Abstract
Keywords: clinical guidelines, colorectal cancer, colorectal surgery, gastrointestinal symptoms
INTRODUCTION
Background
The global incidence of colorectal cancer (CRC) continues to increase, accompanied by improvements in overall and disease‐specific survival. 1 Consequently, there are about 5 million survivors of CRC worldwide, with a range of unmet needs affecting physical, psychological, and social functioning. 2 Gastrointestinal dysfunction is a common problem following surgical treatment for CRC, with a reported incidence of up to 50% at 10 years post‐operatively. 3 It presents with a constellation of symptoms, including abdominal pain and distension and variable bowel habits (e.g. constipation, diarrhoea, fragmentation), all of which require different management strategies. 4 , 5 , 6 These long‐term sequelae can have a significant impact on patients' overall well‐being and quality of life (QoL). Recent studies have shown that a specific cause for gastrointestinal symptoms was found in 80% of patients when examined in a clinic dedicated to late sequelae after colorectal surgery. Additionally, 70% of these patients experienced improvement after treatment. 7 Similar outcomes were observed in a nurse‐led clinic, 8 highlighting the clinical and socio‐economic value of recognising and addressing of these complications.
Gastrointestinal symptoms are a common long‐term consequence of oncological colorectal resections. Different symptom patterns occur, depending on the specific resection type performed, due to the different underlying pathophysiological mechanisms responsible for the gastrointestinal function. A right‐sided hemicolectomy involves the resection of the ileocaecal valve, which has an important role in maintaining normal gastrointestinal function. Right sided colonic resections are associated with bile salt malabsorption which can lead to symptoms of diarrhoea. 4 , 9 , 10 Small bowel bacterial overgrowth may also occur following a right hemicolectomy, which can further exacerbate bowel dysfunction. 4 It is estimated that approximately one in five patients undergoing a right‐sided colectomy experience loose stool, increased bowel frequency and/or increased nocturnal defecation. 4 , 11 Some of these symptoms may improve or resolve spontaneously over time. However, many patients have persistent bowel dysfunction.
Left sided resections have a different symptom profile, which includes diarrhoea, stool fragmentation, a feeling of obstruction and prolonged evacuation time. 4 , 12 It is hypothesised that this is secondary to the reduction in absorptive capacity of water following left sided colonic resections. 9 Additionally, the resection of the rectosigmoid junction, which acts as a high‐pressure barrier that prevents rapid stool transit into the rectum, could also be a contributing factor in developing faecal incontinence. 11 , 13 Functional outcomes after rectal resections have been investigated in several studies. 14 , 15 Low anterior resection syndrome (LARS), although still not clearly defined, is considered to be a condition with a multifactorial aetiology. Key contributing factors include the loss of reservoir function, decrease in anal sphincter function, afferent sensory loss, and autonomic denervation. 6 , 16 Additional contributing factors include the potential compromise of the sensory and motor functions of the colon due to traction and iatrogenic injury to colonic vascularisation and/or innervation during surgery during colonic mobilisation. 17 , 18 Surgical denervation of the left colon, leads to disruption of α‐sympathetic pathways, leading to increased colon motility and loose stool. 19 Furthermore, alterations in the meal response, particularly affecting the rectosigmoid brake cyclic motor pattern, are observed after low anterior resection. 20 These complex and multifactorial pathophysiological mechanisms can lead to symptoms such as diarrhoea, increased frequency, urgency, fragmentation, incomplete evacuation and incontinence for flatus and/or faeces. 15 , 21
Neoadjuvant radiotherapy in rectal cancer patients has improved oncological outcomes. 22 , 23 However, the long‐term adverse effects of radiotherapy may lead to more pronounced bowel dysfunction in irradiated patients compared to patients who undergo surgery alone. 24 , 25 Pre‐operative radiotherapy is associated with long‐term increased bowel frequency, urgency and soiling. 26 , 27 , 28 Whether or not the patient has undergone radiotherapy should be taken into account when composing a treatment plan, since tissue damage due to radiation is not expected to improve over time. Late radiation tissue injury is estimated to affect between 5% and 15% of long‐term survivors who received radiotherapy. 29
We are aware of the significance of preventive measures in addressing these gastrointestinal symptoms, including the consideration of pre‐treatment options. Nevertheless, the primary focus of this guideline is to synthesise the available up‐to‐date evidence on the adequate assessment and management of the gastrointestinal symptoms that manifest after colorectal resections.
Previous guidelines regarding the management of gastrointestinal symptoms after oncological colorectal surgery have primarily focussed on rectal cancer patients experiencing LARS 16 or addressed all possible sequelae (i.e., including urinary incontinence, sexual dysfunction and chemotherapy‐induced symptoms) resulting in a limited focus on pure gastrointestinal symptoms. 30 Therefore, an updated guideline focussing on gastrointestinal symptoms after any oncological colorectal resection, using the best available evidence, was an unmet need. The goal of this project was to create an up‐to‐date joint European, multidisciplinary guideline on the assessment and management of all gastrointestinal symptoms after oncological colorectal resections, using the best available evidence.
Methods
This guideline has been created in collaboration with patient representatives and members of the United European Gastroenterology (UEG), European Society of Coloproctology (ESCP), European Association of Endoscopic Surgery (EAES), European Society for Primary Care Gastroenterology (ESPCG), European Society for Clinical Nutrition and Metabolism (ESPEN), European Society of Neurogastroenterology and Motility (ESNM) and the European Society of Surgical Oncology (ESSO). The patient representatives involved were selected from the target population of individuals intended to use this guideline, as they either currently experienced gastrointestinal symptoms after colorectal surgery or had experienced them in the past. They were represented in each working group, provided feedback on the research (PICO) questions and were asked to vote on the importance of outcomes.
This guideline provides guidance on the role of diagnostic modalities and the effectiveness of treatment options for gastrointestinal symptoms following colorectal surgery. The guideline consists of two parts: Part I—Sequelae to oncological diseases and Part II—Sequelae to benign diseases. Both parts contain the following chapters:
-
‐
Diagnosis
-
‐
First‐line treatment
-
‐
Second‐line therapies | Non‐surgical interventions
-
‐
Second‐line therapies | Surgical interventions
The guideline is intended for use by all healthcare professionals who treat patients who experience gastrointestinal symptoms after colorectal surgery (e.g. nurses, general practitioners, dietitians, gastroenterologists, colorectal surgeons, etc.). It can also serve as a source of information for patients seeking knowledge about the diagnosis and treatment options for their gastrointestinal symptoms in order to improve QoL. This guideline project was funded by the ESCP and UEG. The Guideline Development Group (GDG) had full control over the development of the protocol and the guideline without any influence from the funding body. The full methods are provided in Supporting Information S1: Appendix 1. The evidence‐to‐decision frameworks are provided in Supporting Information S2: Appendix 2. Before presenting the systematic literature review, we provide an overview of the recommendations, including a schematic representation in a treatment algorithm (Figure 1).
FIGURE 1.
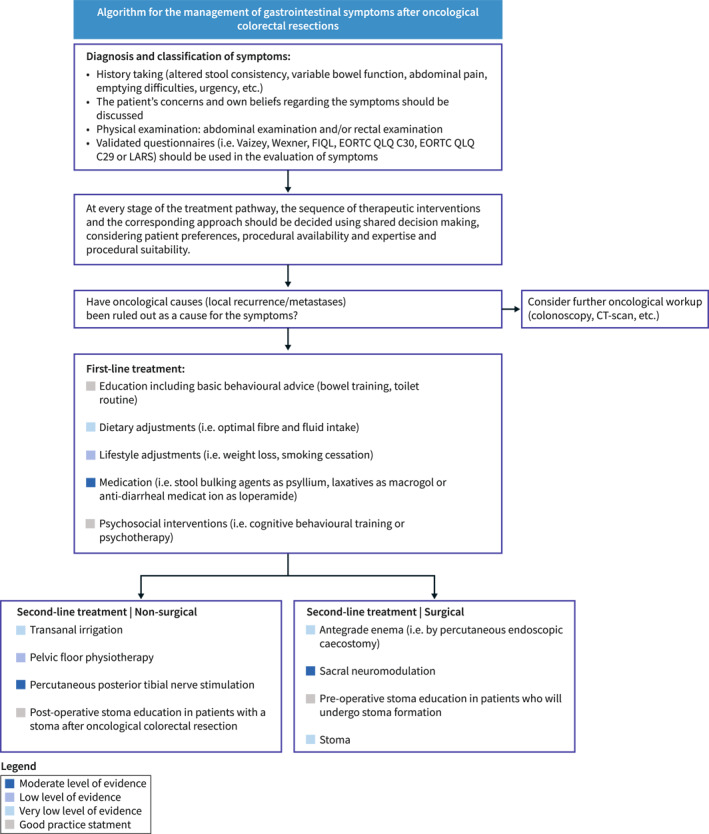
Treatment algorithm gastrointestinal symptoms.
RECOMMENDATIONS
Legend: Wording and colour of recommendations
Diagnosis and classification of gastrointestinal symptoms after oncological colorectal resections

|
Health care professionals should assess other post‐operative symptoms, including altered stool consistency, variable or unpredictable bowel function, emptying difficulties, involuntary loss of faeces or gas and/or urgency. |
| Low level of evidence; upgraded by the GDG (see evidence to decision framework in Supporting Information S2 : Appendix 2) | |

|
Health care professionals should assess postoperative pain, including the frequency, duration and intensity of pain after oncological resections. The impact of pain, including the impact on sleep and daily activities should also be considered |
| Low level of evidence; upgraded by the GDG (see evidence to decision framework in Supporting Information S2 : Appendix 2) | |

|
Physical examination should be performed in patients, including an abdominal examination and (digital) anorectal examination |
| Good Practice Statement, ungraded | |

|
A colonoscopy could be used to rule out anatomical causes (i.e. anastomotic stenosis, local recurrence) |
| Very low level of evidence; upgraded by the GDG (see evidence to decision framework in Supporting Information S2 : Appendix 2) | |

|
Anorectal manometry alone should not be used as a diagnostic modality in patients |
| Very low level of evidence | |

|
Health care professionals should use a validated questionnaire in order to evaluate gastrointestinal symptoms |
| Very low level of evidence |
First‐line treatment of gastrointestinal symptoms after oncological colorectal resections

|
Basic behavioural advice (i.e., toilet routine, bowel training) can be considered |
| Good Practice Statement, ungraded | |

|
Dietary adjustments (i.e., optimal fibre and fluid intake) can be considered |
| Very low level of evidence | |

|
Lifestyle adjustments, especially weight loss in overweight patients and smoking cessation in active smokers, could be used |
| Low level of evidence | |

|
Medication (i.e., stool bulking agents as psyllium, laxatives as macrogol, anti‐diarrheal medication such as loperamide, and/or bile acid binders such as cholestyramine) could be used |
| Low level of evidence | |

|
Psychosocial interventions (i.e., cognitive behavioural training or psychotherapy) can be considered once other pathology has been ruled out |
| Good Practice Statement, ungraded |
Second‐line treatment of gastrointestinal symptoms after oncological colorectal resections | Non‐surgical interventions

|
Transanal irrigation can be considered |
| Very low level of evidence | |

|
Pelvic floor physiotherapy could be used, if an experienced therapist is available to guide the patient |
| Low level of evidence | |

|
Percutaneous posterior tibial nerve stimulation could be used, if expertise is available at the local treatment facility |
| Low level of evidence | |

|
Post‐operative stoma education can be considered in patients with a stoma after oncological colorectal resections |
| Good Practice Statement, ungraded |
Second‐line treatment of gastrointestinal symptoms after oncological colorectal resections | Surgical interventions

|
An antegrade enema (i.e. by percutaneous endoscopic caecostomy) can be considered as treatment for faecal incontinence after a total mesorectal excision for rectal cancer |
| Very low level of evidence | |

|
Sacral neuromodulation could be used in patients with LARS/faecal incontinence |
| Moderate level of evidence; downgraded by the GDG GDG (see evidence to decision framework in Supporting Information S2 : Appendix 2) | |

|
A stoma can be considered in patients with faecal incontinence, for patients with refractory symptoms |
| Very low level of evidence | |

|
Pre‐operative stoma education can be considered in patients who will undergo stoma formation |
| Good Practice Statement, ungraded |
DIAGNOSIS
Introduction
Assessment of gastrointestinal symptoms should be a routine part of cancer follow‐up after surgery for CRC. A detailed patient history regarding symptoms is essential, including onset and severity of symptoms. These should be appropriately investigated using a variety of diagnostic modalities. This chapter focuses on the diagnosis of gastrointestinal symptoms following oncological colorectal resections.
Review questions
The following questions form the basis of our evidence review:
-
Which factors should be assessed during history taking?
-
‐
Altered stool consistency, variable, unpredictable bowel function, abdominal pain, emptying difficulties, involuntary loss of faeces or gas, urgency
-
‐
Which validated questionnaires could be applied?
-
‐
-
Which physical examination should be performed prior to treatment?
-
‐
Value of digital rectal examination
-
‐
Value of abdominal exam
-
‐
-
Which additional diagnostic modalities should be applied prior to treatment?
-
‐
Value of colonoscopy
-
‐
Value of anorectal manometry
-
‐
History taking
The initial step in identifying any symptoms of gastrointestinal dysfunction is a thorough medical history. A detailed history regarding bowel function should be taken, this should include specific questions to explore altered stool consistency, variable or unpredictable bowel function, emptying difficulties, involuntary loss of faeces or gas and/or urgency complaints. For specific information on the diagnosis and treatment options for faecal incontinence, please refer to the European guideline on faecal incontinence. 33 Due to the potentially sensitive and personal nature of these symptoms, all questions should be asked in a sensitive manner, taking into account cultural preferences. 34 , 35
Assessment of pain is important following an oncological colorectal resection, with Mortenson et al. highlighting the relevant aspects of a pain which should be elicited during the process of history taking. 36 A study assessing pain in Danish patients following surgery for rectal cancer patients identified significant domains on pain and their impact on QoL, including; frequency of pain, duration of pain, the intensity of pain throughout daily life, intensity of pain when most intense, pain affecting night's sleep and the abandonment of activities. Independent risk factors for post‐operative pain included, age <50 years, female sex, open surgery and radiotherapy with or without chemotherapy. Prolonged pelvic pain, defined as pain that has persisted for more than 6 months, is reported in up to 23% of CRC survivors. 37 Furthermore, it is important to elicit the multidimensional nature of pain, including the possibility of phantom pain following rectal resection. 38 , 39 Fear of cancer recurrence is important in this patient cohort and must be appropriately explored. 40 , 41 Health care professionals should be aware of the fact stress and anxiety in cancer survivors could be a contributing factor in the experienced symptoms. 42
The broader impact of symptoms on the patient's physical, psychological and social well‐being should be explored. Alongside, this any current or previous treatments and their impact should be appropriately explored and assessed. Understanding patient's preferences, including their ability to adhere/tolerate a specific certain treatment plan should also be explored to appropriately guide shared decision‐making
The use of validated questionnaires to assess for gastrointestinal symptoms post‐operatively is a valuable method to objectively assess and record symptoms. This allows for the objective comparison of symptoms between patients, enables serial monitoring, assesses treatment response and ensures homogeneity in outcome report within research studies. Chen et al. reported many different validated questionnaires, all capable of detecting clinically significant differences in patients with gastrointestinal symptoms. 43 The authors recommended that the LARS score or the Memorial Sloan‐Kettering Cancer Centre Bowel Function Instrument would be the best options to capture anorectal function after surgery for rectal cancer. Due to the brevity and ease of use in daily practice, the LARS score, has become the preferred questionnaire to assess anorectal function. 44 In keeping with these recommendations, the GDG recommends the use of the following validated questionnaires: the Vaizey score, 45 the Wexner score 46 or the Faecal Incontinence Quality of Life scale (FIQL) 47 for evaluating faecal incontinence. For evaluating faecal incontinence. The European Organisation for Research and Treatment of Cancer QoL Questionnaire Core Questionnaire (EORTC QLQ –C30) 48 and its CRC module (‐CR29) 49 could be used to assess all other relevant symptoms and QoL after oncological colorectal resections, in conjunction with the LARS score. 15
Although the management of urinary and sexual dysfunction following oncological colorectal resections is beyond the scope of these guidelines, we would like to advise addressing these symptoms (e.g. voiding dysfunction, urinary incontinence, erectile dysfunction, or dyspareunia) as part of standard history‐taking. If there is any suggestion of post‐operative dysfunction within these domains, we recommend referring the patient to the appropriate specialist for further adequate assessment and management of their symptoms.
Physical examination
There is no high quality evidence assessing the role and importance of physical examination in the diagnosis of gastrointestinal symptoms after oncological colorectal resections. Physical examination of the patient is always warranted and starts with a general observation of the patient. An abdominal examination should be performed, looking for any clinical signs of possible locoregional or metastatic disease, and any stigmata of other gastrointestinal conditions. Palpation of inguinal lymph nodes in rectal cancer patients should be considered. 50 Due to the critical significance of a thorough physical examination in the diagnosis of any medical condition, the GDG has opted to upgrade the level of evidence for this recommendation.
Digital rectal examination could be performed to assess for anastomotic pathology including stenosis in patients with a low anastomosis who present with gastrointestinal symptoms after oncological colorectal resections. Digital rectal examination can be considered in all other patients with a remaining anal sphincter complex after resection and should always include an inspection of the entire anorectal region. It can be performed in several positions as the left lateral position, or lithotomy position. 51 , 52 Due to the importance of a thorough physical examination in the diagnosis of any medical condition, the GDG has opted to upgrade the level of evidence for this recommendation.
Diagnostic modalities
There are a variety of diagnostic modalities which can be used to assess and evaluate gastrointestinal symptoms, including colonoscopy, anorectal manometry or endoanal ultrasound. Bjoern et al. and Vollebregt et al. assessed the clinical utility of manometry in patients with gastrointestinal symptoms after oncological colorectal resections. 53 , 54
Bjoern et al. prospectively analysed 48 Danish rectal cancer patients following transanal or laparoscopic TME (TaTME and LaTME) at a mean follow‐up time of 41 months. The mean anal sphincter resting pressure did not differ significantly between the groups. There was no significant correlation between the LARS score and manometry parameters, including resting or squeeze pressures. However, the resting and squeezing pressures of the anal sphincter were generally lower than that compared to healthy individuals. Vollebregt et al. compared the results of high‐resolution anorectal manometry in 21 consecutive male patients with LARS to 37 healthy men. They conclude that more than 50% of male patients with LARS had altered anal slow‐wave pressure activity and that further research is warranted. A colonoscopy should be used to rule out anatomical causes (i.e. local regrowth or anastomotic stenosis) for the gastrointestinal symptoms. 55 , 56 The use of transanal or endoscopic ultrasound can be considered in the work‐up of gastrointestinal symptoms if the appropriate expertise is available in the institution. 57
Recommendations for the diagnosis and classification of gastrointestinal symptoms after oncological colorectal resections

|
Health care professionals should assess other post‐operative symptoms, including altered stool consistency, variable or unpredictable bowel function, emptying difficulties, involuntary loss of faeces or gas and/or urgency | |
| Low level of evidence; upgraded by the GDG (see evidence to decision framework in Supporting Information S2 : Appendix 2) | ||

|
Health care professionals should assess postoperative pain, including the frequency, duration and intensity of pain after oncological resections. The impact of pain, including the impact on sleep and daily activities should also be considered. | |
| Low level of evidence; upgraded by the GDG (see evidence to decision framework in Supporting Information S2 : Appendix 2) | ||

|
Physical examination should be performed in patients, including an abdominal examination and (digital) anorectal examination | |
| Good Practice Statement, ungraded | ||

|
A colonoscopy could be used to rule out anatomical causes (i.e. anastomotic stenosis, local recurrence) | |
| Very low level of evidence; upgraded by the GDG (see evidence to decision framework in Supporting Information S2 : Appendix 2) | ||

|
Anorectal manometry alone should not be used as a diagnostic modality in patients. | |
| Very low level of evidence | ||

|
Health care professionals should use a validated questionnaire in order to evaluate gastrointestinal symptoms. Questionnaires to consider are: Vaizey, Wexner, FIQL, EORTC QLQ C30, EORTC QLQ CR29, LARS score or chronic pain score. Even with very low level of evidence, expert opinion encourages use of validated questionnaires | |
| Very low level of evidence | ||
FIRST‐LINE TREATMENT
Introduction
Once the patient's concerns have been appropriately identified, the appropriate management should be initiated as ‘first‐line treatment’. These first‐line treatment options aim to alleviate gastrointestinal symptoms after oncological colorectal resections, with the goal of improving QoL. First‐line treatment options include behavioural advice, dietary and lifestyle adjustments, medication (i.e. stool bulking agents or anti‐diarrhoeal medication), and/or psychosocial interventions.
Review questions
The following questions form the basis of our evidence review, including the comparison between any two (or more) of the treatment options as outlined below:
What are the effects of basic behavioural advice/education versus no behavioural advice/education on gastrointestinal symptoms in patients after oncological colorectal resections?
What are the effects of advice on toileting habits versus no advice on toileting habits on gastrointestinal symptoms in patients after oncological colorectal resections?
-
What are the effects of dietary adjustments versus no dietary adjustments on gastrointestinal symptoms in patients after oncological colorectal resections?
-
‐
Optimal water intake
-
‐
Optimal fibre intake
-
‐
Decreased caffeine intake
-
‐
-
What are the effects of lifestyle adjustments versus no lifestyle adjustments on gastrointestinal symptoms in patients after oncological colorectal resections?
-
‐
Weight loss in overweight patients
-
‐
Smoking cessation in smokers
-
‐
-
What are the effects of medication versus no medication on gastrointestinal symptoms in patients after oncological colorectal resections?
-
‐
Metamucil
-
‐
Psyllium
-
‐
Carboxymethylcellulose
-
‐
Gum Arabic
-
‐
Loperamide
-
‐
What are the effects of laxatives versus no laxatives on constipation and associated symptoms in patients after oncological colorectal resections?
What are the effects of psychosocial interventions versus no psychosocial interventions on gastrointestinal symptoms in patients after oncological colorectal resections?
Behavioural advice
There is no high quality evidence assessing, including, observational studies, RCTs or systematic reviews, assessing the impact of basic behavioural advice or toileting habits as a first‐line treatment for gastrointestinal symptoms after oncological colorectal resections. Garfinkle et al. 58 published a study on the predisposing factors and treatment options for LARS in 2022. Among the reported self‐management behaviours are sitz baths, proper toileting habits (i.e. defecating with elevated knees), use of perianal skin creams and/or barriers and avoidance of irritants such as alcoholic wipes. 7
Van der Heijden et al. 59 assessed a standardised postoperative treatment protocol for LARS, comprising of three key aspects; adequate provision of information, adequate screening by means of LARS score and Bristol stool score and structured treatment options including an evaluation of the treatment effect. The main goals of this treatment pathway are to create awareness, enhance patient self‐management, improve their coping mechanisms and to adequately treat what bother most.
Adequate patient education, providing up‐to‐date information on behavioural advice and guidance by an expert as a fixed point of contact are deemed very important in creating optimal coping‐mechanisms for CRC patients. This counselling could begin pre‐operatively in patients identified as at high risk of developing gastrointestinal symptoms after oncological colorectal resections, as demonstrated in the bowel rehabilitation programme. 60
Dietary adjustments
There is limited evidence on the effects of dietary adjustments as a first‐line treatment for gastrointestinal symptoms after oncological colorectal resections, with one mixed‐method analysis with pooled data by Sun et al. from 2009 61 and two narrative reviews by Lundby et al. from 2011 62 and by Rosen et al. from 2023. 63 The mixed‐method analysis by Sun et al. assessed health‐related quality of life (HRQoL) in a cohort of 856 CRC survivors across multiple studies. Using validated questionnaires, they found that CRC survivors made substantial dietary adjustments after surgery, regardless of their ostomy status. They classified foods into three different categories:
-
‐
Foods to be avoided: this category included dairy products, spicy and fatty foods since they could result in constipation or diarrhoea. Additionally, carbonated beverages were reported to be troublesome in some patients.
-
‐
Specific foods to avoid: items such as popcorn, unions, corn, beans, lettuce and peanuts were intentionally omitted from the diet.
-
‐
Helpful foods: adequate fluid consumption, foods rich in dietary fibre and prune juice were identified as beneficial in avoiding gastrointestinal symptoms after oncological colorectal resections.
Furthermore, strategies like deliberate chewing, eating smaller meals and meal planning based on social activities were considered helpful. These findings are supported by Lundby et al. 62 who emphasised the necessity of advising patients to avoid food that provokes frequent bowel movements or loose stool. However, this review did not identify foods or types of food that might have these effects.
The narrative review by Rosen et al. reports that dietary modification is regarded as first‐line therapy for patients suffering from LARS. The article identified caffeine, spicy and fatty foods, or alcohol as potential causes of soft stools. They also identified that the consumption of high‐fibre foods could enhance stool consistency, thus improving symptoms of diarrhoea and subsequent incontinence. The authors of the article warn that the increase in dietary fibre intake might potentially lead to a deterioration of symptoms due to bloating and an increased frequency of bowel movements.
Since these dietary adjustments can be quite challenging and the fact that they are very much patient‐specific, we would advise to consult a dietician to identify an optimal, patient‐specific dietary regimen. This dietary guidance is essential for new ostomy patients to ensure appropriate and timely dietary adaptation, considering that ensuring optimal fluid balance and avoiding specific foods (i.e. mushrooms) is a particular challenge in patients with an ileostomy in order to prevent complications as dehydration or blockages. 64
Lifestyle adjustments
The current evidence is limited with regards to lifestyle adjustments as a first‐line treatment for gastrointestinal symptoms after oncological colorectal resections, with only one observational study identified by Anderson et al. from 2010. 65 This small prospective case series consisted of 18 CRC patients after completing curative treatment with a BMI of ≥25 kg/m2, approximately 6–10 months post‐operative. Participants were provided with a personalised 3‐month intervention programme, which consisted of a supervised programme of physical activity and nutrition. Participants reported improvements across a range of symptoms, including reduced symptoms of constipation, increased levels of energy, improved sleeping patterns, and an average weight loss of 1.2 kg. Although, promising, the results from this observational study should be interpreted with caution due to the small population.
There is no high‐quality evidence assessing the effects of smoking cessation on gastrointestinal symptoms in active smokers. However, it is widely acknowledged that nicotine exposure can result in gastrointestinal symptoms, including nausea and diarrhoea, due to increased intestinal motor activity. 66 Furthermore, studies have shown that diarrhoea following oncological colorectal resections is associated with habitual smoking. 67 Therefore, smoking cessation should be considered as part of first line treatment for gastrointestinal symptoms after oncological colorectal resections.
Medication
The current evidence base on the effects of different types of medication as a first‐line treatment for gastrointestinal symptoms after oncological colorectal resections consists of one narrative review by Lundby et al. from 2011 62 and one RCT by Ryoo et al. from 2021 68 on the effects of different types of medication as a first‐line treatment for gastrointestinal symptoms after oncological colorectal resections.
The narrative review by Lundby et al. briefly addressed the role of fibres and bulking agents, that is, methyl cellulose or natural psyllium, in potentially enhancing stool consistency and augmenting the volume of stool per bowel movement. They recommended the incorporation of these therapeutic agents as first‐line treatment for the management of symptoms of faecal incontinence. This is based on the rationale that solid stool leads to less incontinence than loose stool. However, it is important to acknowledge that the evidence for antidiarrheal medications such as fibre supplements and loperamide comes from studies on chronic idiopathic diarrhoea, predominantly within the context of irritable bowel syndrome and is not specific for CRC patients after colorectal resections. 30 , 69 Furthermore, the evidence supporting the use of osmotic and peristaltic laxatives as a first‐line treatment is derived from studies targeting chronic idiopathic constipation. 30 For further information on the treatment options for chronic diarrhoea, we would like to refer to the UEG Guideline on functional bowel disorders with diarrhoea. 70
Ryoo et al. included 98 male patients diagnosed with LARS at least 1 month after low anterior resection for rectal cancer. Patients were eligible for inclusion after ileostomy reversal and adjuvant chemo‐ and or radiotherapy. In total, 98 patients were randomised to receive either 4 weeks of treatment with ramosetron or placebo. The incidence of major LARS was found to be 58% in the ramosetron group, compared to 82% in the placebo group. No major adverse events were reported in the intervention group, with minor adverse events reported in 5 patients. Reported minor events were hard stool, frequent stool and anal pain. These manifestations did not differ between the two groups. An important limitation of this study is the exclusion of female patients, as ramosetron was exclusively approved for administration to male patients by the Korean Food and Drug Administration (FDA) at the time of study. Furthermore, no patients with colon cancer were included.
For patients with bile salt malabsorption, following ileocaecal resection, the most suitable treatment approach is the use of a bile acid binder. Cholestyramine is typically the preferred initial choice. 12 , 71 It is crucial to initiate treatment with a gradual titration of the dosage in order to achieve maximum effect while minimising potential (gastrointestinal) side effects. In cases where cholestyramine proves ineffective, colesevelam could be considered, as it has shown positive responses in up to 70% of patients who did not respond to the cholestyramine. 12 Furthermore, it may be beneficial to explore the implementation of a low fat diet as guided by a dietitian. Low fat diets have been reported to significantly reduce abdominal pain and nocturnal defecation in patients with bile salt malabsorption after ileocaecal resections. 72 , 73
Small intestinal bacterial overgrowth is another consequence of an ileocaecal resection, potentially resulting in loose stools and bloating. Typically, this condition is addressed with a brief course of high‐dose antibiotics. 30 , 74 Ciprofloxacin (500 mg × 2 for 7 days) and rifaximine (600 mg × 2 for 6 days) are the antibiotics with the best established and dose‐related effect. 71 , 75 , 76 However, the specific choice of antibiotics may also depend on the local antibiotic guidelines.
There is a growing trend in the use of central neuromodulators, such as antidepressants or antipsychotics (i.e. tricyclic antidepressants such as amitriptyline, serotonin noradrenaline uptake inhibitors (SSRIs) or atypical antipsychotics such as quetiapine) for the treatment of functional gastrointestinal disorders. These medications have not yet been extensively investigated for patients following oncological colorectal resection. Nonetheless, if there is access to specialist expertise such as neurogastroenterology these types of medication can be considered to help managing gastrointestinal symptoms. It is important that the administration of such medications is overseen and monitored by an experienced healthcare professional to ensure their effectiveness and safety. For more in‐depth information on the use of neuromodulators in the pain management for functional gastrointestinal disorders, please refer to the Rome Foundation working team report. 77
Psychosocial interventions
There is limited evidence regarding the effects of psychosocial interventions on gastrointestinal symptoms after oncological colorectal resections. A systematic review from Mosher et al. from 2016 78 reported on the effects of psychosocial interventions on QoL and psychosocial outcomes. Fourteen RCTs were identified, reporting at least one psychosocial or QoL outcomes, across 2476 individual CRC patients across all disease stages. Cognitive behavioural training or group and/or individual psychotherapy were included as psychosocial interventions. Of these 14 included RCTs, only three studies reported a significant effect of the psychosocial intervention on more than one mental health outcome. 79 , 80 , 81 The authors of the systematic review suggest that future large‐scale trials are warranted to draw definitive conclusion on the effectiveness of the administered psychosocial interventions.
Recommendations for the first‐line treatment of gastrointestinal symptoms after oncological colorectal resections

|
Basic behavioural advice (i.e., toilet routine, bowel training) can be considered |
| Good Practice Statement, ungraded | |

|
Dietary adjustments (i.e., optimal fibre and fluid intake) can be considered |
| Very low level of evidence | |

|
Lifestyle adjustments, especially weight loss in overweight patients and smoking cessation in active smokers, could be used |
| Low level of evidence | |

|
Medication (i.e., stool bulking agents as psyllium, laxatives as macrogol, anti‐diarrheal medication such as loperamide, and/or bile acid binders such as cholestyramine) could be used |
| Low level of evidence | |

|
Psychosocial interventions (i.e., cognitive behavioural training or psychotherapy) can be considered once other pathology has been ruled out |
| Good Practice Statement, ungraded |
SECOND‐LINE THERAPIES | NON‐SURGICAL INTERVENTIONS FOR GASTROINTESTINAL SYMPTOMS
Introduction
For patients in whom first‐line treatment has failed to generate satisfactory improvement of symptoms, additional treatment options should be considered. We suggest that, in general, health care providers should initially aim for the least invasive treatment options, that is, the non‐surgical interventions, before progressing to the often more intrusive surgical alternatives, since the latter are often associated with a higher risk of complications and cost. However, depending on the patient and physician preferences, as well as the availability of expertise in specific treatment modalities, bypassing non‐surgical second‐line treatment options and directly opting for surgical interventions subsequent to first‐line treatment options may be consider reasonable in select patients. This chapter addresses second‐line non‐surgical treatment options such as TAI, pelvic floor physiotherapy, and percutaneous posterior tibial nerve stimulation for gastrointestinal symptoms after oncological colorectal resections.
Review questions
The following questions form the basis of our evidence review, including the comparison between any two (or more) of the treatment options as mentioned below:
What are the effects of TAI versus no trans anal irrigation on gastrointestinal symptoms in patients after oncological colorectal resections?
What are the effects of stoma irrigation versus no stoma irrigation on gastrointestinal symptoms in patients with a stoma after surgery for CRC?
What are the effects of pelvic floor physiotherapy versus no pelvic floor physiotherapy on gastrointestinal symptoms in patients after oncological colorectal resections?
What are the effects of PPTNS versus no PPTNS on gastrointestinal symptoms in patients after oncological colorectal resections?
What are the effects of post‐operative stoma education on gastrointestinal symptoms in patients with a stoma after oncological colorectal resections?
Irrigation methods
Transanal irrigation
The current evidence base consists of four systematic reviews and three RCTs on the effects of TAI on symptoms associated with LARS after oncological colorectal resections. The first systematic review by Christensen et al. from 2010 82 included a total of 17 studies that have evaluated TAI in 1229 adult patients. TAI was regarded as successful treatment in 658 cases (53%). Based on symptom profiles, the most clinical impact was achieved in patients with mixed symptoms (59%), followed by faecal incontinence (47%) and constipation (45%).
The second review by Burch et al. from 2021 83 included 30 studies published between 1996 and 2020, reporting on 853 individual patients. Significant heterogeneity was observed amongst the studies included. TAI was described as a treatment method in five of these included studies without elaborating on specific treatment results. Only one of these 5 studies was an RCT, which has been included separately for this guideline. 84 They compared TAI (n = 13) to PPTNS (n = 14) for a treatment period up to 6 months. They conclude that both treatments improved LARS scores, however, TAI was associated with a statistically significant reduction in LARS score, improving from 35 to 30.
The third narrative review by Rosen et al. 63 reports on the first introduction of TAI as a treatment for chronic LARS in 1989. 85 This is followed by an RCT conducted by the same group in 2020. 86 Rosen et al. randomised 18 patients for TAI and 19 patients for supportive therapy as a control group. Nine patients stopped TAI due to the length of time of the emptying process (n = 8) or pain (n = 1). After one year, the 10 patients who continued TAI reported a lower number of bowel movements during day and night. However, there was no concurrent improvement in the Wexner score, LARS score or SF‐36. The results of this trial should be interpreted with caution due to the high number of adverse events, significant attrition rate and overall small sample size.
The most recent RCT by Pieniowski et al. from 2023 87 randomised 45 patients with LARS after rectal cancer surgery. Twenty two patients were randomised to receive TAI and 23 patients were randomised to receive standard care as part of the control group. After 1 year of treatment, patients in the TAI group had significantly improved LARS scores and Cleveland Clinic Florida Fecal Incontinence Score. They also had better QoL scores on the EORTC QLQ‐C30 questionnaire compared to patients receiving standard treatment.
The narrative review by Lundby et al. from 2011 62 made a brief statement that the TAI procedure is simple and generally well‐tolerated for long‐term treatment, with only minor complications. Overall, treatment results have been reported to be effective in patients with faecal incontinence after oncological rectal resections.
Stoma irrigation
There were no studies identified regarding the effects of stoma irrigation on any gastrointestinal symptoms after oncological colorectal resections. Since this is potentially applicable to a select cohort of patients, with a lack of current evidence and further clinical studies warranted, we refrain from providing any endorsement regarding the integration of stoma irrigation into a standardised treatment pathway for this patient population.
Pelvic floor physiotherapy
Four systematic reviews 88 , 89 , 90 , 91 and one recently published RCT 92 which examined the effects of pelvic floor therapy on gastrointestinal symptoms after oncological colorectal resections. The first review and meta‐analysis by Li et al. from 2022 88 included 12 studies, of which two were RCTs, with a total of 561 patients suffering from bowel dysfunction after rectal cancer treatment. Key findings included significant improvements in Wexner and Vaizey scores as well as HRQoL following pelvic floor physiotherapy. The systematic review of Chan et al. from 2021 89 included 11 studies, of which five were overlapping with Li et al. Symptoms of faecal incontinence improved in seven studies and five studies reported a decreased frequency of bowel movements. All of these reviews report that included studies had several methodological limitations and that further research is warranted.
The RCT by Asnong et al. from 2022 92 was not yet included in these systematic reviews. They performed a multi‐centre, single‐blind RCT comparing pelvic floor muscle training (n = 50) with a control group (n = 54) after TME for rectal cancer, with a minimal follow‐up of 1 year. The proportion of participants with an improvement in LARS category was significantly higher after pelvic floor physiotherapy compared to the control group after 4 and 6 months, however, this effect was no longer evident at 1 year. Asnong et al. conclude that pelvic floor physiotherapy mainly results in a faster recovery of bowel symptoms after colorectal resections and/or stoma reversal, justifying it as an early treatment option for bowel symptoms after oncological rectal resections.
Percutaneous posterior tibial nerve stimulation
Two systematic reviews 93 , 94 have evaluated the impact of PPTNS on LARS. The meta‐analysis by Liapis et al. from 2023 consisted three RCTs and two observational studies, reporting on a total of 74 patients with LARS that received PPTNS. 93 This identified a significant reduction of LARS scores, with improvement in defecation, functionality and QoL with (PPTNS). These results need to be interpreted with caution since only five studies with relatively small population sizes were included. The review by Bulfone et al. 94 included five studies, of which three were evaluating sacral nerve stimulation, and only two reporting on PPTNS. Those two studies are also included in the meta‐analysis by Liapis et al.
Stoma education
Only one systematic review from Faury et al. from 2017 95 identified the impact of pre‐ and post‐operative stoma education on gastrointestinal symptoms in patients with a stoma after oncological colorectal resections. This systematic review included 15 studies (6 RCTs) that delivered a variety of patient education interventions before and/or after surgery. Patient education interventions were defined as ‘a structured, standardised and condition‐specific intervention, which is different from routine clinical education due to its structured characteristics’. Education was provided by a range of health care providers, including, dedicated stoma care nurses, surgeons, or trained expert patients. Ten studies offered individual training/information sessions, four offered group education and one study applied both methods of training/education. The frequency and length of education sessions was variable. Five of these studies examined QoL outcomes, with three studies reporting statistically significant improvements in QoL after stoma education sessions. 80 , 96 , 97 Patient education was identified to have a positive impact on self‐management skills. Education interventions should be integrated into daily clinical practice in the treatment and prevention of gastrointestinal symptoms for patients with a stoma after oncological colorectal resections.
Recommendations for the second‐line treatment of gastrointestinal symptoms after oncological colorectal resections | Non‐surgical interventions

|
Transanal irrigation can be considered |
| Very low level of evidence | |

|
Pelvic floor physiotherapy could be used, if an experienced therapist is available to guide the patient |
| Low level of evidence | |

|
Percutaneous posterior tibial nerve stimulation could be used, if expertise is available at the local treatment facility |
| Low level of evidence | |

|
Post‐operative stoma education can be considered in patients with a stoma after oncological colorectal resections |
| Good Practice Statement, ungraded |
SECOND‐LINE THERAPIES | SURGICAL INTERVENTIONS FOR GASTROINTESTINAL SYMPTOMS
Introduction
If first‐line treatment and/or subsequent second‐line non‐surgical treatment options have failed to produce satisfactory outcomes, or if second‐line non‐surgical options are not favoured or accessible, surgical interventions should be considered. Surgical interventions should be considered on an individual patient basis. We outline types and timing of surgical interventions to consider as second‐line treatment for gastrointestinal symptoms after oncological colorectal resections.
Review questions
The following questions form the basis of our systematic review, including the comparison between any two (or more) of the treatment options as mentioned below:
When should surgical interventions be considered in treatment for gastrointestinal symptoms in patients after oncological colorectal resections?
What is the most appropriate surgical procedure when conservative treatments fail?
What are the effects of sacral neuromodulation (SNM) versus no SNM on gastrointestinal symptoms in patients after oncological colorectal resections?
What are the effects of a stoma versus no stoma in patients with a primary anastomosis, in which conservative and other surgical interventions have failed on patient satisfaction and QoL?
Timing of surgical interventions
There were no studies identified studies identified regarding the timing of surgical interventions as second‐line treatment for gastrointestinal symptoms after oncological colorectal resections. It is important to stress that the deliberation of any surgical intervention and its timing must be tailored to the patient's unique circumstances, with any decision‐making made jointly between the patient and healthcare professionals. It is essential that the patient are appropriately counselled and understands the potential risks of complications and implications of surgery prior to embarking on any surgical interventions.
Sacral neuromodulation
Three systematic reviews assessed the effect of SNM as a second‐line surgical treatment for gastrointestinal symptoms after oncological colorectal resections. 94 , 98 , 99
The systematic review and meta‐analysis by Huang et al. from 2019 98 included 10 studies (four prospective cohort studies, 5 case series, and one case report) with a total of 75 patients. The Inclusion criteria were not well defined, although the authors indicate that all patients ‘had failed conservative management’ for bowel dysfunction following low anterior resections before opting for SNM. Overall median follow‐up was 18 months after implantation. Overall, there was a significant improvement in the Cleveland Clinic Incontinence score (used in all 10 studies) and the LARS score (applied in three studies) which was 67% for both validated scores.
The systematic review and meta‐analysis by Ram et al. from 2020 99 included three new studies besides the 10 previously identified by Huang et al, with a new total of 114 individual patients. These three studies were prospective cohort studies on the efficacy of SNM in the treatment of LARS. This systematic review reported significant improvement in anal continence as measured by several clinical and functional parameters, including the Wexner score. Manometric resting pressure, maximum squeeze pressure and maximum tolerated volume. QoL questionnaires also demonstrated a significant improvement, however the 36‐item Short Form Health Survey (SF‐36) was only reported in a small group of patients (n = 6). The systematic review by Bulfone et al. from 2020 94 also included three studies on SNM. However, all three included articles were also analysed by Huang et al. and Ram et al.
The overall quality of the evidence for this research question was classified as ‘moderate’ according to the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) methodology. Given the small number of eligible patients for SNM, combined with limited experience and funding for this particular treatment globally, the GDG decided to downgrade the level of evidence and thus the recommendation regarding SNM is as a second‐line surgical treatment.
Antegrade enema
One systematic review and one prospective cohort study, not included in this review, evaluated the impact of an antegrade enema as a second‐line surgical treatment for gastrointestinal symptoms after oncological colorectal resections.
The systematic review by Pape et al. from 2023 100 included 12 articles published between 2013 and 2021 reporting on intervention pathways for the treatment of LARS following sphincter‐preserving rectal cancer surgery. Antegrade irrigation was reported in two of these studies, and subsequently included in the proposed treatment pathways. This included both antegrade continence enemas and percutaneous endoscopic caecostomies.
The prospective cohort study by Didailler et al. from 2018 101 reports on 25 patients who underwent percutaneous endoscopic caecostomies for refractory LARS and faecal incontinence after a TME. Two patients (8%) developed post‐operative abscess managed by antibiotic treatment. Following a median follow up of 8 months, four catheters (15%) had been removed on patient request, two patients did not have an effective response, one patient experienced too significant pain and one patient had locally recurrent rectal cancer. Three patients went on to have a permanent stoma. Four patients (15%) died during follow‐up due to causes unrelated to the antegrade lavage. LARS score, Wexner score, and the Gastrointestinal QoL Index all improved significantly after percutaneous endoscopic caecostomy. The authors conclude that an enema could be a promising treatment for refractory LARS and faecal incontinence, in patients trying to avoid a definitive stoma. However, the results from this observational study should be interpreted with caution due to the small population and relative high number of adverse events.
Stoma
No studies were identified regarding the impact of stoma formation in patients with a primary anastomosis, in which conservative and/or other surgical interventions had failed in the management of gastrointestinal symptoms after oncological colorectal resections. It is important to emphasise that a dedicated, multidisciplinary team is of great value in determining the therapeutic strategy in patients with refractory gastrointestinal symptoms after oncological colorectal resections. Many patients can achieve improvement with appropriate adherence to treatment plans and guidance by dedicated health care professionals. However, if all else fails, several studies, conclude that stoma formation can be considered as a final treatment option in patients with severe LARS or other gastrointestinal complaints with refractory symptoms and impaired QoL. 16 , 30 , 62 , 102
It is important to emphasise the role of stoma care nurse or any other health care practitioner dedicated to stoma education, with regards to counselling for stoma formation, with stoma education provided pre‐operatively.
Recommendations for the second‐line treatment of gastrointestinal symptoms after oncological colorectal resections | Surgical interventions

|
An antegrade enema (i.e. by percutaneous endoscopic caecostomy) can be considered as treatment for faecal incontinence after a total mesorectal excision for rectal cancer |
| Very low level of evidence | |

|
Sacral neuromodulation could be used in patients with LARS/faecal incontinence |
| Moderate level of evidence; downgraded by the GDG (see evidence to decision framework in Supporting Information S2 : Appendix 2) | |

|
A stoma can be considered in patients with faecal incontinence, for patients with refractory symptoms |
| Very low level of evidence | |

|
Pre‐operative stoma education can be considered in patients who will undergo stoma formation |
| Good Practice Statement, ungraded |
DISCUSSION
This is an up‐to‐date, European, multidisciplinary clinical practice guideline for the assessment and management of gastrointestinal symptoms after oncological colorectal resections. We included 19 recommendations on the assessment and management of gastrointestinal symptoms after oncological colorectal resections. A treatment algorithm (Figure 1) has been created in order to provide a schematic overview of the most important recommendations. The development of this algorithm involved visually representing the formulated recommendations, which were derived from a systematic and rigorous review of the best available evidence. In instances where literature was lacking, recommendations (or Good Practice Statements) were informed by the expert opinions of the GDG members involved. The GDG suggests using these treatment algorithms as a guide when exploring which diagnostic modalities or treatment options are applicable.
The multimodality treatment for CRC, including (chemo)radiation therapy and/or surgical resection, can result in chronic symptoms affecting multiple organ systems (i.e. bowel dysfunction, urinary incontinence, or sexual dysfunction). The need for a multidisciplinary care team to address the issues of increasing number of CRC survivors is currently an unmet need across multiple healthcare systems. 103 We would like to emphasise the added value of incorporating a diverse array of specialised healthcare providers in aiding patients and their families in navigating through every aspect of CRC survivorship. Gastroenterologists, colorectal surgeons, general practitioners, ostomy care nurses, urologists, gynaecologists, pelvic floor physiotherapists, social workers and psychologists, dietitians, as well as patient support groups and associations, could all potentially contribute valuable insights to these multidisciplinary teams. With an ever‐increasing number of CRC survivors, it is imperative not only to standardise oncological follow‐up procedures, but also to develop long‐term follow‐up protocols addressing the long‐term functional outcomes. 104 , 105 It is essential to integrate QoL and psychosocial aspects into our follow‐up protocols. 106 , 107 Patient‐reported outcome measures could serve as a valuable instrument in establishing a core outcome set for CRC survivors, facilitating the reporting and monitoring of functional outcomes. 108 , 109 We recommend the use of validated questionnaires in the evaluation of symptoms and treatment‐effect monitoring. Nevertheless, the interpretation of responses remains subject to healthcare professionals' discretion, potentially resulting in variability.
The potential financial repercussions of CRC on patients and their families should also be recognised. Research indicates that up to 40% of CRC survivors reported adverse financial consequences associated with their cancer diagnosis. 110 Nearly one in three CRC patients transitioned to part‐time employment or stopped working, as cancer impacted their ability to work and depressed their income. 111 This cancer‐related financial strain was found to be associated with lower HRQoL. 112 Given the increasing incidence of CRC in individuals below the age of 50, who are more frequently part of the working population, recognising this financial burden is crucial among the challenges faced by CRC survivors. 113 , 114
As previously highlighted, it is essential to consider preventive measures in the management of bowel, urinary, and/or sexual dysfunction. Given the complex association between various long‐term sequelae and the specific type of surgical resection performed, there is a critical need to further explore the potential of organ preservation in CRC patients and the watch‐and‐wait approach for rectal cancer. 115 , 116 Furthermore, major advancements in imaging, systemic therapy and radiation delivery enable a more personalised approach to the treatment of CRC. 117 A patient‐specific approach in chemoradiotherapy and tailored surgical innovations may collectively contribute to improving the burden of long‐term sequelae after CRC treatment and therefore enhance patients' QoL. 118 , 119
The key strength of this guideline is the multidisciplinary and international approach, maximising the experience with various treatment options, across multiple perspectives and healthcare systems. Furthermore, patients have participated in an active manner in composing this guideline to ensure that all important aspects were covered. The guideline is limited by small evidence base, which consists of low quality studies. Consequently, we have had to rely on this very low level of evidence or even expert opinion for several recommendations or good practice statements where this was the only available evidence. We recognise that this introduces a potential bias into the guideline, notwithstanding our efforts to mitigate it through a multidisciplinary and international approach. Our guideline does not address preventive measurements, including the consideration of pre‐treatment options, for gastrointestinal symptoms after oncological colorectal resections. We have performed a systematic literature search and included the best available evidence. In order to improve the level of evidence and therefore, the strength of the recommendations, future high‐quality prospective trials are warranted.
All UEG channels will be utilised for the widespread dissemination of this guideline. The guideline and treatment algorithm will be available in the UEG Guideline app, with very minimal resources required to access these documents. Additional support from all participating societies will contribute to the broad distribution and implementation of the guideline. Local adaptation of this guideline, in collaboration with local stakeholders, could potentially help overcome economic or infrastructural challenges in the implementation. This guideline will be updated in consultation with the UEG Quality of Care committee, provided sufficient funding has been allocated. The update process will adhere to a systematic and methodologically rigorous approach conducted in collaboration with the UEG and other participating associations. The literature search will be repeated annually in order to identify new evidence. In case this new evidence would substantially impact the recommendations in this guideline, then an update will be provided.
AUTHOR CONTRIBUTIONS
Stephanie O. Breukink, Daniel Keszthelyi and Deena Harji were the lead authors responsible for the assembly of the GDG and drafting of the guidelines protocol. The initial list of research questions and core outcomes to be covered by these guidelines were drafted by Stephanie O. Breukink, Deena Harji, Daniel Keszthelyi, Anke H.C. Gielen and methodologist Jos Kleijnen. All research questions and intended outcomes were revised by all GDG members. The literature search was conducted by Anke H.C. Gielen under supervision of the methodologist Jos Kleijnen. Screening and selection of the articles was independently performed by Anke H.C. Gielen and Coco Smit. Data extraction was performed by Anke H.C. Gielen and verified by Coco Smit. The quality of evidence of the included articles was systematically appraised according to the GRADE method by Anke H.C. Gielen and verified by Jos Kleijnen. All GDG members and the external reviewer (Marc Gladman) discussed the results and reached a consensus on the recommendations. The lead authors Stephanie O. Breukink, Deena Harji, Daniel Keszthelyi and Anke H.C. Gielen drafted this manuscript, which was reviewed, revised, and approved by all aforementioned GDG members.
CONFLICT OF INTEREST STATEMENT
The authors would like to report the following potential conflict(s) of interest:
D. Keszthelyi, ZonMw (Dutch government), Dutch Foundation for Gastroenterology (MLDS), Allergan, Rome Foundation Horizon 2020, speaking at event Falk Foundation; J. Melenhorst, ZonMw (Dutch government); S.O. Breukink, ZonMw (Dutch government), Nationale Fonds tegen Kanker (National fund against Cancer) C. Kontovounisios, stakeholder One Welbeck hospital; A. Weimann, receipt of research supports B. Braun, Mucos and Seca, speaker at events of Abbott, Baxter, B. Braun, Fresenius Kabi and the Falk Foundation; H. Mohan, International Medical Robotics Academy consultation fees; J. Kleijnen, ESCP consultation fees, owner of Kleijnen Systematic Reviews Ltd; M. Gladman, grant from the Colorectal Surgical Society of Australia & New Zealand. These conflicts of interest were disclosed and addressed prior the start of the guideline development, ensuring that they did not influence the guideline process and development of recommendations.
DISCLAIMER
These guidelines have been developed with reasonable care and with the best of knowledge available to the authors at the time of preparation. They are intended to assist healthcare professionals and allied healthcare professionals as an educational tool to provide information that may support them in providing care to patients. Patients or other community members using these guidelines shall do so only after consultation with a health professional and shall not mistake these guidelines as professional medical advice. These guidelines must not substitute seeking professional medical and health advice from a health professional. These guidelines may not apply to all situations and should be interpreted in the light of specific clinical situations and resource availability. It is up to every clinician to adapt these guidelines to local regulations and to each patient's individual circumstances and needs. The information in these guidelines shall not be relied upon as being complete, current or accurate, nor shall it be considered as inclusive of all proper treatments or methods of care or as a legal standard of care. UEG makes no warranty, express or implied, in respect of these guidelines and cannot be held liable for any damages resulting from the application of these guidelines, in particular for any loss or damage (whether direct or indirect) resulting from a treatment based on the guidance given herein. UEG shall not be held liable to the utmost extent permissible according to the applicable laws for any content available on such external websites, which can be accessed by using the links included herein.
Supporting information
Supporting Information S1
Supporting Information S2
Supporting Information S3
ACKNOWLEDGEMENTS
United European Gastroenterology & European Society of Coloproctology Activity Grant.
Members of the guideline development group
Stavros A. Antoniou: Department of Surgery, Papageorgiou General Hospital, Thessaloniki, Greece.
Geerard L. Beets: School for Oncology and Reproduction (GROW), Maastricht University, Maastricht, The Netherlands; Department of Surgery, The Netherlands Cancer Institute, Amsterdam, The Netherlands.
Stephanie O. Breukink: Department of Surgery, Maastricht University (Maastricht University, Including Maastricht UMC+), Maastricht, The Netherlands; School of Nutrition and Translational Research in Metabolism (NUTRIM), Maastricht University, Maastricht, The Netherlands; School for Oncology and Reproduction (GROW), Maastricht University, Maastricht, The Netherlands.
Suzanne Dore: Patient Advisory Board Representative, UK.
Asbjørn M. Drewes: Mech‐Sense, Department of Gastroenterology & Hepatology, Aalborg University Hospital, Aalborg, Denmark; Danish Cancer Society Centre for Research on Survivorship and Late Adverse Effects After Cancer in the Pelvic Organs, Aarhus, Denmark.
Hannah Garside: Department of Colorectal Surgery, Western General Hospital, Edinburgh, UK.
Marc A. Gladman: Faculty of Health & Medical Sciences, The University of Adelaide, Adelaide Medical School, Adelaide, South Australia, Australia.
Deena Harji: Department of Colorectal Surgery, Manchester University NHS Foundation Trust, Manchester, UK.
Goran Hauser: Department of Gastroenterology, Clinical Hospital Centre Rijeka, Faculty of Medicine, University of Rijeka, Rijeka, Croatia.
Therese Juul: Danish Cancer Society Centre for Research on Survivorship and Late Adverse Effects After Cancer in the Pelvic Organs, Aarhus, Denmark; Department of Surgery, Aarhus University Hospital, Aarhus, Denmark.
Daniel Keszthelyi: School of Nutrition and Translational Research in Metabolism (NUTRIM), Maastricht University, Maastricht, The Netherlands; Division of Gastroenterology‐Hepatology, Department of Internal Medicine, Maastricht University Medical Centre, Maastricht, The Netherlands.
Jos Kleijnen; School for Oncology and Reproduction (GROW), Maastricht University, Maastricht, The Netherlands.
Christos Kontovounisios: Department of Surgery, Papageorgiou General Hospital, Thessaloniki, Greece; Department of Colorectal Surgery, Chelsea and Westminster Hospital NHS Foundation Trust, London, UK.
Laura Lorenzon: General Surgery Unit, Fondazione Policlinico Universitario “A. Gemelli” ‐ IRCCS, Rome, Italy.
Lisa Massey: Department of Colorectal Surgery, Nottingham University Hospitals, Nottingham, UK.
Jarno Melenhorst: Department of Surgery, Maastricht University (Maastricht University, Including Maastricht UMC+), Maastricht, The Netherlands; hool of Nutrition and Translational Research in Metabolism (NUTRIM), Maastricht University, Maastricht, The Netherlands; School for Oncology and Reproduction (GROW), Maastricht University, Maastricht, The Netherlands.
Helen M. Mohan: Department of Colorectal Surgery, Western General Hospital, Edinburgh, UK.
Jean Muris: Department of General Practice, Care and Public Health Research Institute, Maastricht University, Maastricht, The Netherlands.
Coco Smit: Faculty of Health, Medicine and Life Sciences, Maastricht University, Maastricht, The Netherlands.
Yvonne Tillotson: Patient Advisory Board Representative, The Netherlands.
Arved Weimann: Department of General, Visceral and Oncological Surgery, St. George Hospital, Leipzig, Germany.
Marco Zelic: Department of Abdominal Surgery, Clinical Hospital Centre Rijeka, Rijeka, Croatia.
Contributor Information
Anke H. C. Gielen, Email: anke.gielen@mumc.nl.
the Guideline Development Group:
Stavros A. Antoniou, Geerard L. Beets, Stephanie O. Breukink, Suzanne Dore, Asbjørn M. Drewes, Hannah Garside, Marc A. Gladman, Deena Harji, Goran Hauser, Therese Juul, Daniel Keszthelyi, Jos Kleijnen, Christos Kontovounisios, Laura Lorenzon, Lisa Massey, Jarno Melenhorst, Helen M. Mohan, Jean Muris, Coco Smit, Yvonne Tillotson, Arved Weimann, and Marco Zelic
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this guideline, since no new data were created or analysed in this project. All results as presented in this manuscript were directly derived from data as presented in the original articles. These are all included in the list of references.
REFERENCES
- 1. Xi Y, Xu P. Global colorectal cancer burden in 2020 and projections to 2040. Translational Oncol. 2021;14(10):101174. 10.1016/j.tranon.2021.101174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Denlinger CS, Barsevick AM. The challenges of colorectal cancer survivorship. J Natl Compr Cancer Netw. 2009;7(8):883–894. 10.6004/jnccn.2009.0058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lam D, Jones O. Changes to gastrointestinal function after surgery for colorectal cancer. Best Pract Res Clin Gastroenterol. 2020;48:101705. 10.1016/j.bpg.2020.101705 [DOI] [PubMed] [Google Scholar]
- 4. Hope C, Reilly J, Lund J, Andreyev H. Systematic review: the effect of right hemicolectomy for cancer on postoperative bowel function. Support Care Cancer. 2020;28(10):4549–4559. 10.1007/s00520-020-05519-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pape E, Vlerick I, Van Nieuwenhove Y, Pattyn P, Van de Putte D, van Ramshorst G, et al. Experiences and needs of patients with rectal cancer confronted with bowel problems after stoma reversal: a systematic review and thematic‐synthesis. Eur J Oncol Nurs. 2021;54:102018. 10.1016/j.ejon.2021.102018 [DOI] [PubMed] [Google Scholar]
- 6. Verkuijl SJ, Jonker JE, Trzpis M, Burgerhof JG, Broens PM, Furnee EJ. Functional outcomes of surgery for colon cancer: a systematic review and meta‐analysis. Eur J Surg Oncol. 2021;47(5):960–969. 10.1016/j.ejso.2020.11.136 [DOI] [PubMed] [Google Scholar]
- 7. Larsen HM, Borre M, Christensen P, Mohr Drewes A, Laurberg S, Krogh K, et al. Clinical evaluation and treatment of chronic bowel symptoms following cancer in the colon and pelvic organs. Acta Oncol. 2019;58(5):776–781. 10.1080/0284186x.2018.1562211 [DOI] [PubMed] [Google Scholar]
- 8. Mekhael M, Larsen HM, Lauritzen MB, Thorlacius‐Ussing O, Laurberg S, Krogh K, et al. Bowel dysfunction following pelvic organ cancer: a prospective study on the treatment effect in nurse‐led late sequelae clinics. Acta Oncologica. 2023;62(1):70–79. 10.1080/0284186x.2023.2168214 [DOI] [PubMed] [Google Scholar]
- 9. Magdeburg J, Glatz N, Post S, Kienle P, Rickert A. Long‐term functional outcome of colonic resections: how much does faecal impairment influence quality of life? Colorectal Dis. 2016;18(11):O405–O413. 10.1111/codi.13526 [DOI] [PubMed] [Google Scholar]
- 10. Yde J, Larsen HM, Laurberg S, Krogh K, Moeller HB. Chronic diarrhoea following surgery for colon cancer—frequency, causes and treatment options. Int J Colorectal Dis. 2018;33(6):683–694. 10.1007/s00384-018-2993-y [DOI] [PubMed] [Google Scholar]
- 11. Wright HK. The functional consequences of colectomy. Am J Surg. 1975;130(5):532–534. 10.1016/0002-9610(75)90506-1 [DOI] [PubMed] [Google Scholar]
- 12. Larsen HM, Mekhael M, Juul T, Borre M, Christensen P, Mohr Drewes A, et al. Long‐term gastrointestinal sequelae in colon cancer survivors: prospective pilot study on identification, the need for clinical evaluation and effects of treatment. Colorectal Dis. 2021;23(2):356–366. 10.1111/codi.15544 [DOI] [PubMed] [Google Scholar]
- 13. Schoetz DJ. Postcolectomy syndromes. World J Surg. 1991;15(5):605–608. 10.1007/bf01789206 [DOI] [PubMed] [Google Scholar]
- 14. Bryant CL, Lunniss PJ, Knowles CH, Thaha MA, Chan CL. Anterior resection syndrome. Lancet Oncol. 2012;13(9):e403–e408. 10.1016/s1470-2045(12)70236-x [DOI] [PubMed] [Google Scholar]
- 15. Emmertsen KJ, Laurberg S. Low anterior resection syndrome score: development and validation of a symptom‐based scoring system for bowel dysfunction after low anterior resection for rectal cancer. Ann Surg. 2012;255(5):922–928. 10.1097/sla.0b013e31824f1c21 [DOI] [PubMed] [Google Scholar]
- 16. Christensen P, Im Baeten C, Espín‐Basany E, Martellucci J, Nugent KP, Zerbib F, et al. Management guidelines for low anterior resection syndrome–the MANUEL project. Colorectal Dis. 2021;23(2):461–475. 10.1111/codi.15517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thorsen Y, Stimec BV, Lindstrom JC, Nesgaard JM, Oresland T, Ignjatovic D. Bowel motility after injury to the superior mesenteric plexus during D3 extended mesenterectomy. J Surg Res. 2019;239:115–124. 10.1016/j.jss.2019.02.004 [DOI] [PubMed] [Google Scholar]
- 18. Ng KS, Gladman M. Patient‐reported and physician‐recorded bowel dysfunction following colorectal resection and radical cystectomy: a prospective, comparative study. Colorectal Dis. 2020;22(10):1336–1347. 10.1111/codi.15041 [DOI] [PubMed] [Google Scholar]
- 19. Lee WY, Takahashi T, Pappas T, Mantyh CR, Ludwig KA. Surgical autonomic denervation results in altered colonic motility: an explanation for low anterior resection syndrome? Surgery. 2008;143(6):778–783. 10.1016/j.surg.2008.03.014 [DOI] [PubMed] [Google Scholar]
- 20. Keane C, Paskaranandavadivel N, Vather R, Rowbotham D, Arkwright J, Dinning P, et al. Altered colonic motility is associated with low anterior resection syndrome. Colorectal Dis. 2021;23(2):415–423. 10.1111/codi.15465 [DOI] [PubMed] [Google Scholar]
- 21. Hernandez MC, Wong P, Melstrom K. Low anterior resection syndrome. J Surg Oncol. 2023;127(8):1271–1276. 10.1002/jso.27261 [DOI] [PubMed] [Google Scholar]
- 22. Rahbari NN, Elbers H, Askoxylakis V, Motschall E, Bork U, Büchler MW, et al. Neoadjuvant radiotherapy for rectal cancer: meta‐analysis of randomized controlled trials. Ann Surg Oncol. 2013;20(13):4169–4182. 10.1245/s10434-013-3198-9 [DOI] [PubMed] [Google Scholar]
- 23. Feeney G, Sehgal R, Sheehan M, Hogan A, Regan M, Joyce M, et al. Neoadjuvant radiotherapy for rectal cancer management. World J Gastroenterol. 2019;25(33):4850–4869. 10.3748/wjg.v25.i33.4850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gegechkori N, Haines L, Lin JJ. Long‐term and latent side effects of specific cancer types. Med Clin. 2017;101(6):1053–1073. 10.1016/j.mcna.2017.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Peeters K, Van De Velde C, Leer J, Martijn H, Junggeburt J, Kranenbarg EK, et al. Late side effects of short‐course preoperative radiotherapy combined with total mesorectal excision for rectal cancer: increased bowel dysfunction in irradiated patients—a Dutch colorectal cancer group study. J Clin Oncol. 2005;23(25):6199–6206. 10.1200/jco.2005.14.779 [DOI] [PubMed] [Google Scholar]
- 26. Knowles G, Haigh R, McLean C, Phillips HA, Dunlop MG, Din FV. Long term effect of surgery and radiotherapy for colorectal cancer on defecatory function and quality of life. Eur J Oncol Nurs. 2013;17(5):570–577. 10.1016/j.ejon.2013.01.010 [DOI] [PubMed] [Google Scholar]
- 27. Marijnen CA, Van De Velde CJ, Putter H, Van Den Brink M, Maas CP, Martijn H, et al. Impact of short‐term preoperative radiotherapy on health‐related quality of life and sexual functioning in primary rectal cancer: report of a multicenter randomized trial. J Clin Oncol. 2005;23(9):1847–1858. 10.1200/jco.2005.05.256 [DOI] [PubMed] [Google Scholar]
- 28. Lange MM, den Dulk M, Bossema ER, Maas CP, Peeters KCMJ, Rutten HJ, et al. Risk factors for faecal incontinence after rectal cancer treatment. J Br Surg. 2007;94(10):1278–1284. 10.1002/bjs.5819 [DOI] [PubMed] [Google Scholar]
- 29. Bennett MH, Feldmeier J, Hampson NB, Smee R, Milross C. Hyperbaric oxygen therapy for late radiation tissue injury. Cochrane Database Syst Rev. 2016;2018(4). 10.1002/14651858.cd005005.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Haas S, Mikkelsen AH, Kronborg CJS, Oggesen BT, Møller PF, Fassov J, et al. Management of treatment‐related sequelae following colorectal cancer. Colorectal Dis. 2023;25(3):458–488. 10.1111/codi.16299 [DOI] [PubMed] [Google Scholar]
- 31. Group GW. Grading quality of evidence and strength of recommendations. Bmj. 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Santesso N, Glenton C, Dahm P, Garner P, Akl EA, Alper B, et al. GRADE guidelines 26: informative statements to communicate the findings of systematic reviews of interventions. J Clin Epidemiol. 2020;119:126–135. 10.1016/j.jclinepi.2019.10.014 [DOI] [PubMed] [Google Scholar]
- 33. Assmann SL, Keszthelyi D, Kleijnen J, Anastasiou F, Bradshaw E, Brannigan AE, et al. Guideline for the diagnosis and treatment of Faecal Incontinence—a UEG/ESCP/ESNM/ESPCG collaboration. United Eur Gastroenterol J. 2022;10(3):251–286. 10.1002/ueg2.12213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wilson M. Asking sensitive questions: accessing the ‘private’account. Nurse Res. 2009;16(4):31–39. 10.7748/nr2009.07.16.4.31.c7159 [DOI] [PubMed] [Google Scholar]
- 35. Krishan Aggarwal N, Chen D, Lewis‐Fernández R. If you don’t ask, they don’t tell: the cultural formulation interview and patient perceptions of the clinical relationship. Am J Psychother. 2022;75(3):108–113. 10.1176/appi.psychotherapy.20210040 [DOI] [PubMed] [Google Scholar]
- 36. Mortensen A, Thyø A, Emmertsen K, Laurberg S. Chronic pain after rectal cancer surgery–development and validation of a scoring system. Colorectal Dis. 2019;21(1):90–99. 10.1111/codi.14436 [DOI] [PubMed] [Google Scholar]
- 37. Lowery AE, Starr T, Dhingra LK, Rogak L, Hamrick‐Price JR, Farberov M, et al. Frequency, characteristics, and correlates of pain in a pilot study of colorectal cancer survivors 1–10 years post‐treatment. Pain Med. 2013;14(11):1673–1680. 10.1111/pme.12223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fingren J, Lindholm E, Carlsson E. Perceptions of phantom rectum syndrome and health‐related quality of life in patients following abdominoperineal resection for rectal cancer. J Wound, Ostomy Cont Nurs. 2013;40(3):280–286. 10.1097/won.0b013e31827e8b20 [DOI] [PubMed] [Google Scholar]
- 39. Kuhlmann L, Teo K, Olesen SS, Phillips AE, Faghih M, Tuck N, et al. Development of the comprehensive pain assessment tool short form for chronic pancreatitis: validity and reliability testing. Clin Gastroenterol Hepatol. 2022;20(4):e770–e783. 10.1016/j.cgh.2021.05.055 [DOI] [PubMed] [Google Scholar]
- 40. Harnas SJ, Booij SH, Csorba I, Nieuwkerk PT, Knoop H, Braamse AM. Which symptom to address in psychological treatment for cancer survivors when fear of cancer recurrence, depressive symptoms, and cancer‐related fatigue co‐occur? Exploring the level of agreement between three systematic approaches to select the focus of treatment. J Cancer Surviv‐res Pract. 2023:1–13. 10.1007/s11764-023-01423-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Prins JB, Deuning‐Smit E, Custers JA. Interventions addressing fear of cancer recurrence: challenges and future perspectives. Curr Opin Oncol. 2022;34(4):279–284. 10.1097/cco.0000000000000837 [DOI] [PubMed] [Google Scholar]
- 42. Emery J, Butow P, Lai‐Kwon J, Nekhlyudov L, Rynderman M, Jefford M. Management of common clinical problems experienced by survivors of cancer. Lancet. 2022;399(10334):1537–1550. 10.1016/s0140-6736(22)00242-2 [DOI] [PubMed] [Google Scholar]
- 43. Chen TY.‐T, Emmertsen KJ, Laurberg S. What are the best questionnaires to capture anorectal function after surgery in rectal cancer? Curr colorectal Cancer Rep. 2015;11(1):37–43. 10.1007/s11888-014-0217-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Quezada‐Diaz FF, Elfeki H, Emmertsen KJ, Pappou EP, Jimenez‐Rodriguez R, Patil S, et al. Comparative analysis of the memorial sloan kettering bowel function instrument and the low anterior resection syndrome questionnaire for assessment of bowel dysfunction in rectal cancer patients after low anterior resection. Colorectal Dis. 2021;23(2):451–460. 10.1111/codi.15515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vaizey C, Carapeti E, Cahill J, Kamm M. Prospective comparison of faecal incontinence grading systems. Gut. 1999;44(1):77–80. 10.1136/gut.44.1.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jorge JMN, Wexner SD. Etiology and management of fecal incontinence. Dis Colon Rectum. 1993;36(1):77–97. 10.1007/bf02050307 [DOI] [PubMed] [Google Scholar]
- 47. Rockwood TH, Church JM, Fleshman JW, Kane RL, Mavrantonis C, Thorson AG, et al. Fecal Incontinence Quality of Life Scale: quality of life instrument for patients with fecal incontinence. Dis Colon Rectum. 2000;43(1):9–16. 10.1007/bf02237236 [DOI] [PubMed] [Google Scholar]
- 48. Fayers P, Bottomley A, Group EqoL . Quality of life research within the EORTC—the EORTC QLQ‐C30. Eur J Cancer. 2002;38:125–133. 10.1016/s0959-8049(01)00448-8 [DOI] [PubMed] [Google Scholar]
- 49. Gujral S, Conroy T, Fleissner C, Sezer O, King P, Avery K, et al. Assessing quality of life in patients with colorectal cancer: an update of the EORTC quality of life questionnaire. Eur J Cancer. 2007;43(10):1564–1573. 10.1016/j.ejca.2007.04.005 [DOI] [PubMed] [Google Scholar]
- 50. Shiratori H, Nozawa H, Kawai K, Hata K, Tanaka T, Kaneko M, et al. Risk factors and therapeutic significance of inguinal lymph node metastasis in advanced lower rectal cancer. Int J Colorectal Dis. 2020;35(4):655–664. 10.1007/s00384-020-03520-2 [DOI] [PubMed] [Google Scholar]
- 51. Maas M, Lambregts DM, Nelemans PJ, Heijnen LA, Martens MH, Leijtens JW, et al. Assessment of clinical complete response after chemoradiation for rectal cancer with digital rectal examination, endoscopy, and MRI: selection for organ‐saving treatment. Ann Surg Oncol. 2015;22(12):3873–3880. 10.1245/s10434-015-4687-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sabbagh C, Mauvais F, Vecten A, Ainseba N, Cosse C, Diouf M, et al. What is the best position for analyzing the lower and middle rectum and sphincter function in a digital rectal examination? A randomized, controlled study in men. Dig Liver Dis. 2014;46(12):1082–1085. 10.1016/j.dld.2014.08.045 [DOI] [PubMed] [Google Scholar]
- 53. Bjoern M, Perdawood S. Manometric assessment of anorectal function after transanal total mesorectal excision. Tech Coloproctol. 2020;24(3):231–236. 10.1007/s10151-020-02147-3 [DOI] [PubMed] [Google Scholar]
- 54. Vollebregt PF, Wiklendt L, Ang D, Dinning PG, Knowles CH, Scott SM. Altered slow‐wave pressure activity in low anterior resection syndrome: new mechanistic insight into pathophysiology? 2020. [DOI] [PubMed]
- 55. Mokhles S, Macbeth F, Farewell V, Fiorentino F, Williams N, Younes R, et al. Meta‐analysis of colorectal cancer follow‐up after potentially curative resection. J Br Surg. 2016;103(10):1259–1268. 10.1002/bjs.10233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lauretta A, Montori G, Guerrini GP. Surveillance strategies following curative resection and non‐operative approach of rectal cancer: how and how long? Review of current recommendations. World J Gastrointest Surg. 2023;15(2):177–192. 10.4240/wjgs.v15.i2.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cârțână ET, Gheonea DI, Săftoiu A. Advances in endoscopic ultrasound imaging of colorectal diseases. World J Gastroenterol. 2016;22(5):1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Garfinkle R, Boutros M. Low anterior resection syndrome: predisposing factors and treatment. Surg Oncol. 2022;43:101691. 10.1016/j.suronc.2021.101691 [DOI] [PubMed] [Google Scholar]
- 59. van der Heijden JA, van Heinsbergen M, Thomas G, Caers F, Slooter GD, Maaskant‐Braat AJ. Implementation of a postoperative screening and treatment guidance for the low anterior resection syndrome: preliminary results. Dis Colon Rectum. 2019;62(9):1033–1042. 10.1097/dcr.0000000000001428 [DOI] [PubMed] [Google Scholar]
- 60. Harji D, Fernandez B, Boissieras L, Berger A, Capdepont M, Zerbib F, et al. A novel bowel rehabilitation programme after total mesorectal excision for rectal cancer: the BOREAL pilot study. Colorectal Dis. 2021;23(10):2619–2626. 10.1111/codi.15812 [DOI] [PubMed] [Google Scholar]
- 61. Sun V, Grant M, Wendel CS, McMullen CK, Bulkley JE, Altschuler A, et al. Dietary and behavioral adjustments to manage bowel dysfunction after surgery in long‐term colorectal cancer survivors. Ann Surg Oncol. 2015;22(13):4317–4324. 10.1245/s10434-015-4731-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lundby L, Duelund‐Jakobsen J. Management of fecal incontinence after treatment for rectal cancer. Curr Opin Support Palliat Care. 2011;5(1):60–64. 10.1097/spc.0b013e3283435dd4 [DOI] [PubMed] [Google Scholar]
- 63. Rosen H, Sebesta CG, Sebesta C. Management of low anterior resection syndrome (LARS) following resection for rectal cancer. Cancers. 2023;15(3):778. 10.3390/cancers15030778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mitchell A, England C, Perry R, Lander T, Shingler E, Searle A, et al. Dietary management for people with an ileostomy: a scoping review. JBI Evid Synth. 2021;19(9):2188–2306. 10.11124/jbies-20-00377 [DOI] [PubMed] [Google Scholar]
- 65. Anderson A, Caswell S, MacAskill S, Steele R, Wells M. LiveWell, a feasibility study of a personalized lifestyle programme for colorectal cancer survivors. Support Care Cancer. 2010;18(4):409–415. 10.1007/s00520-009-0677-4 [DOI] [PubMed] [Google Scholar]
- 66. Das SK, Chisti MJ, Ahmed AS, Malek MA, Ahmed S, Shahunja K, et al. Diarrhoea and smoking: an analysis of decades of observational data from Bangladesh. BMC Publ Health. 2015;15:1–9. 10.1186/s12889-015-1906-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ohta H, Miyake T, Ueki T, Kojima M, Kawasaki M, Tatsuta T, et al. Predictors and clinical impact of postoperative diarrhea after colorectal cancer surgery: a prospective, multicenter, observational study (SHISA‐1602). Int J Colorectal Dis. 2022;37(3):657–664. 10.1007/s00384-022-04097-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ryoo S.‐B, Park J, Lee D, Lee M, Kwon Y.‐H, Kim M, et al. Anterior resection syndrome: a randomized clinical trial of a 5‐HT3 receptor antagonist (ramosetron) in male patients with rectal cancer. Br J Surg. 2021;108(6):644–651. 10.1093/bjs/znab071 [DOI] [PubMed] [Google Scholar]
- 69. Larsen HM, Elfeki H, Emmertsen KJ, Laurberg S. Long‐term bowel dysfunction after right‐sided hemicolectomy for cancer. Acta Oncol. 2020;59(10):1240–1245. 10.1080/0284186x.2020.1772502 [DOI] [PubMed] [Google Scholar]
- 70. Savarino E, Zingone F, Barberio B, Marasco G, Akyuz F, Akpinar H, et al. Functional bowel disorders with diarrhoea: clinical guidelines of the united European gastroenterology and European society for neurogastroenterology and motility. United Eur Gastroenterol J. 2022;10(6):556–584. 10.1002/ueg2.12259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Larsen HM, Krogh K, Borre M, Gregersen T, Mejlby Hansen M, Arveschoug AK, et al. Chronic loose stools following right‐sided hemicolectomy for colon cancer and the association with bile acid malabsorption and small intestinal bacterial overgrowth. Colorectal Dis. 2023;25(4):600–607. 10.1111/codi.16409 [DOI] [PubMed] [Google Scholar]
- 72. Gupta A, Muls AC, Lalji A, Thomas K, Watson L, Shaw C, et al. Outcomes from treating bile acid malabsorption using a multidisciplinary approach. Support Care Cancer. 2015;23(10):2881–2890. 10.1007/s00520-015-2653-5 [DOI] [PubMed] [Google Scholar]
- 73. Jackson A, Lalji A, Kabir M, Muls A, Gee C, Vyoral S, et al. The efficacy of a low‐fat diet to manage the symptoms of bile acid malabsorption–outcomes in patients previously treated for cancer. Clin Med. 2017;17(5):412–418. 10.7861/clinmedicine.17-5-412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Gasbarrini A, Lauritano EC, Gabrielli M, Scarpellini E, Lupascu A, Ojetti V, et al. Small intestinal bacterial overgrowth: diagnosis and treatment. Dig Dis. 2007;25(3):237–240. 10.1159/000103892 [DOI] [PubMed] [Google Scholar]
- 75. Lauritano EC, Gabrielli M, Lupascu A, Santoliquido A, Nucera G, Scarpellini E, et al. Rifaximin dose‐finding study for the treatment of small intestinal bacterial overgrowth. Aliment Pharmacol Ther. 2005;22(1):31–35. 10.1111/j.1365-2036.2005.02516.x [DOI] [PubMed] [Google Scholar]
- 76. Lauritano E, Gabrielli M, Scarpellini E, Ojetti V, Roccarina D, Villita A, et al. Antibiotic therapy in small intestinal bacterial overgrowth: rifaximin versus metronidazole. Eur Rev Med Pharmacol Sci. 2009;13(2). [PubMed] [Google Scholar]
- 77. Drossman DA, Tack J, Ford AC, Szigethy E, Törnblom H, Van Oudenhove L. Neuromodulators for functional gastrointestinal disorders (disorders of gut− brain interaction): a Rome foundation working team report. Gastroenterology. 2018;154(4):1140–1171.e1. 10.1053/j.gastro.2017.11.279 [DOI] [PubMed] [Google Scholar]
- 78. Mosher CE, Winger JG, Given BA, Shahda S, Helft PR. A systematic review of psychosocial interventions for colorectal cancer patients. Support Care Cancer. 2017;25(7):2349–2362. 10.1007/s00520-017-3693-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Carmack CL, Basen‐Engquist K, Yuan Y, Greisinger A, Rodriguez‐Bigas M, Wolff RA, et al. Feasibility of an expressive‐disclosure group intervention for post‐treatment colorectal cancer patients: results of the Healthy Expressions study. Cancer. 2011;117(21):4993–5002. 10.1002/cncr.26110 [DOI] [PubMed] [Google Scholar]
- 80. Cheung YL, Molassiotis A, Chang AM. The effect of progressive muscle relaxation training on anxiety and quality of life after stoma surgery in colorectal cancer patients. Psycho Oncol: J Psychol Soc Behav Dimensions Cancer. 2003;12(3):254–266. 10.1002/pon.638 [DOI] [PubMed] [Google Scholar]
- 81. Zhang M, Chan SW.‐c, You L, Wen Y, Peng L, Liu W, et al. The effectiveness of a self‐efficacy‐enhancing intervention for Chinese patients with colorectal cancer: a randomized controlled trial with 6‐month follow up. Int J Nurs Stud. 2014;51(8):1083–1092. 10.1016/j.ijnurstu.2013.12.005 [DOI] [PubMed] [Google Scholar]
- 82. Christensen P, Krogh K. Transanal irrigation for disordered defecation: a systematic review. Scand J Gastroenterol. 2010;45(5):517–527. 10.3109/00365520903583855 [DOI] [PubMed] [Google Scholar]
- 83. Burch J, Swatton A, Taylor C, Wilson A, Norton C. Managing bowel symptoms after sphincter‐saving rectal cancer surgery: a scoping review. J Pain Symptom Manage. 2021;62(6):1295–1307. 10.1016/j.jpainsymman.2021.05.022 [DOI] [PubMed] [Google Scholar]
- 84. Enriquez‐Navascues J, Labaka‐Arteaga I, Aguirre‐Allende I, Artola‐Etxeberria M, Saralegui‐Ansorena Y, Elorza‐Echaniz G, et al. A randomized trial comparing transanal irrigation and percutaneous tibial nerve stimulation in the management of low anterior resection syndrome. Colorectal Dis. 2020;22(3):303–309. 10.1111/codi.14870 [DOI] [PubMed] [Google Scholar]
- 85. Iwama T, Imajo M, Yaegashi K, Mishima Y. Self washout method for defecational complaints following low anterior rectal resection. Jpn J Surg. 1989;19(2):251–253. 10.1007/bf02471596 [DOI] [PubMed] [Google Scholar]
- 86. Rosen H, Boedecker C, Fürst A, Krämer G, Hebenstreit J, Kneist W. “Prophylactic” transanal irrigation (TAI) to prevent symptoms of low anterior resection syndrome (LARS) after rectal resection: results at 12‐month follow‐up of a controlled randomized multicenter trial. Tech Coloproctol. 2020;24(12):1247–1253. 10.1007/s10151-020-02261-2 [DOI] [PubMed] [Google Scholar]
- 87. Pieniowski EH, Bergström CM, Nordenvall CA, Westberg KS, Johar AM, Tumlin Ekelund SF, et al. A randomized controlled clinical trial of transanal irrigation versus conservative treatment in patients with low anterior resection syndrome after rectal cancer surgery. Ann Surg. 2023;277(1):30–37. 10.1097/sla.0000000000005482 [DOI] [PubMed] [Google Scholar]
- 88. Li H, Guo C, Gao J, Yao H. Effectiveness of biofeedback therapy in patients with bowel dysfunction following rectal cancer surgery: a systemic review with meta‐analysis. Therapeut Clin Risk Manag. 2022;18:71–93. 10.2147/tcrm.s344375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Chan K, Suen M, Coulson S, Vardy JL. Efficacy of pelvic floor rehabilitation for bowel dysfunction after anterior resection for colorectal cancer: a systematic review. Support Care Cancer. 2021;29(4):1795–1809. 10.1007/s00520-020-05832-z [DOI] [PubMed] [Google Scholar]
- 90. Lin KY, Granger CL, Denehy L, Frawley HC. Pelvic floor muscle training for bowel dysfunction following colorectal cancer surgery: a systematic review. Neurourol Urodyn. 2015;34(8):703–712. 10.1002/nau.22654 [DOI] [PubMed] [Google Scholar]
- 91. Visser WS, Te Riele WW, Boerma D, Van Ramshorst B, Van Westreenen HL. Pelvic floor rehabilitation to improve functional outcome after a low anterior resection: a systematic review. Ann Coloproctol. 2014;30(3):109. 10.3393/ac.2014.30.3.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Asnong A, D’Hoore A, Van Kampen M, Wolthuis A, Van Molhem Y, Van Geluwe B, et al. The role of pelvic floor muscle training on low anterior resection syndrome: a multicenter randomized controlled trial. Ann Surg. 2022;276(5):761–768. 10.1097/sla.0000000000005632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Liapis SC, Baloyiannis I, Perivoliotis K, Lytras D, Theodoropoulos G, Tzovaras G. The role of percutaneous tibial nerve stimulation (PTNS) in low anterior resection syndrome (LARS): a systematic review and meta‐analysis. J Gastrointest Cancer. 2023;54(4):1–12. 10.1007/s12029-023-00910-x [DOI] [PubMed] [Google Scholar]
- 94. Bulfone G, Del Negro F, Del Medico E, Cadorin L, Bressan V, Stevanin S. Rehabilitation strategies for low anterior resection syndrome. A Systematic Review Annali Dell'istituto Superiore di Sanità. 2020;56(1):38–47. [DOI] [PubMed] [Google Scholar]
- 95. Faury S, Koleck M, Foucaud J, M’Bailara K, Quintard B. Patient education interventions for colorectal cancer patients with stoma: a systematic review. Patient Educ Couns. 2017;100(10):1807–1819. 10.1016/j.pec.2017.05.034 [DOI] [PubMed] [Google Scholar]
- 96. Altuntas Y, Kement M, Gezen C, Eker H, Aydin H, Sahin F, et al. The role of group education on quality of life in patients with a stoma. Eur J Cancer Care. 2012;21(6):776–781. 10.1111/j.1365-2354.2012.01360.x [DOI] [PubMed] [Google Scholar]
- 97. Karadağ A, Menteş BB, Üner A, Irkörücü O, Ayaz S, Özkan S. Impact of stomatherapy on quality of life in patients with permanent colostomies or ileostomies. Int J Colorectal Dis. 2003;18(3):234–238. 10.1007/s00384-002-0462-z [DOI] [PubMed] [Google Scholar]
- 98. Huang Y, Koh C. Sacral nerve stimulation for bowel dysfunction following low anterior resection: a systematic review and meta‐analysis. Colorectal Dis. 2019;21(11):1240–1248. 10.1111/codi.14690 [DOI] [PubMed] [Google Scholar]
- 99. Ram E, Meyer R, Carter D, Gutman M, Rosin D, Horesh N. The efficacy of sacral neuromodulation in the treatment of low anterior resection syndrome: a systematic review and meta‐analysis. Tech Coloproctol. 2020;24(8):803–815. 10.1007/s10151-020-02231-8 [DOI] [PubMed] [Google Scholar]
- 100. Pape E, Burch J, van Ramshorst GH, Van Nieuwenhove Y, Taylor C. Intervention pathways for low anterior resection syndrome after sphincter‐saving rectal cancer surgery: a systematic scoping review. Colorectal Dis. 2023;25(4):538–548. 10.1111/codi.16412 [DOI] [PubMed] [Google Scholar]
- 101. Didailler R, Denost Q, Loughlin P, Chabrun E, Ricard J, Picard F, et al. Antegrade enema after total mesorectal excision for rectal cancer: the last chance to avoid definitive colostomy for refractory low anterior resection syndrome and fecal incontinence. Dis Colon Rectum. 2018;61(6):667–672. 10.1097/dcr.0000000000001089 [DOI] [PubMed] [Google Scholar]
- 102. Dulskas A, Smolskas E, Kildusiene I, Samalavicius NE. Treatment possibilities for low anterior resection syndrome: a review of the literature. Int J Colorectal Dis. 2018;33(3):251–260. 10.1007/s00384-017-2954-x [DOI] [PubMed] [Google Scholar]
- 103. Chan H, Savoie MB, Munir A, Moslehi J, Anwar M, Laffan A, et al. Multi‐disciplinary management in rectal cancer survivorship: a clinical practice review. J Gastrointest Cancer. 2023;54(4):1–14. 10.1007/s12029-022-00885-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Hardiman KM, Felder SI, Friedman G, Migaly J, Paquette IM, Feingold DL. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the surveillance and survivorship care of patients after curative treatment of colon and rectal cancer. Dis Colon Rectum. 2021;64(5):517–533. 10.1097/dcr.0000000000001984 [DOI] [PubMed] [Google Scholar]
- 105. Bräuner AB, Avellaneda N, Christensen P, Drewes AM, Emmertsen KJ, Krogh K, et al. Prospective evaluation of bowel function and quality of life after colon cancer surgery–is it time for routine screening for late sequelae? Acta Oncol. 2023;62(9):1132–1142. 10.1080/0284186x.2023.2246102 [DOI] [PubMed] [Google Scholar]
- 106. Santin O, Murray L, Prue G, Gavin A, Gormley G, Donnelly M. Self‐reported psychosocial needs and health‐related quality of life of colorectal cancer survivors. Eur J Oncol Nurs. 2015;19(4):336–342. 10.1016/j.ejon.2015.01.009 [DOI] [PubMed] [Google Scholar]
- 107. Gonzalez‐Saenz de Tejada M, Bilbao A, Baré M, Briones E, Sarasqueta C, Quintana J, et al. Association of social support, functional status, and psychological variables with changes in health‐related quality of life outcomes in patients with colorectal cancer. Psycho Oncol. 2016;25(8):891–897. 10.1002/pon.4022 [DOI] [PubMed] [Google Scholar]
- 108. Kotronoulas G, Papadopoulou C, MacNicol L, Simpson M, Maguire R. Feasibility and acceptability of the use of patient‐reported outcome measures (PROMs) in the delivery of nurse‐led supportive care to people with colorectal cancer. Eur J Oncol Nurs. 2017;29:115–124. 10.1016/j.ejon.2017.06.002 [DOI] [PubMed] [Google Scholar]
- 109. McNair A, Whistance R, Forsythe R, Rees J, Jones J, Pullyblank A, et al. Synthesis and summary of patient‐reported outcome measures to inform the development of a core outcome set in colorectal cancer surgery. Colorectal Dis. 2015;17(11):O217–O229. 10.1111/codi.13021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Sharp L, O’Leary E, O’Ceilleachair A, Skally M, Hanly P. Financial impact of colorectal cancer and its consequences: associations between cancer‐related financial stress and strain and health‐related quality of life. Dis Colon Rectum. 2018;61(1):27–35. 10.1097/dcr.0000000000000923 [DOI] [PubMed] [Google Scholar]
- 111. Mols F, Thong MS, Vissers P, Nijsten T, van de Poll‐Franse LV. Socio‐economic implications of cancer survivorship: results from the PROFILES registry. Eur J Cancer. 2012;48(13):2037–2042. 10.1016/j.ejca.2011.11.030 [DOI] [PubMed] [Google Scholar]
- 112. Ó Céilleachair A, Costello L, Finn C, Timmons A, Fitzpatrick P, Kapur K, et al. Inter‐relationships between the economic and emotional consequences of colorectal cancer for patients and their families: a qualitative study. BMC Gastroenterol. 2012;12:1–10. 10.1186/1471-230x-12-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Siegel RL, Jakubowski CD, Fedewa SA, Davis A, Azad NS. Colorectal cancer in the young: epidemiology, prevention, management. Am Soc Clin Oncol Educ Book. 2020;40:e75–e88. 10.1200/edbk_279901 [DOI] [PubMed] [Google Scholar]
- 114. Blum‐Barnett E, Madrid S, Burnett‐Hartman A, Mueller SR, McMullen CK, Dwyer A, et al. Financial burden and quality of life among early‐onset colorectal cancer survivors: a qualitative analysis. Health Expect. 2019;22(5):1050–1057. 10.1111/hex.12919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Garcia‐Aguilar J, Patil S, Gollub MJ, Kim JK, Yuval JB, Thompson HM, et al. Organ preservation in patients with rectal adenocarcinoma treated with total neoadjuvant therapy. J Clin Oncol. 2022;40(23):2546–2556. 10.1200/jco.22.00032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Custers PA, Hupkens BJ, Grotenhuis BA, Kuhlmann KF, Breukink SO, Beets GL, et al. Selected stage IV rectal cancer patients managed by the watch‐and‐wait approach after pelvic radiotherapy: a good alternative to total mesorectal excision surgery? Colorectal Dis. 2022;24(4):401–410. 10.1111/codi.16034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Roeder F, Meldolesi E, Gerum S, Valentini V, Rödel C. Recent advances in (chemo‐) radiation therapy for rectal cancer: a comprehensive review. Radiat Oncol. 2020;15(1):1–21. 10.1186/s13014-020-01695-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Hewish M, Lord CJ, Martin SA, Cunningham D, Ashworth A. Mismatch repair deficient colorectal cancer in the era of personalized treatment. Nat Rev Clin Oncol. 2010;7(4):197–208. 10.1038/nrclinonc.2010.18 [DOI] [PubMed] [Google Scholar]
- 119. Mirnezami R, Chang GJ, Das P, Chandrakumaran K, Tekkis P, Darzi A, et al. Intraoperative radiotherapy in colorectal cancer: systematic review and meta‐analysis of techniques, long‐term outcomes, and complications. Surg Oncol. 2013;22(1):22–35. 10.1016/j.suronc.2012.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Supporting Information S2
Supporting Information S3
Data Availability Statement
Data sharing is not applicable to this guideline, since no new data were created or analysed in this project. All results as presented in this manuscript were directly derived from data as presented in the original articles. These are all included in the list of references.


