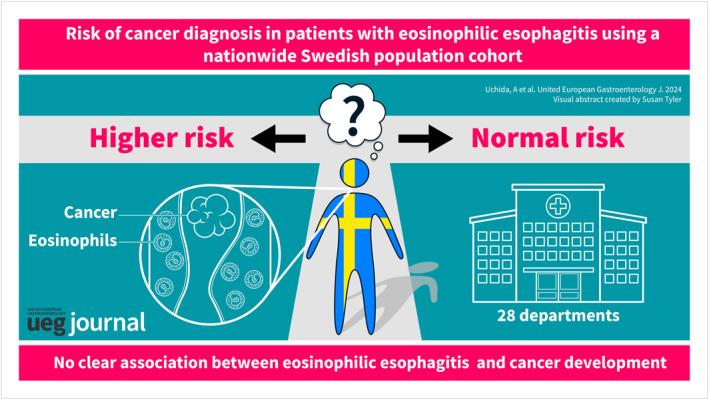
ABSTRACT
Background
Eosinophilic esophagitis (EoE) is a chronic, inflammatory disease of the esophagus. Chronic inflammation has been linked to cancer development. We aimed to study the potential association between EoE and later cancer diagnosis.
Methods
In this nationwide population‐based cohort study, we identified 1580 individuals with EoE diagnosed between 1990–2017 through Sweden's 28 pathology departments. Up to five general population reference individuals were matched on age and sex (n = 7533). A Cox regression analysis estimated adjusted hazard ratios (aHRs) for cancer up until December 31, 2020. To reduce potential intrafamilial confounding, we also compared EoE individuals with their unaffected siblings.
Results
During a median follow‐up of 7 years, 47 individuals with EoE (3.9/1000 person‐years) developed cancer versus 183 (3.2/1000 person‐years) reference individuals. This corresponded to a non‐significant aHR of 1.11 (95% CI = 0.80–1.53). Incidence rates were independent of budesonide and proton‐pump inhibitor use. Individuals with EoE however did have an increased risk of esophageal cancer where two EoE versus one reference individual were diagnosed (aHR = 25.20; 95% CI = 2.28–278.80), and also Barrett's esophagus risk was also increased in EoE (HR = 18.18; 95% CI = 6.75–48.95). Non‐esophageal gastrointestinal (GI) cancer occurred in 11 EoE versus 24 reference individuals: aHR = 2.03 (95% CI = 0.99–4.18). We found no increased risk of cancers from the skin (EoE n = 10), lung (n = 0), breast (n = 4), or blood (n = 0). Sibling analyses supported these findings.
Conclusion
We did not find any overall association between EoE and cancer development. EoE was associated with esophageal cancer, but this was very rare with wide confidence interval and few cases therefore we urge caution with generalization of these findings.
Keywords: allergy, autoimmunity, Barrett's esophagus, EoE, epidemiology, esophageal cancer, inflammation, malignancy, onset, risk factor
1.
Summary.
-
Summarise the established knowledge on this subject
-
◦
EoE is an increasingly common chronic inflammatory disease of the esophagus.
-
◦
Chronic inflammation is associated in increased cancer risk.
-
◦
-
What are the significant and/or new findings of this study?
-
◦
EoE was not associated with an overall risk of cancer in this nationwide histopathologic cohort.
-
◦
Subgroup analyses identified an increased risk of esophageal cancer in EoE patients; however, the absolute risk was low with only 1 in about 800 EoE patients developing such cancer during a median follow‐up of 7 years; therefore, we urge much caution with generalization of these findings.
-
◦
Abbreviations
- aHR
adjusted HR
- CI
confidence interval
- EoE
eosinophilic esophagitis
- ESPRESSO
epidemiology strengthened by histopathology reports in Sweden (cohort study)
- GI
gastrointestinal
- HPF
high power field
- HR
hazard ratio
- IBD
inflammatory bowel disease
- ICD
international classification of diseases
- IQR
interquartile range
- IR
incidence rate
- PIN
personal identity number
- PPI
proton pump inhibitor
- SNOMED
systematized nomenclature of medicine
2. Introduction
Eosinophilic esophagitis is an allergic disease of the esophagus that affects all ages, sexes, and races. It is characterized by clinical symptoms of esophageal dysfunction and histologic findings of basal cell hyperplasia, dilated intercellular spaces, and the presence of ≥ 15 intraepithelial eosinophils per high power field (eos/HPF) [1]. EoE is increasingly prevalent given the rising incidence noted in multiple populations and does not appear to be life‐limiting [2, 3, 4]. As EoE is a relatively new disease, establishing an international classification of disease (ICD) code in the United States only in 2008, the field has a growing need for understanding potential long‐term health consequences such as cancer. Additionally, an earlier validation study found that 1 in 4 EoE patients had a diagnostic delay of more than 10 years, which may also affect the development of malignancy [5].
It is well‐known in other gastrointestinal (GI) diseases that chronic inflammation and fibrotic diseases increases the risk for cancer of that organ such as inflammatory bowel disease (IBD) with colorectal cancer, cirrhosis with hepatocellular carcinoma (HCC), and even in allergic inflammation such as atopic dermatitis and skin cancer [6, 7, 8]. Conversely, eosinophils are suspected in some malignancies to be anti‐tumoral, where in many cases these tumor infiltrating eosinophils are associated with a favorable prognosis or augmentation of anti‐cancer therapy [9, 10, 11, 12, 13]. Though, the relationship of eosinophils and cancer is understood to be complex and likely cancer type or eosinophil type‐dependent [14]. Few studies to date have investigated EoE and cancer directly. In 2003, Straumann et al. reported a cohort of 30 patients with EoE who were followed for on average 7.2 years and did not identify any malignant risk in these patients [15]. These same findings were described in a smaller study of 13 patients with EoE followed for almost 14 years [16], as well as cross‐sectional population‐based study examining esophageal cancer in particular over a 5 year period [17]. While these studies are reassuring for a lack of increased cancer risk, they are smaller, lacking statistical power to examine specific cancer types or followed for a short period of time. However, a meta‐analysis by Muir et al. highlights the possibility of an association between atopic diseases at mucosal surfaces and the development of cancer and supports further exploration of EoE and cancer risk [18]. Given the few investigations of EoE and cancer directly, the ESPRESSO cohort's analysis provides novel data to the field from a different population.
Given that EoE causes chronic, eosinophilic inflammation in the esophagus and studies of EoE and cancer to date have been limited, we utilized a nationwide histopathology cohort of patients with biopsy‐confirmed EoE in Sweden. We hypothesized that EoE is not associated with an increased risk of overall cancer given the findings in studies to date, and we performed subgroup analyses to investigate specific malignancies such as esophageal, other GI and hematologic cancers. Better understanding the relationship between EoE and cancer is vital in counseling patients about their disease and its natural history to inform treatment plans.
3. Methods
3.1. Study Design and Patient Cohort
We utilized the ESPRESSO (Epidemiology Strengthened by histopathology Reports in Sweden) cohort, which is a nationwide population cohort that contains prospectively collected data from all Swedish health and welfare registers. ESPRESSO contains GI biopsies with accompanying systemized nomenclature of medicine (SNOMED) clinical terms collected from all 28 pathology departments in Sweden between the years of 1965–2017 [5]. All Swedes are assigned a personal identity number (PIN), which is a unique number that allows for large‐scale linkages and epidemiological research [19]. We linked data on all EoE cases in the ESPRESSO cohort (Topography T62, Morphology M47150) to the nationwide Swedish healthcare registers including the Patient Register [5, 20, 21, 22]. A major component of the diagnosis of EoE is histologic, defined as ≥ 15 intraepithelial esophageal eos/HPF. Upon ESPRESSO cohort validation, the EoE cohort derived from ESPRESSO was found to have a positive predictive value of 89% [5]. The ESPRESSO cohort validation analysis study by Rojler et al. had ethical approval to scrutinize individual patient charts and confirm adequate esophageal eos/HPF as well as clinical documentation of EoE‐specific symptoms, test results, and treatment plans [5]. This high positive predictive value is reassuring for the adequacy of this cohort and application to EoE.
We examined patients for new diagnoses of EoE (referred to as index biopsy) based on the above histopathologic criteria in the ESPRESSO cohort between the years of 1990–2017. These years were chosen as there was generally low awareness of EoE prior to 1990s, and to allow years of follow up time after new diagnosis, respectively. Follow up for incident cancer diagnosis occurred until December 31, 2020; this date was chosen because it was the longest follow up available within the Swedish Cancer Register.
3.2. Reference Individuals
Our primary control group included general population reference individuals from the Swedish Total Population Register (covering the complete Swedish population) [23]. EoE individuals were matched with up to five reference individuals based on age, sex, calendar year of index biopsy, and county of residence by the government agency Statistics Sweden.
In a separate analysis, we used unaffected full siblings to EoE individuals as controls. We did so to minimize intrafamilial confounders (shared genetic and some early environmental factors). Siblings of individuals with EoE were identified through the Swedish Multigeneration Register, a sub‐section of the Total Population Register. Sibling data were available on all individuals born since 1932 and registered as residents of Sweden in 1961 or later.
3.3. Exclusion Criteria
EoE individuals were excluded if they had a prior diagnosis of cancer or death before index EoE diagnosis (index biopsy), or if they emigrated prior to EoE diagnosis/matching date, since this would prohibit evaluating their history prior to EoE (and an earlier cancer diagnosis may then be missed).
3.4. Outcome Measures
Our primary outcome was incident cancer diagnosis of any type as ascertained through the Swedish Cancer Register using ICD7 codes (ICD7 = 140–208; Table S1). The Swedish Cancer Register accounts for over 96% of all cancer cases in Sweden, and cases are confirmed and classified by specialists using established histopathological and/or radiographic criteria [24]. Secondary outcomes included examining risk for developing the various cancer subtypes as follows: esophageal cancer (ICD7 = 150), any non‐esophageal GI cancer including liver cancer (ICD7 = 151–159), melanoma or non‐melanoma skin cancer (ICD7 = 190, 191), lung cancer (ICD7 = 162), breast cancer (ICD7 = 170), extra‐hepatic hematologic cancers (ICD7 = 200–208). In secondary analyses, we compared EoE individuals to their full siblings. We also examined the odds of having a diagnosis of any cancer prior to EoE in a case‐control fashion to better understand the inverse relationship of cancer diagnosis prior to EoE. We also assessed for frequency of follow up upper endoscopies after EoE diagnosis (codes found in Table S2).
3.5. Covariates
We collected detailed information regarding demographics. We adjusted for sex, age, calendar year of biopsy and county as well as level of education as obtained through the Longitudinal Integrated Database for Health Insurance and Labor Market Studies (LISA) [25]. We also estimated the risk of Barrett's esophagus (defined as having an ICD‐10 code of K22.7) as a proxy for esophageal adenocarcinoma (as opposed to squamous cell carcinoma) risk.
3.6. Statistical Analyses
All analyses were performed with R 4.0.5 primarily using the survival package [26, 27]. A Cox proportional hazard modeling was utilized to determine hazard ratios (HRs) and 95% confidence intervals (95% CI) for any cancer diagnosis. Adjusted models were conditioned for age at index biopsy of EoE diagnosis, sex, calendar period, county, education, and other immune‐mediated disease diagnosis at baseline (EoE diagnosis date and matching date) (see Table S3 for immune‐mediated diagnoses and relevant ICD codes). Sensitivity analyses using EoE siblings as comparators were run using a stratified Cox regression adjusting for age and sex.
3.7. Ethics
This study was approved by the Stockholm Ethics board. Informed consent was waived since the study was strictly register‐based [28].
4. Results
4.1. Study Cohort
The baseline characteristics of 1580 EoE individuals and 7533 general population reference individuals are outlined in Table 1. Approximately 75% of EoE individuals were male and the mean age of EoE diagnosis was 37 years. At baseline, 2.1% of EoE patients had prescriptions for topical steroids (budesonide) and 18.5% for PPI. For the vast majority of EoE patients (93%), follow‐up ended December 31, 2020, whereas death and development of cancer accounted for 2.5% and 3.0% of patients, respectively. Some 51% of EoE individuals had no follow‐up upper endoscopy, 33% had 1%, 2%, and 16% had ≥ 3 follow‐up endoscopies.
TABLE 1.
Baseline characteristics of histologically defined EoE patients and matched general population controls.
| General population | ||
|---|---|---|
| EoE | Reference individuals | |
| n [%] | n [%] | |
| Total | 1580 [100.0] | 7533 [100.0] |
| Male | 1191 [75.4] | 5730 [76.1] |
| Female | 389 [24.6] | 1803 [23.9] |
| Age at start follow up | ||
| Mean [SD] years | 36.6 [19.9] | 35.5 [19.4] |
| Median [IQR] years | 37.0 [19.0–51.0] | 36.0 [18.0–50.0] |
| < 18 years | 251 [15.9] | 1241 [16.5] |
| 18 < 50 years | 821 [52.0] | 4028 [53.5] |
| ≥ 50 years | 508 [32.2] | 2264 [30.1] |
| Years of follow up | ||
| Mean [SD] years | 7.6 [3.0] | 7.6 [3.1] |
| Median [IQR] years | 7.1 [5.6–9.1] | 7.0 [5.6–9.1] |
| < 1 year | 13 [0.8] | 87 [1.2] |
| 1 < 5 years | 223 [14.1] | 1098 [14.6] |
| 5 < 10 years | 1056 [66.8] | 4955 [65.8] |
| ≥ 10 years | 288 [18.2] | 1393 [18.5] |
| Year of start follow up | ||
| 1990–2005 | 37 [2.3] | 176 [2.3] |
| 2006–2013 | 810 [51.3] | 3868 [51.3] |
| 2014–2017 | 733 [46.4] | 3489 [46.3] |
| Country of birth | ||
| Nordic | 1501 [95.0] | 6304 [83.7] |
| Other | 79 [5.0] | 1229 [16.3] |
| Education | ||
| Compulsory school, ≤ 9 years | 249 [15.8] | 1418 [18.8] |
| Upper secondary school (10–12 years) | 556 [35.2] | 2648 [35.2] |
| College or university (≥ 13 years) | 485 [30.7] | 1919 [25.5] |
| Missing data | 290 [18.4] | 1548 [20.5] |
| Reason for end of follow‐up | ||
| Death | 40 [2.5] | 157 [2.1] |
| Emigration | 13 [0.8] | 166 [2.2] |
| 31 December 2020 | 1470 [93.0] | 6958 [92.4] |
| Cancer | 47 [3.0] | 183 [2.4] |
| Developed EoE | 0 [0.0] | 3 [0.0] |
Abbreviation: EoE, eosinophilic esophagitis.
4.2. Cancer Risk
During a median follow‐up of 7.1 years, 47 individuals with EoE (3.9/1000 person‐years) developed any type of cancer versus 183 (3.2/1000 person‐years) among reference individuals (Table 2). This was equivalent to a HR of 1.11 (95% CI = 0.81–1.53) (Tables 2 and 3). After multivariable adjustment, the adjusted hazard ratio (aHR) remained 1.11 (95% CI = 0.80–1.53) (Tables 2 and 3). Similarly, subgroup analyses by sex, years of follow‐up, age at diagnosis, country of origin, and education level were not significantly different between EoE or population reference individuals (Table 3).
TABLE 2.
Risk of incident cancer among individuals with histologically defined EoE compared to general population controls.
| Cancer | EoE | General population reference individuals | |
|---|---|---|---|
| Any cancer | N | 1580 | 7533 |
| Event | 47 | 183 | |
| IR [95% CI] | 3.9 [3.0–5.1] | 3.2 [2.8–3.7] | |
| IRD [95% CI] | 0 [−1.6 to 1.6] | −0.7 [−1.9 to 0.5] | |
| HR [95% CI] | NA | 1.11 [0.81–1.53] | |
| aHR [95% CI] | NA | 1.11 [0.80–1.53] | |
| Esophageal cancer | Event | 2 | 1 |
| IR [95% CI] | 0.2 [0.1–0.5] | 0.0 [0.0–0.1] | |
| IRD [95% CI] | 0 [−0.3 to 0.3] | −0.1 [−0.4 to 0.1] | |
| HR [95% CI] | NA | 18.35 [1.66–202.97] | |
| aHR [95% CI] | NA | 25.20 [2.28–278.80] | |
| Gastrointestinal cancer a (not esophageal) | Event | 11 | 24 |
| IR [95% CI] | 0.9 [0.5–1.5] | 0.4 [0.3–0.6] | |
| IRD [95% CI] | 0 [−0.8 to 0.8] | −0.5 [−1.1 to 0.1] | |
| HR [95% CI] | NA | 2.09 [1.02–4.28] | |
| aHR [95% CI] | NA | 2.03 [0.99–4.18] | |
| Skin cancer | Event | 10 | 30 |
| IR [95% CI] | 0.8 [0.5–1.4] | 0.5 [0.4–0.7] | |
| IRD [95% CI] | 0 [−0.7 to 0.7] | −0.3 [−0.9 to 0.2] | |
| HR [95% CI] | NA | 1.37 [0.67–2.82] | |
| aHR [95% CI] | NA | 1.31 [0.63–2.70] | |
| Lung cancer | Event | 0 | 7 |
| IR [95% CI] | 0 | 0.1 [0.1–0.2] | |
| IRD [95% CI] | 0 | 0.1 [0–0.2] | |
| HR [95% CI] | NA | NA | |
| aHR [95% CI] | NA | NA | |
| Breast cancer | Event | 4 | 10 |
| IR [95% CI] | 0.3 [0.1–0.7] | 0.2 [0.1–0.3] | |
| IRD [95% CI] | 0 [−0.5 to 0.5] | −0.2 [−0.5 to 0.2] | |
| HR [95% CI] | NA | 1.68 [0.53–5.35] | |
| aHR [95% CI] | 1.67 [0.52–5.33] | ||
| Hematologic cancers b | Event | 0 | 13 |
| IR [95% CI] | 0.0 [0.0–0.0] | 0.2 [0.1–0.4] | |
| IRD [95% CI] | NA | 0.2 [0.1–0.3] | |
| HR [95% CI] | NA | NA | |
| aHR [95% CI] | NA | NA |
Note: NA values could not be calculated due to insufficient data.
Abbreviations: aHR, adjusted HR for age at EoE, sex, calendar year, county where biopsy obtained, education and immune‐mediated diagnosis at baseline (EoE diagnosis date or matching date in reference individuals, Table S2); HR, hazard ratio adjusted for age, sex, calendar year, county, and education; IR, incidence rate per 1000 person‐years; IRD, incidence rate difference per 1000 person‐years.
Any GI cancer including liver cancer but excludes esophageal (see Table S1 for ICD list of codes: gastric, small bowel, colon, liver, biliary, pancreas, peritoneum, unspecified digestive organ).
Extrahepatic hematological cancer.
TABLE 3.
Cancer incidence rates and hazard ratios for histologically defined EoE patients and general population controls.
| IR EoE/1000 person‐years | IR general population | aHR [95% CI] | |
|---|---|---|---|
| Reference individuals/1000 person‐years | |||
| Total | 3.9 [3.0–5.1] | 3.2 [2.8–3.7] | 1.11 [0.80–1.53] |
| Sex | |||
| Males | 3.5 [2.5–4.9] | 2.9 [2.4–3.4] | 1.14 [0.77–1.68] |
| Females | 5.2 [3.1–8.1] | 4.3 [3.3–5.5] | 1.12 [0.63–1.97] |
| Follow up (years) | |||
| < 1 | 6.4 [3.5–10.9] | 3.7 [2.6–5.3] | 1.54 [0.75–3.18] |
| 1 < 5 | 3.1 [2.0–4.7] | 3.0 [2.5–3.7] | 0.96 [0.58–1.59] |
| 5 < 10 | 3.8 [2.3–6.1] | 3.2 [2.4–4.1] | 1.08 [0.60–1.95] |
| ≥ 10 | 6.3 [2.6–13.8] | 4.0 [2.4–6.5] | 1.30 [0.39–4.38] |
| Age at start follow up (years) | |||
| < 18 | 0.3 [0.1–1.2] | 0.2 [0.1–0.5] | 1.81 [0.19–17.42] |
| 18–49 | 2.3 [1.4–3.7] | 1.9 [1.4–2.4] | 1.21 [0.65–2.24] |
| ≥ 50 | 10.5 [7.5–14.4] | 9.2 [7.8–10.9] | 1.09 [0.74–1.60] |
| Year of start follow up | |||
| 1990–2005 | 12.4 [6.1–23.1] | 4.8 [2.9–7.6] | 3.19 [1.20–8.51] |
| 2006–2013 | 3.4 [2.3–4.9] | 3.4 [2.8–4.0] | 0.93 [0.60–1.43] |
| 2014–2017 | 3.7 [2.3–5.8] | 2.7 [2.0–3.5] | 1.26 [0.70–2.24] |
| Country | |||
| Nordic | 4.0 [3.0–5.2] | 3.5 [3.0–4.0] | 1.06 [0.76–1.48] |
| Non‐Nordic | 3.6 [1.1–10.1] | 1.7 [1.0–2.6] | NA [NA–NA] |
| Education (years) | |||
| Compulsory school (≤ 9) | 5.7 [3.1–9.7] | 3.7 [2.7–5.0] | 1.45 [0.58–3.59] |
| Upper secondary school (10–12) | 4.7 [3.1–7.0] | 4.3 [3.4–5.2] | 0.94 [0.54–1.65] |
| College or university (≥ 13) | 4.6 [2.9–7.1] | 3.8 [2.9–5.0] | 1.13 [0.56–2.28] |
| Missing data | 0.4 [0.1–1.5] | 0.5 [0.2–0.9] | 2.55 [0.23–28.14] |
Note: NA values could not be calculated due to insufficient data.
Abbreviations: aHR, adjusted hazard ratio; EoE, eosinophilic esophagitis; IR, incidence rate.
EoE patients had a significantly higher risk of developing esophageal cancer with an aHR of 25.20 (95% CI = 2.28–278.80), though this was based on only two events in EoE (equal to 1 in about 800 EoE patients) and one in the reference individuals. The median age of EoE patients who developed esophageal cancer was 57 at EoE diagnosis and 59 at esophageal cancer diagnosis. The median EoE duration before cancer development was 2 years. In attempts to discern esophageal cancer risk type, we performed an analysis for risk of Barrett's esophagus as these patients are at higher risk for adenocarcinoma of the esophagus. We found that compared to matched controls (n = 5; 0.07%), patients with EoE (n = 19; 1.2%) were at an 18‐fold increased risk of Barret's esophagus (HR = 18.18; 95% CI = 6.75–48.95).
During follow‐up, 11 EoE patients and 24 reference individuals developed non‐esophageal GI cancer corresponding to an aHR of 2.03 (95% CI = 0.99–4.18). Of these 11 and 24 individuals, one and two, respectively, had a cancer diagnosis in the first 6 months after EoE diagnosis/matching date. Our ethics approval did not allow us to specify type of cancer in these three individuals since that would increase the risk that individual patients were identified. Skin, lung, breast, and extra‐hepatic hematologic malignancies were not significantly different from population reference individuals and in some cases such as lung or hematologic cancers, no cases occurred.
Incidence rates (IRs) and corresponding aHRs according to sex, age, and years of follow‐up, country, and education between EoE and control individuals are shown in Table 3. After adjustment, there were no significant differences between groups.
4.3. Sibling Analyses
In secondary analyses, we compared 1194 EoE individuals (76% male) and 1997 full siblings (52% male) (Table 4). The mean age of EoE diagnosis and initiation of follow up in sibling analyses was similar to our primary analysis. After adjustment (also for sex, age, calendar year and county since the sibling analyses were unmatched), and taking family stratum into account, we again found no significant risk of overall cancer when comparing EoE patients to their siblings, and the risk of esophageal cancer diminished and lost significance in sibling analyses (Table 5). There were no significant risk associations between any subtype of cancer examined. We did not find a significantly increased odds of having a previous cancer diagnosis and later developing EoE (Table S4).
TABLE 4.
Summary statistics for histologically defined EoE patients and their full siblings.
| EoE | Siblings | |
|---|---|---|
| n [%] | n [%] | |
| Total | 1194 [100.0] | 1997 [100.0] |
| Male | 907 [76.0] | 1053 [52.7] |
| Female | 287 [24.0] | 944 [47.3] |
| Age at start follow up (years) | ||
| Mean [SD] | 34.8 [18.5] | 36.0 [19.3] |
| Median [IQR] | 35.0 [18.0–49.0] | 37.0 [20.0–50.0] |
| < 18 | 194 [16.2] | 261 [13.1] |
| 18 < 50 | 658 [55.1] | 1098 [55.0] |
| ≥ 50 | 342 [28.6] | 638 [31.9] |
| Years of follow up (years) | ||
| Mean [SD] | 7.6 [2.9] | 7.6 [3.1] |
| Median [IQR] | 7.1 [5.7–9.2] | 7.2 [5.7–9.1] |
| < 1 | 8 [0.7] | 21 [1.1] |
| 1 < 5 | 151 [12.6] | 263 [13.2] |
| 5 < 10 | 820 [68.7] | 1351 [67.7] |
| ≥ 10 | 215 [18.0] | 360 [18.0] |
| Year of start follow up | ||
| 1990–2005 | 25 [2.1] | 45 [2.3] |
| 2006–2013 | 621 [52.0] | 1072 [53.7] |
| 2014–2017 | 548 [45.9] | 880 [44.1] |
| Country of birth | ||
| Nordic | 1168 [97.8] | 1925 [96.4] |
| Other | 26 [2.2] | 72 [3.6] |
| Education (years) | ||
| Compulsory school, ≤ 9 | 172 [14.4] | 290 [14.5] |
| Upper secondary school (10–12) | 422 [35.3] | 714 [35.8] |
| College or university (≥ 13) | 371 [31.1] | 607 [30.4] |
| Missing | 229 [19.2] | 386 [19.3] |
| Reason for end of follow‐up | ||
| Death | 19 [1.6] | 39 [2.0] |
| Emigration | 8 [0.7] | 18 [0.9] |
| 31 December 2020 | 1133 [94.9] | 1870 [93.6] |
| Cancer | 27 [2.3] | 55 [2.8] |
| Developed EoE | 0 [0.0] | 6 [0.3] |
TABLE 5.
Risk of incident cancer among individuals with histologically defined EoE compared to full siblings.
| Cancer | EoE | Siblings | |
|---|---|---|---|
| Any cancer | N | 1194 | 1997 |
| Event | 27 | 55 | |
| IR [95% CI] | 3.0 [2.0–4.2] | 3.6 [2.8–4.6] | |
| IRD [95% CI] | 0 [−1.6 to 1.6] | 0.6 [−0.8 to 2.1] | |
| HR [95% CI] | 0.87 [0.54–1.41] | ||
| aHR [95% CI] | 1.10 [0.65–1.85] | ||
| Esophageal cancer | Event | 1 | 0 |
| IR [95% CI] | 0.1 [0.0–0.4] | 0.0 [0.0–0.0] | |
| IRD [95% CI] | 0 [−0.3 to 0.3] | −0.1 [−0.3 to 0.1] | |
| HR [95% CI] | 1 | NA | |
| aHR [95% CI] | 1 | NA | |
| Gastrointestinal cancer a (not esophageal) | Event | 6 | 4 |
| IR [95% CI] | 0.7 [0.3–1.3] | 0.3 [0.1–0.6] | |
| IRD [95% CI] | 0 [−0.7 to 0.7] | −0.4 [−1 to 0.2] | |
| HR [95% CI] | 1 | 2.86 [0.78–10.50] | |
| aHR [95% CI] | 1 | NA | |
| Skin cancer | Event | 6 | 10 |
| IR [95% CI] | 0.7 [0.3–1.3] | 0.6 [0.4–1.1] | |
| IRD [95% CI] | 0 [−0.7 to 0.7] | 0 [−0.7 to 0.7] | |
| HR [95% CI] | 1 | 1.16 [0.40–3.37] | |
| aHR [95% CI] | 1 | 0.92 [0.27–3.07] | |
| Breast cancer | Event | 1 | 9 |
| IR [95% CI] | 0.1 [0.0–0.4] | 0.6 [0.3–1.0] | |
| IRD [95% CI] | 0 [−0.3 to 0.3] | 0.5 [0–0.9] | |
| HR [95% CI] | 1 | ||
| aHR [95% CI] | 1 | 0.52 [0.04–6.30] |
Note: NA values could not be calculated due to insufficient data#Extrahepatic hematological cancer. Please note that hazard ratios for lung cancer and extrahepatic hematological cancer were not calculated due to insufficient statistical power seen already in the general population comparison (see Table 2).
Abbreviations: aHR, adjusted HR for age at EoE, sex, calendar year, county where biopsy obtained, education and immune‐mediated diagnosis at baseline (EoE diagnosis date or matching date in reference individuals, Table S2); HR, hazard ratio adjusted for age, sex, calendar year, county, and education; IR, incidence rate; IRD, incidence rate difference.
Any GI cancer including liver cancer but excludes esophageal (see Table S1 for ICD list of codes: gastric, small bowel, colon, liver, biliary, pancreas, peritoneum, unspecified digestive organ).
5. Discussion
In this nationwide cohort, biopsy‐verified EoE was not associated with future cancer. In cancer subtype analyses however, there was a significantly increased risk for esophageal cancer (aHR 25.50, 95% CI = 2.28–278.80), and non‐esophageal GI cancer diagnoses fell just short of significance (aHR 2.03; 95% CI = 0.99–4.18). It is important to note that absolute numbers particularly for cancer subtypes were small (for instance only 1 in about 800 EoE patients had a later diagnosis of esophageal cancer), which should be comforting to patients and providers. We urge much caution with generalization of these findings without further studies. The increased risk of esophageal cancer in EoE patients did not maintain when EoE individuals were compared to their full siblings. This suggests there may be shared genetic factors or early life environmental factors among EoE individuals and their siblings that contributed to detection of cancer. However, it should also be noted that the sibling analysis had lower statistical power than the main analysis. Given that we were unable to discern more detailed information about the cancer type due to the risk of revealing patient identity, we performed an analysis of Barrett's esophagus risk in EoE, as these patients are at risk for developing adenocarcinoma of the esophagus. The risk for Barrett's esophagus was increased in EoE suggesting Barrett's could be a possible mediator for esophageal cancer risk. Lastly, earlier cancer diagnosis was not associated with an increased in EoE, suggesting that at a population level undiagnosed EoE does not predispose to cancer.
Our findings are in line with the few earlier reports where no increased risk of overall cancer was identified in patients with EoE. Our study adds to the field by utilizing a nationwide biopsy‐verified cohort with larger numbers, and cancer‐specific analyses. In two previous studies, Straumann et al. and Lipka et al. examined EoE patients over time to understand the natural history and broad scope of long‐term clinical outcomes of EoE [15, 16]. The Straumann et al. study examined 30 EoE patients over a mean period of 7.2 years (max of 11.5 years) and did not observe cancer in any patients [15]. While we had a similar mean follow‐up, we examined more than 1500 patients with EoE, out of which 47 developed cancer. Lipka et al. examined a smaller group of EoE patients; 13 met study criteria of patients treated with dilation and PPI and were retrospectively examined over approximately a 14‐year history [16]. Similarly, no patients developed cancer during the study period.
Notably, our cancer subtype analyses contrast with the only study to our knowledge that specifically examined esophageal cancer and EoE [17]. This study by Syed, Maradey‐Romero, and Fass was a cross‐sectional population study using healthcare codes in an electronic medical record platform (Explorys Platform) and inquired about various esophageal disorders such as Barrett's esophagus, gastroesophageal reflux, and EoE, and assessed their risk for esophageal cancer [17]. This study included over 27 million subjects, of which approximately 5300 had EoE and none of these patients had concomitant diagnostic codes for esophageal cancer. The Syed et al. study looked only at a single time point and can only determine the prevalence of EoE and esophageal cancer comorbidity. Here, we performed a nationwide population‐based cohort study where EoE patients had a histopathologically verified diagnosis, rather than a medical code. We followed EoE patients over a 31‐year period with a mean follow up of 7.6 years, and assessed for later incidence rate of cancer diagnoses. This method is of particular importance given that there was low awareness of EoE in the early 1990s and 2000s, and that in general, malignant transformation can take many years to develop, particularly in the setting of chronic inflammation. Still, we urge caution given our study was not powered to detect secondary outcomes such as esophageal cancer. The low number of incident diagnoses confirms this and should be an area of future research.
There is precedence for the development of cancer in organs chronically afflicted by inflammation and therefore the association of esophageal cancer with EoE is mechanistically and biologically plausible [6, 7]. Many other GI and non‐GI diseases rooted in chronic inflammation have well‐known risks for development of cancer in the organ affected by inflammation such as colorectal cancer in IBD or hepatocellular carcinoma in cirrhosis for example, where cancer screening is actively employed [6, 7]. However, we detected very few incident cancer events and therefore clinical interpretation and generalization should be limited prior to additional studies.
Moreover, this is the first cohort study to examine subtypes of cancer in EoE. The majority of cancer subtypes examined here did not have a significant association with earlier EoE diagnosis (breast, lung, skin, and hematologic), and non‐esophageal GI cancers including liver cancer fell just short of significance. Of note, the association with esophageal cancer dissipated when we used siblings as reference individuals. While imperfect, sibling comparisons provide a unique opportunity to examine disease associations where factors such as genetics, early environmental factors (such as diet, air‐, land‐ or water‐borne substances), and healthcare seeking behaviors are generally shared between groups. The lack of association in our study suggests that esophageal cancer risk may be attributable to one or several such shared factors.
It is worth noting, particularly given our null primary outcome, but positive secondary outcomes of esophageal cancer, that the role eosinophils play in EoE‐associated cancer remains elusive. Some studies have suggested eosinophils may be anti‐tumorigenic, including breast and colorectal cancers that when abundant with eosinophils are associated with better clinical outcomes [29, 30]. Additionally, in mouse models of EoE, investigators have inquired mechanistic impacts of EoE and esophageal cancer development and found that the esophageal epithelial remodeling events inherent to murine EoE may itself limit esophageal carcinogenesis [31]. To note, mouse modeling of esophageal squamous cell carcinoma by Sun et al. demonstrated the anti‐tumorigenic role of eosinophils through the release of reactive oxygen species in response to IL‐17 suppression, which is important for GI mucosal health [32].
Our study is strengthened by utilizing ESPRESSO, which is a nationwide population‐based cohort where disease status is rooted on strict histopathological findings, and potential confounding variables are recorded in country registers [5]. This approach and the comparatively large sample size compared to prior studies allowed for prolonged follow up time and exploration of subtypes of cancer. Our use of general population and sibling comparisons allows for the potential to examine intrafamilial factors and their associations with diseases. Together these approaches enabled us to examine disease associations while minimizing potential bias from residual confounding and shared familial factors.
Despite these strengths, we acknowledge several limitations of our study. First, EoE is a clinicopathological disease, meaning both clinical (symptom) and histological criteria are required for diagnosis and ESPRESSO is based on histopathological criteria. While this is true, we note that upon validation, the EoE ESPRESSO cohort had a positive predictive value of 89%, which is more than adequate for epidemiological studies [5]. This validation was performed according to the current post 2018 AGREE guideline criteria [1]. We note while our cohort is nationwide, the number of EoE patients who met inclusion criteria was limited to 1580 individuals. However, it is worth remarking that this cohort size is notably larger than most of the comparative studies on EoE and cancer. While the aHR for esophageal cancer was markedly high (aHR = 25.2), we urge caution with interpretation. Additionally, noting the type (such as adenocarcinoma or squamous cell carcinoma) of esophageal cancer could help with biological underpinnings of possible associations, however our ethical approval for this study did not allow us to specify the type of cancer since that would increase the risk that individual patients be identified [28]. We were unable to compare EoE patients with endoscopy controls. An earlier study on patients with normal mucosa may however serve as a reference for such controls and found a 1.07‐fold increased risk of any cancer in patients with normal mucosa [32]. That study did not specifically calculate the risk for esophageal cancer but looked at any GI cancer (HR = 1.03; 95% CI = 1.00–1.07) as well as gastric cancer (HR = 1.07; 95% CI = 0.97–1.19). Meanwhile we found no association between EoE and any cancer (HR = 1.11), but an increased risk of esophageal cancer (HR = 25.20). Unfortunately, we were unable to examine the impact of medication use for cancer risk in EoE. We also acknowledge the lack of data on smoking, alcohol consumption and dietary habits. In a validation paper on our EoE cohort, smoking (current or past) was noted in 14% and alcohol use in 22% of EoE patients [5]. Lastly, our data are a representation of a single country (Sweden) that is predominantly Caucasian, and conclusions therefore may not be generalizable to all populations.
In conclusion, within this population‐based study of 1580 individuals with biopsy‐confirmed EoE in Sweden and matched reference individuals, EoE was not associated with an overall risk of cancer development. Patients with EoE were, however, at an increased risk of later esophageal cancer, although absolute risks were small. While future studies are needed to confirm risk for specific cancer types and EoE, our findings can inform conversations with patients about their long‐term health risks.
Author Contributions
Writing assistance: A.M.U. and S.S.S. constructed the first draft of the manuscript. J.F.L. completed the final version of the manuscript and is the guarantor of the article. All authors read, edited, and agreed with the final version of the manuscript.
Ethics Statement
This study was approved by the Stockholm Ethics Review Board August 27, 2014 (2014/1287‐31/4) with an amendment May 7, 2018 (2018/972‐32).
Conflicts of Interest
Dr. Uchida is an advisor/consultant for Sanofi‐Regeneron, AstraZeneca, Takeda, and Uniquity (unrelated to this study). Dr. Ludvigsson has coordinated an unrelated study on behalf of the Swedish IBD quality register (SWIBREG). That study received funding from Janssen corporation. Dr. Ludvigsson has also received financial support from MSD developing a paper reviewing national healthcare registers in China, and has an ongoing research collaboration with Takeda about celiac disease. Dr. Carlson has received speaker's fees from ViforPharma. She is the national PI for clinical trials for AstraZeneca. None of these activities have any relation to the present study.
Supporting information
Tables S1–S4
Acknowledgments
All authors approved the final version of the article, including the authorship list.
Funding: A.M.U. has support from the Consortium of Eosinophilic Gastrointestinal Disease Researcher (CEGIR) Training Program. J.F.L. was supported by Karolinska Institutet.
Amiko M. Uchida and Sophia S. Schuman are co‐first authorship.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
- 1. Dellon E. S., Liacouras C. A., Molina‐Infante J., et al., “Updated International Consensus Diagnostic Criteria for Eosinophilic Esophagitis: Proceedings of the AGREE Conference,” Gastroenterology 155, no. 4 (2018): 1022–1033.e10. Epub 20180906, PubMed PMID: 30009819; PMCID: PMC6174113, 10.1053/j.gastro.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dellon E. S., Jensen E. T., Martin C. F., Shaheen N. J., and Kappelman M. D., “Prevalence of Eosinophilic Esophagitis in the United States,” Clinical Gastroenterology and Hepatology 12, no. 4 (2014): 589–596.e1. Epub 20130911, PubMed PMID: 24035773; PMCID: PMC3952040, 10.1016/j.cgh.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Garber J. J., Lochhead P. J., Uchida A. M., et al., “Increasing Incidence of Eosinophilic Esophagitis in Sweden: A Nationwide Population Study,” Esophagus 19, no. 4 (2022): 535–541. Epub 20220602, PubMed PMID: 35654916, 10.1007/s10388-022-00926-5. [DOI] [PubMed] [Google Scholar]
- 4. Rojler L., Garber J. J., Roelstraete B., Walker M. M., and Ludvigsson J. F., “Mortality in Eosinophilic Esophagitis – A Nationwide, Population‐Based Matched Cohort Study From 2005 to 2017,” Upsala Journal of Medical Sciences 126, no. 1 (2021). Epub 20210831, PubMed PMID: 34540144; PMCID: PMC8431988, 10.48101/ujms.v126.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rojler L., Glimberg I., Walker M. M., Garber J. J., and Ludvigsson J. F., “Validation of the Diagnosis of Eosinophilic Esophagitis Based on Histopathology Reports in Sweden,” Upsala Journal of Medical Sciences 126, no. 1 (2021). Epub 20210813, PubMed PMID: 34471483; PMCID: PMC8383932, 10.48101/ujms.v126.7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Flemming J. A., Yang J. D., Vittinghoff E., Kim W. R., and Terrault N. A., “Risk Prediction of Hepatocellular Carcinoma in Patients With Cirrhosis: The ADRESS‐HCC Risk Model,” Cancer 120, no. 22 (2014): 3485–3493. Epub 20140716, PubMed PMID: 25042049; PMCID: PMC4553222, 10.1002/cncr.28832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stidham R. W. and Higgins P. D. R., “Colorectal Cancer in Inflammatory Bowel Disease,” Clinics in Colon and Rectal Surgery 31, no. 3 (2018): 168–178. Epub 20180401, PubMed PMID: 29720903; PMCID: PMC5929884, 10.1055/s-0037-1602237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhu Y., Wang H., He J., et al., “Atopic Dermatitis and Skin Cancer Risk: A Systematic Review,” Dermatologic Therapy 12, no. 5 (2022): 1167–1179. Epub 20220417, PubMed PMID: 35430723; PMCID: PMC9110609, 10.1007/s13555-022-00720-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jacobse J., Aziz Z., Sun L., et al., “Eosinophils Exert Antitumorigenic Effects in the Development of Esophageal Squamous Cell Carcinoma,” Cellular and Molecular Gastroenterology and Hepatology 16, no. 6 (2023): 961–983. Epub 20230811, PubMed PMID: 37574015; PMCID: PMC10630122, 10.1016/j.jcmgh.2023.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Davis B. P. and Rothenberg M. E., “Eosinophils and Cancer,” Cancer Immunology Research 2, no. 1 (2014): 1–8. PubMed PMID: 24778159, 10.1158/2326-6066.CIR-13-0196. [DOI] [PubMed] [Google Scholar]
- 11. Harbaum L., Pollheimer M. J., Kornprat P., Lindtner R. A., Bokemeyer C., and Langner C., “Peritumoral Eosinophils Predict Recurrence in Colorectal Cancer,” Modern Pathology 28, no. 3 (2015): 403–413. Epub 20140912, PubMed PMID: 25216222, 10.1038/modpathol.2014.104. [DOI] [PubMed] [Google Scholar]
- 12. Reichman H., Itan M., Rozenberg P., et al., “Activated Eosinophils Exert Antitumorigenic Activities in Colorectal Cancer,” Cancer Immunology Research 7, no. 3 (2019): 388–400. Epub 20190121, PubMed PMID: 30665890, 10.1158/2326-6066.CIR-18-0494. [DOI] [PubMed] [Google Scholar]
- 13. Blomberg O. S., Spagnuolo L., Garner H., et al., “IL‐5‐Producing CD4(+) T Cells and Eosinophils Cooperate to Enhance Response to Immune Checkpoint Blockade in Breast Cancer,” Cancer Cell 41, no. 1 (2023): 106–123.e10. Epub 20221215, PubMed PMID: 36525971, 10.1016/j.ccell.2022.11.014. [DOI] [PubMed] [Google Scholar]
- 14. Varricchi G., Galdiero M. R., Loffredo S., et al., “Eosinophils: The Unsung Heroes in Cancer?,” OncoImmunology 7, no. 2 (2018): e1393134. Epub 20171113, PubMed PMID: 29308325; PMCID: PMC5749653, 10.1080/2162402X.2017.1393134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Straumann A., Spichtin H. P., Grize L., Bucher K. A., Beglinger C., and Simon H. U., “Natural History of Primary Eosinophilic Esophagitis: A Follow‐Up of 30 Adult Patients for Up to 11.5 Years,” Gastroenterology 125, no. 6 (2003): 1660–1669. PubMed PMID: 14724818, 10.1053/j.gastro.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 16. Lipka S., Keshishian J., Boyce H. W., Estores D., and Richter J. E., “The Natural History of Steroid‐Naive Eosinophilic Esophagitis in Adults Treated With Endoscopic Dilation and Proton Pump Inhibitor Therapy Over a Mean Duration of Nearly 14 Years,” Gastrointestinal Endoscopy 80, no. 4 (2014): 592–598. Epub 20140402, PubMed PMID: 24703087, 10.1016/j.gie.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 17. Syed A., Maradey‐Romero C., and Fass R., “The Relationship Between Eosinophilic Esophagitis and Esophageal Cancer,” Diseases of the Esophagus 30, no. 7 (2017): 1–5. PubMed PMID: 30052901, 10.1093/dote/dox050. [DOI] [PubMed] [Google Scholar]
- 18. Muir A. B., Whelan K. A., Dougherty M. K., et al., “The Potential for Malignancy From Atopic Disorders and Allergic Inflammation: A Systematic Review and Meta‐Analysis,” Clinical and Experimental Allergy 50, no. 2 (2020): 147–159. Epub 20191220, PubMed PMID: 31743536; PMCID: PMC6994341, 10.1111/cea.13537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ludvigsson J. F., Otterblad‐Olausson P., Pettersson B. U., and Ekbom A., “The Swedish Personal Identity Number: Possibilities and Pitfalls in Healthcare and Medical Research,” European Journal of Epidemiology 24, no. 11 (2009): 659–667. Epub 20090606, PubMed PMID: 19504049; PMCID: PMC2773709, 10.1007/s10654-009-9350-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brooke H. L., Talback M., Hornblad J., et al., “The Swedish Cause of Death Register,” European Journal of Epidemiology 32, no. 9 (2017): 765–773. Epub 20171005, PubMed PMID: 28983736; PMCID: PMC5662659, 10.1007/s10654-017-0316-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cnattingius S., Ericson A., Gunnarskog J., and Kallen B., “A Quality Study of a Medical Birth Registry,” Scandinavian Journal of Social Medicine 18, no. 2 (1990): 143–148. PubMed PMID: 2367825, 10.1177/140349489001800209. [DOI] [PubMed] [Google Scholar]
- 22. Ludvigsson J. F., Andersson E., Ekbom A., et al., “External Review and Validation of the Swedish National Inpatient Register,” BMC Public Health 11, no. 1 (2011): 450. Epub 20110609, PubMed PMID: 21658213; PMCID: PMC3142234, 10.1186/1471-2458-11-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ludvigsson J. F., Almqvist C., Bonamy A. K., et al., “Registers of the Swedish Total Population and Their Use in Medical Research,” European Journal of Epidemiology 31, no. 2 (2016): 125–136. Epub 20160114, PubMed PMID: 26769609, 10.1007/s10654-016-0117-y. [DOI] [PubMed] [Google Scholar]
- 24. Barlow L., Westergren K., Holmberg L., and Talback M., “The Completeness of the Swedish Cancer Register: A Sample Survey for Year 1998,” Acta Oncologica 48, no. 1 (2009): 27–33. PubMed PMID: 18767000, 10.1080/02841860802247664. [DOI] [PubMed] [Google Scholar]
- 25. Ludvigsson J. F., Svedberg P., Olen O., Bruze G., and Neovius M., “The Longitudinal Integrated Database for Health Insurance and Labour Market Studies (LISA) and Its Use in Medical Research,” European Journal of Epidemiology 34, no. 4 (2019): 423–437. Epub 20190330, PubMed PMID: 30929112; PMCID: PMC6451717, 10.1007/s10654-019-00511-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. R Core Team , R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2021), https://www.R‐project.org. [Google Scholar]
- 27. Therneau T., “_A Package for Survival Analysis in R_. R package Version 3.2‐10 U” (2021), https://CRAN.R‐project.org/package=survival.
- 28. Ludvigsson J. F., Haberg S. E., Knudsen G. P., et al., “Ethical Aspects of Registry‐Based Research in the Nordic Countries,” Clinical Epidemiology 7 (2015): 491–508. Epub 20151123, PubMed PMID: 26648756; PMCID: PMC4664438, 10.2147/CLEP.S90589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Prizment A. E., Vierkant R. A., Smyrk T. C., et al., “Tumor Eosinophil Infiltration and Improved Survival of Colorectal Cancer Patients: Iowa Women's Health Study,” Modern Pathology 29, no. 5 (2016): 516–527. Epub 20160226, PubMed PMID: 26916075; PMCID: PMC4848192, 10.1038/modpathol.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ghebeh H., Elshenawy M. A., AlSayed A. D., and Al‐Tweigeri T., “Peripheral Blood Eosinophil Count Is Associated With Response to Chemoimmunotherapy in Metastatic Triple‐Negative Breast Cancer,” Immunotherapy 14, no. 4 (2022): 189–199. Epub 20220105, PubMed PMID: 34984928, 10.2217/imt-2021-0149. [DOI] [PubMed] [Google Scholar]
- 31. Fuller A. D., Karami A. L., Kabir M. F., et al., “Eosinophilic Esophagitis‐Associated Epithelial Remodeling May Limit Esophageal Carcinogenesis,” Frontiers in Allergy 4 (2023): 1086032. Epub 20230329, PubMed PMID: 37064719; PMCID: PMC10090679, 10.3389/falgy.2023.1086032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sun J., Fang F., Olen O., et al., “Normal Gastrointestinal Mucosa at Biopsy and Subsequent Cancer Risk: Nationwide Population‐Based, Sibling‐Controlled Cohort Study,” BMC Cancer 22, no. 1 (2022): 890. Epub 20220813, PubMed PMID: 35964121; PMCID: PMC9375922, 10.1186/s12885-022-09992-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S4
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


