection rate. Therefore, the numbers of T1 CRCs with LVI in this study may not be representative of piecemeal resected T1 CRCs. Finally, as mentioned earlier, specific details on the method of LVI diagnosis (H&E or IHC) were lacking in our dataset, thereby impeding us to draw a more robust conclusion regarding the impact of the use of additional staining on LVI detection rates and treatment.
In conclusion, the findings of this study demonstrate a substantial variation in the detection of LVI among locally resected T1 CRCs at a national level. Moreover, the study reveals that higher levels of LVI detection are associated with an increased risk of unnecessary completion surgery, as compared to low and average detection levels. Notably, there were no significant differences in cancer recurrence rates after local excision among the three LVI detection groups, implying that lower levels of LVI detection accompanied by fewer completion surgeries do not compromise oncological outcomes. These findings challenge that, concerning LVI detection in T1 CRCs, ‘more is better’ and in fact suggest that ‘less may not matter’. For clinicians, this study highlights the importance of possessing a comprehensive understanding of how the LVI was detected and what brought the pathologist to his or her conclusions, so that the actual risk of LNM can be weighed against the risk of surgical morbidity and mortality, while also considering the individual preferences of the patient.
CONFLICT OF INTEREST STATEMENT
There are no conflicts of interest.
Supporting information
Supporting Information S1
Figure S1
ACKNOWLEDGEMENTS
None.
van der Schee L, Verbeeck A, Deckers IAG, Kuijpers CCHJ, Offerhaus GJA, Seerden TCJ, et al. Variation in the detection of lymphovascular invasion in T1 colorectal cancer and its impact on treatment: a nationwide Dutch study. United European Gastroenterol J. 2024;12(10):1429–39. 10.1002/ueg2.12670
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Hashiguchi Y, Muro K, Saito Y, Ito Y, Ajioka Y, Hamaguchi T, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol. 2020;25(1):1–42. 10.1007/s10147-019-01485-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ferlitsch M, Moss A, Hassan C, Bhandari P, Dumonceau JM, Paspatis G, et al. Colorectal polypectomy and endoscopic mucosal resection (EMR): European Society of Gastrointestinal Endoscopy (ESGE) clinical guideline. Endoscopy. 2017;49(3):270–297. 10.1055/s-0043-102569 [DOI] [PubMed] [Google Scholar]
- 3. Yasue C, Chino A, Takamatsu M, Namikawa K, Ide D, Saito S, et al. Pathological risk factors and predictive endoscopic factors for lymph node metastasis of T1 colorectal cancer: a single‐center study of 846 lesions. J Gastroenterol. 2019;54(8):708–717. 10.1007/s00535-019-01564-y [DOI] [PubMed] [Google Scholar]
- 4. Bosch S, Teerenstra S, De Wilt JW, Cunningham C, Nagtegaal I. Predicting lymph node metastasis in pT1 colorectal cancer: a systematic review of risk factors providing rationale for therapy decisions. Endoscopy. 2013;45(10):827–834. [Internet]. [Cited 2020 Jul 27]. 10.1055/s-0033-1344238 [DOI] [PubMed] [Google Scholar]
- 5. Kajiwara Y, Oka S, Tanaka S, Nakamura T, Saito S, Fukunaga Y, et al. Nomogram as a novel predictive tool for lymph node metastasis in T1 colorectal cancer treated with endoscopic resection: a nationwide, multicenter study. Gastrointest Endosc. 2023;97(6):1119–1128.e5. [Internet]. 10.1016/j.gie.2023.01.022 [DOI] [PubMed] [Google Scholar]
- 6. Ebbehøj AL, Smith HG, Jørgensen LN, Krarup PM. Prognostic factors for lymph node metastases in pT1 colorectal cancer differ according to tumor morphology: a nationwide cohort study. Ann Surg. 2023;277(1):127–135. 10.1097/sla.0000000000005684 [DOI] [PubMed] [Google Scholar]
- 7. Kudo SE, Ichimasa K, Villard B, Mori Y, Misawa M, Saito S, et al. Artificial intelligence system to determine risk of T1 colorectal cancer metastasis to lymph node. Gastroenterology. 2021;160(4):1075–1084.e2. [Internet]. [Cited 2023 May 9]. 10.1053/j.gastro.2020.09.027 [DOI] [PubMed] [Google Scholar]
- 8. Davenport A, Morris J, Pritchard SA, Salmo E, Scott M, Haboubi NY. Interobserver variability amongst gastrointestinal pathologists in assessing prognostic parameters of malignant colorectal polyps: a cause for concern. Tech Coloproctol. 2016;20(9):647–652. 10.1007/s10151-016-1513-8 [DOI] [PubMed] [Google Scholar]
- 9. Komuta K, Batts K, Jessurun J, Snover D, Garcia‐Aguilar J, Rothenberger D, et al. Interobserver variability in the pathological assessment of malignant colorectal polyps. Br J Surg. 2004;91(11):1479–1484. [Internet]. [Cited 2020 Jul 27]. 10.1002/bjs.4588 [DOI] [PubMed] [Google Scholar]
- 10. Turner JK, Williams GT, Morgan M, Wright M, Dolwani S. Interobserver agreement in the reporting of colorectal polyp pathology among bowel cancer screening pathologists in Wales. Histopathology. 2013;62(6):916–924. 10.1111/his.12110 [DOI] [PubMed] [Google Scholar]
- 11. Kai K, Aishima S, Aoki S, Takase Y, Uchihashi K, Masuda M, et al. Cytokeratin immunohistochemistry improves interobserver variability between unskilled pathologists in the evaluation of tumor budding in T1 colorectal cancer. Pathol Int. 2016;66(2):75–82. 10.1111/pin.12374 [DOI] [PubMed] [Google Scholar]
- 12. Kirsch R, Messenger DE, Riddell RH, Pollett A, Cook M, Al‐Haddad S, et al. Venous invasion in colorectal cancer: impact of an elastin stain on detection and interobserver agreement among gastrointestinal and nongastrointestinal pathologists. Am J Surg Pathol. 2013;37(2):200–210. [Internet]. [Cited 2020 Jun 22]. 10.1097/pas.0b013e31826a92cd [DOI] [PubMed] [Google Scholar]
- 13. Harris EI, Lewin DN, Wang HL, Lauwers GY, Srivastava A, Shyr Y, et al. Lymphovascular invasion in colorectal cancer: an interobserver variability study. Am J Surg Pathol. 2008;32(12):1816–1821. [Internet]. [Cited 2021 Feb 15]. 10.1097/pas.0b013e3181816083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bae HJ, Ju H, Lee HH, Kim J, Lee BI, Lee SH, et al. Long‐term outcomes after endoscopic versus surgical resection of T1 colorectal carcinoma. Surg Endosc. 2023;37(2):1231–1241. 10.1007/s00464-022-09649-1 [DOI] [PubMed] [Google Scholar]
- 15. Kim JK, Rhee YY, Bae JM, Kim JH, Koh SJ, Lee HJ, et al. Composite scoring system and optimal tumor budding cut‐off number for estimating lymph node metastasis in submucosal colorectal cancer. BMC Cancer. 2022;22(1):861. 10.1186/s12885-022-09957-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Morini A, Annicchiarico A, De Giorgi F, Ferioli E, Romboli A, Montali F, et al. Local excision of T1 colorectal cancer: good differentiation, absence of lymphovascular invasion, and limited tumor radial infiltration (≤4.25 mm) may allow avoiding radical surgery. Int J Colorectal Dis. 2022;37(12):2525–2533. 10.1007/s00384-022-04279-4 [DOI] [PubMed] [Google Scholar]
- 17. Ozeki T, Shimura T, Ozeki T, Ebi M, Iwasaki H, Kato H, et al. The risk analyses of lymph node metastasis and recurrence for submucosal invasive colorectal cancer: novel criteria to skip completion surgery. Cancers (Basel). 2022;14(3):822. 10.3390/cancers14030822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nuttall D, Parkin D, Devlin N. Inter‐provider comparison of patient‐reported outcomes: developing an adjustment to account for differences in patient case mix. Health Econ (UK). 2015;24(1):41–54. 10.1002/hec.2999 [DOI] [PubMed] [Google Scholar]
- 19. Van Der Heiden‐Van Der Loo M, De ML, Visser O, Westenend PJ, Van Dalen T, Menke MB, et al. Variation between hospitals in surgical margins after first breast‐conserving surgery in The Netherlands. Breast Cancer Res Treat. 2012;131(2):691–698. 10.1007/s10549-011-1809-3 [DOI] [PubMed] [Google Scholar]
- 20. Washington MK, Berlin J, Branton P, Burgart LJ, Carter DK, Fitzgibbons PL, et al. Protocol for the examination of specimens from patients with primary carcinoma of the colon and rectum. Arch Pathol Lab Med. 2009;133(10):1539–1551. [Internet]. 10.5858/133.10.1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rosty C, Brown I, Cooper H, Dekker E, Driman D, Gonzalez R, et al. Colorectal excisional biopsy (polypectomy) histopathology reporting guide. Sydney: International Collaboration on Cancer Reporting; 2020. [Google Scholar]
- 22. Richtlijn Colorectaal carcinoom (CRC) versie 4.0; 2019. [Internet]. Available from: https://richtlijnendatabase.nl/richtlijn/colorectaal_carcinoom_crc/startpagina_‐_crc.html
- 23. Colorectal cancer structured reporting protocol. 4th ed. Sydney: RCPA; 2020. [Google Scholar]
- 24. Ervine AJ, McBride HA, Kelly PJ, Loughrey MB. Double immunohistochemistry enhances detection of lymphatic and venous invasion in early‐stage colorectal cancer. Virchows Arch. 2015;467(3):265–271. 10.1007/s00428-015-1792-x [DOI] [PubMed] [Google Scholar]
- 25. Nederlandse Vereniging voor Heelkunde . Richtlijn Colorectaal Carcinoom (CRC); 2023. p. 5–18. [Internet]. [Cited 2023 Nov 14]. Available from: https://richtlijnendatabase.nl/richtlijn/colorectaal_carcinoom_crc/pathologie_bij_crc/histologische_risicofactoren_bij_t1_crc.html
- 26. Loughrey MB, Quirke P, Shepherd NA. Dataset for histopathological reporting of colorectal cancer, version 5. 2023. [Internet]. Available from: https://www.rcpath.org/static/c8b61ba0‐ae3f‐43f1‐85ffd3ab9f17cfe6/c19a5cd7‐3485‐44c2‐b5e1c87154830582/G049‐Dataset‐for‐histopathological‐reporting‐of‐colorectal‐cancer.pdf
- 27. Ronnow CF, Arthursson V, Toth E, Krarup PM, Syk I, Thorlacius H. Lymphovascular infiltration, not depth of invasion, is the critical risk factor of metastases in early colorectal cancer: retrospective population‐based cohort study on prospectively collected data, including validation. Ann Surg. 2022;275(1):E148–E154. [Internet]. [Cited 2023 May 9]. 10.1097/sla.0000000000003854 [DOI] [PubMed] [Google Scholar]
- 28. Mochizuki K, Kudo SE, Ichimasa K, Kouyama Y, Matsudaira S, Takashina Y, et al. Left‐sided location is a risk factor for lymph node metastasis of T1 colorectal cancer: a single‐center retrospective study. Int J Colorectal Dis. 2020;35(10):1911–1919. 10.1007/s00384-020-03668-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ijspeert JEG, Madani A, Overbeek LIH, Dekker E, Nagtegaal ID. Implementation of an e‐learning module improves consistency in the histopathological diagnosis of sessile serrated lesions within a nationwide population screening programme. Histopathology. 2017;70(6):929–937. 10.1111/his.13155 [DOI] [PubMed] [Google Scholar]
- 30. Van Dooijeweert C, Van Diest PJ, Baas IO, Van Der Wall E, Deckers IAG. Grading variation in 2,934 patients with ductal carcinoma in situ of the breast: the effect of laboratory: the pathologist‐specific feedback reports. Diagn Pathol. 2020;15(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. van Dooijeweert C, Deckers IAG, de Ruiter EJ, ter Hoeve ND, Vreuls CPH, van der Wall E, et al. The effect of an e‐learning module on grading variation of (pre)malignant breast lesions. Mod Pathol. 2020;33(10):1961–1967. [Internet]. 10.1038/s41379-020-0556-6 [DOI] [PubMed] [Google Scholar]
- 32. Madani A, Kuijpers CCHJ, Sluijter CE, Von der Thüsen JH, Grünberg K, Lemmens VEPP, et al. Decrease of variation in the grading of dysplasia in colorectal adenomas with a national e‐learning module. Histopathology. 2019;74(6):925–932. 10.1111/his.13834 [DOI] [PubMed] [Google Scholar]
- 33. Flach RN, Egevad L, Eklund M, Van Der Kwast TH, Delahunt B, Samaratunga H, et al. Use of the ISUP e‐learning module improves interrater reliability in prostate cancer grading. J Clin Pathol. 2022;77(1):22–26. 10.1136/jcp-2022-208506 [DOI] [PubMed] [Google Scholar]
- 34. Naxerova K, Reiter JG, Brachtel E, Lennerz JK, van de Wetering M, Rowan A, et al. Origins of lymphatic and distant metastases in human colorectal cancer. Science (1979). 2017;357(6346):55–60. [Internet]. 10.5061/dryad.vv53d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kandagatla P, Maguire LH, Hardiman KM. Biology of nodal spread in colon cancer: insights from molecular and genetic studies. Eur Surg Res. 2019;59(5–6):361–370. 10.1159/000494832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vermeer NCA, Backes Y, Snijders HS, Bastiaannet E, Liefers GJ, Moons LMG, et al. National cohort study on postoperative risks after surgery for submucosal invasive colorectal cancer. BJS Open. 2019;3(2):210–217. [Internet]. [Cited 2021 Feb 17]. 10.1002/bjs5.50125 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Figure S1
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


