ABSTRACT
Diclofenac (DCF), a commonly used anti‐inflammatory medication, presents environmental concerns due to its presence in water bodies, resistance to conventional wastewater treatment methods, and detection at increasing concentrations (ng/L to µg/L) that highlight DCF as a global emerging pollutant. While microalgae have been effective in degrading DCF in wastewater, immobilization into a matrix offers a promising approach to enhance treatment retention and efficiency. This study aimed to evaluate the efficacy of DCF removal using immobilized freshwater microalgae. Two algal species, Chlamydomonas reinhardtii (Chlamydomonas) and Scenedesmus obliquus (Scenedesmus), were tested for 6 days in both free and immobilized forms to determine if immobilized algae could degrade DCF comparably to free cells. The findings indicate that by Day 3, immobilized Chlamydomonas and Scenedesmus removed 78.0% and 80.1% of DCF, outperforming free‐cell cultures. Mixed cultures demonstrated synergistic effects, with removal amounts of 91.4% for free and 92.3% for immobilized systems. By Day 6, all conditions achieved complete DCF removal (100%). Mechanistic analysis showed 80.0% biodegradation and 20.0% bioaccumulation in free Chlamydomonas and 56.8% biodegradation with 43.2% bioaccumulation in Scenedesmus. Immobilization shifted pathways slightly: in Chlamydomonas, 61.6% of DCF removal occurred via biodegradation, 18.3% via bioaccumulation, and 20.1% via abiotic degradation. For Scenedesmus, immobilization achieved 45.6% biodegradation, 36.6% bioaccumulation, and 17.8% abiotic degradation, enhancing abiotic degradation while maintaining biodegradation efficiency. This research serves as a proof of concept for utilizing immobilized algae in DCF removal and suggests an avenue for improved wastewater treatment of emerging contaminants.
Keywords: alginate beads, biodegradation, Chlamydomonas reinhardtii, diclofenac, Scenedesmus obliquus
Immobilized freshwater microalgae, specifically a combination of Chlamydomonas reinhardtii and Scenedesmus obliquus, exhibit superior efficiency in the removal of diclofenac from water compared to their free‐floating counterparts. This research validates the concept of employing immobilized algal cocktails to effectively degrade diclofenac in wastewater treatment processes. “Created with BioRender.com”.
1. Introduction
DCF is a widely prescribed nonsteroidal anti‐inflammatory drug (NSAID) known for its efficacy in pain management and reduction of inflammation (Altman et al. 2015). However, its extensive and persistent presence in aquatic environments has raised significant environmental concerns (Sathishkumar et al. 2020). DCF is frequently detected in freshwater ecosystems worldwide, at concentrations ranging from nanograms to micrograms per liter (ng/L–µg/L) (Mirzaee et al. 2021). Despite its therapeutic benefits, DCF's resistance to conventional wastewater treatment processes, continual discharge from sewage effluents and inputs from agricultural runoff or pharmaceutical manufacturing facilities, contribute to its accumulation in surface waters (Vieno and Sillanpää 2014; Pan et al. 2024). Studies have shown adverse effects on aquatic organisms, including fish, mussels, and algae, even at low (ng/L) concentrations (Bouly et al. 2022; Harshkova et al. 2021). Furthermore, the long‐term ecological consequences of DCF contamination remain uncertain, warranting attention to mitigate potential environmental impacts in receiving aquatic ecosystems (Patel et al. 2019). Most conventional municipal water treatment methods cannot fully eliminate pharmaceuticals including DCF, highlighting the need for new and sustainable remediation approaches (Mussa et al. 2022; Hejna, Kapuścińska, and Aksmann 2022; Oluwole, Omotola, and Olatunji 2020).
Microalgae can fulfill a critical role in pharmaceutical contaminant removal from wastewater, owing to their capacity to uptake and metabolize a wide variety of organic pollutants such as antibiotics, hormones, and NSAIDs like diclofenac (Abdelfattah et al. 2022; Goh et al. 2022). However, in the realm of wastewater treatment, the effective utilization of microalgae presents a dual challenge: harnessing their potent bioremediation capabilities while ensuring their containment to prevent unintended environmental release. Additionally, microalgae require prolonged exposure to efficiently degrade DCF, as evidenced by varying removal efficiencies across different species, media, and incubation periods (summarized in Table 1). For instance, Chlorella vulgaris achieved 85.5% DCF removal in Bold Basal Medium (BBM) over 27 days (Sánchez‐Sandoval et al. 2022), while Chlorella sorokiniana reached 91.5% removal in BG‐11 media after 9 days at an initial concentration of 10 mg/L (Sharma et al. 2023). However, removal efficiency decreased with higher concentrations, such as 71.7% at 100 mg/L (Sharma et al. 2023). Similarly, anaerobically treated wastewater required 31 days for Chlorella sorokiniana to achieve 40%–60% removal of 147 µg/L DCF (de Wilt et al. 2016).
Table 1.
Examples of diclofenac removed by algae species.
| Culture condition | Initial diclofenac concentration | Microalgae | Cultivation system | Diclofenac removal efficiency (%) | Incubation time | References |
|---|---|---|---|---|---|---|
| BG‐11 media | 10 mg/L | Chlorella sorokiniana | Batch cultures | 91.5 | 9 days | Sharma et al. (2023) |
| 100 mg/L | Chlorella sorokiniana | 71.7 | ||||
| High Salt Medium | 32.7 mg/L | Chlamydomonas reinhardtii | Batch cultures | 37.7 | 4 days | Liakh et al. (2023) |
| Bold basal medium (BBM) | 10 µg/mL | Chlorella vulgaris | Batch cultures | 85.5 | 27 days | Sánchez‐Sandoval et al. (2022) |
| Nannochloropsis oculata | 80.5 | |||||
| 8.7 µg/mL | Scenedesmus acutus | 85.6 | ||||
| Scenedesmus obliquus | 90.5 | |||||
| BBM | 25 mg/L | Graesiella sp. | Batch cultures | 73 | 5 days | Ben Ouada et al. (2019) |
| 50 mg/L | 42 | |||||
| 75 mg/L | 25 | |||||
| Zarrouk medium | 25 mg/L | Picocystis sp. | 52 | |||
| 50 mg/L | 28 | |||||
| 75 mg/L | 24 | |||||
| Mann and Myers | 25 mg/L | Chlorella sorokiniana | Bubbling column photobioreactors (PBRs) 300 mL capacity | 30 | 10 days | Escapa et al. (2016) |
| Chlorella vulgaris | 21.6 | |||||
| Scenedesmus obliquus | 79.1 | |||||
| Anaerobically treated wastewater | 147 ± 9 µg/L | Chlorella sorokiniana | Batch cultures | 40–60 | 31 days | de Wilt et al. (2016) |
Indigenous microalgae commonly found in wastewater treatment plants (WWTPs) include Chlorococcum sp., Chlorella sp., Scenedesmus sp., and Tetradesmus sp. (Pereira et al. 2018). Besides microalgae, bacterial communities are also present, predominantly from the phyla Proteobacteria, Bacteroidetes, Acidobacteria, Firmicutes, and Nitrospirae, with Proteobacteria typically being the most abundant (Numberger et al. 2019). Specific genera such as Pseudomonas, Acinetobacter, Bacillus, and Nitrosomonas that are present in WWTPs are known for their capabilities in breaking down complex pharmaceuticals including DCF (Xie et al. 2021). Whereas the pharmaceutical degradation efficiency varies significantly depending on the microbial species and environmental conditions, the degradation rates of DCF in conventional WWTPs are generally low (Vieno and Sillanpää 2014). Studies report removal amounts of DCF below 40% in standard treatment processes like activated sludge or anaerobic fermentation (Ma et al. 2020). Hence, this inefficiency leads to the presence of DCF in the effluents of WWTPs, subsequently affecting receiving surface waters.
Immobilization techniques have gained attention as a means to enhance the efficacy and longevity of microalgae‐based remediation systems (Ruiz‐Marin, Mendoza‐Espinosa, and Stephenson 2010). Immobilization of algae is a technique that restricts cell mobility by attaching the cells to a solid support or entrapping them within a polymer matrix (Girijan and Kumar 2019). Immobilization enables microalgae retention within a matrix, ensuring continuous contact with contaminants and enhancing removal efficiency (Melnikova et al. 2022; Encarnação et al. 2020; Mollamohammada, Aly Hassan, and Dahab 2021). In the treatment of wastewater from pharmaceutical manufacturing plants, immobilized algae have been shown to effectively remove antibiotics and other persistent organic pollutants (Obaid, Salman, and Kadhim 2023). For instance, immobilized C. vulgaris (Chlorella) demonstrated greater sulfamethoxazole tolerance and removal efficiency compared to suspended cells, promoting a symbiotic relationship with bacterial populations and enhancing contaminant degradation (Xie et al. 2020). Additionally, in aquaculture settings, immobilized algae systems are used to manage nutrient loads by removing excess nitrogen and phosphorus from water, thus maintaining water quality and preventing eutrophication (Obaid, Salman, and Kadhim 2023). For example, immobilized Scenedesmus was able to remove 90% of ammonium within 4 h and 100% of phosphate within 2 h from typical effluent, highlighting its potential for tertiary wastewater treatment (Chevalier and De la Noüe 1985). Moreover, Travieso et al. (1996) used immobilized Chlorella for secondary wastewater treatment in municipal treatment, demonstrating its efficacy over 6 months of operation. Furthermore, the utilization of microalgal cocktails, comprising multiple algal species, has demonstrated synergistic effects on pollutant removal, offering potential advantages over single‐species systems (Avila et al. 2021; Abdel‐Razek et al. 2019). These examples underscore the broad applicability and effectiveness of immobilized algae in various environmental and industrial contexts, making it a promising strategy for sustainable bioremediation and wastewater treatment.
Although the literature has explored the use of immobilized algae for bioremediation, a significant knowledge gap exists in the comparative analysis of free algae cells versus immobilized algae cells and also when using multiple algae in cocktails for bioremediation processes. While previous studies have investigated the free and immobilized approaches for bioremediation separately, there is a notable absence of direct comparisons between the two methods. This lack of comparative data and the algae cocktail approach limits our understanding of their relative performances and hinders the optimization of algal bioremediation techniques. A thorough comparative analysis of free algal cells, immobilized algal cells, and the algal combinatorial approach offers critical insights into their respective efficiencies, constraints, and potential applications across diverse remediation contexts. This comprehensive evaluation enhances our understanding of these methodologies, facilitating the optimization of algal‐based bioremediation strategies for various environmental scenarios.
In this study, we evaluate the efficiency of DCF removal by free and immobilized Chlamydomonas and Scenedesmus, two model microalgal species, when used individually and in combination. Through the immobilization of microalgae within a matrix and the utilization of a cocktail approach, we demonstrate their effective degradation of DCF, with the algae cocktail exhibiting a faster removal amount compared to free cells. Also, our findings demonstrate the potential of the algal cocktail remediation strategy for pharmaceutical contaminant removal.
2. Materials and Methods
2.1. Algal Cells and Culturing Conditions
The microalgae strains used in this study were Chlamydomonas reinhardtii (CC‐400) from Chlamydomonas Resource Center and Scenedesmus obliquus (UTEX 393) from UTEX Culture Collection of Algae. All the cultures were single colonies isolated before the experiments to have plated monocultures. Cells were maintained under a medium light level (50 μmol photons/m2/s) at 23°C on Tris‐acetate phosphate (TAP) medium (Harris 1989) containing 1.5% Bacto agar (purchased from Fisher Scientific, USA). DCF (purchased from Cayman Chemical Company Inc., USA) was dissolved in double distilled H2O (ddH2O) and added to cell cultures at the beginning of each experiment to obtain a final concentration of 150 µM (47.7 mg/L). For our experiments, we selected a DCF concentration of 150 µM (47.7 mg/L), which lies between the EC10 (32.7 mg/L) and EC25 (65.75 mg/L) values of DCF for Chlamydomonas as reported by Harshkova et al. (2021). This concentration was chosen after confirming that it did not impair cell growth compared to cells grown in TAP medium. Cell growth was assessed using hemocytometer‐based cell counts, which were conducted over a period of 6 days (Table A1). The results showed no significant difference in growth rates between the DCF‐treated cells and the TAP‐grown control cells for both Chlamydomonas and Scenedesmus cultures (Table A1).
2.2. Preparation of Liquid and Bead Cultures
Chlamydomonas and Scenedesmus were inoculated in 2 × 105 cells/mL concentration in 200 mL TAP cultures without agar and grown for 3 days under continuous light (50 μmol photons/m2/s) at continuous shaking (120 rpm). At the beginning of the experiment, the asynchronized cell population was diluted to a starting cell concentration between 4.5 × 106 and 4.7 × 106 cells/mL and divided into four sub‐populations (Figure 1). Two of the sub‐populations, were used in liquid/free culture experiments while the remaining two were used in immobilization. For immobilization, each pellet was resuspended in 5 mL of 2% (w/v) Na‐alginate (Sigma‐Aldrich, CA). The gel droplets were introduced gradually using a 1 mL micropipette into a 250 mL beaker with a 2% CaCl2 (≥ 97%, Sigma‐Aldrich, CA) solution, where they polymerized to form beads approximately 4 mm in diameter (Melnikova et al. 2022). Each culture had 20 beads. After polymerization, beads were washed three times with sterilized ddH2O water before introducing into the DCF media. TAP media without DCF and algae cells was used as the negative control, while TAP + 150 µM DCF without algae cells was used as a positive control. For the immobilization experiments, empty beads without algae in TAP + 150 µM DCF were used as a positive control. All the experiments were carried out under continuous light (50 μmol photons/m2/s) at continuous shaking (120 rpm). Data for each condition were collected from three biological replicates.
Figure 1.
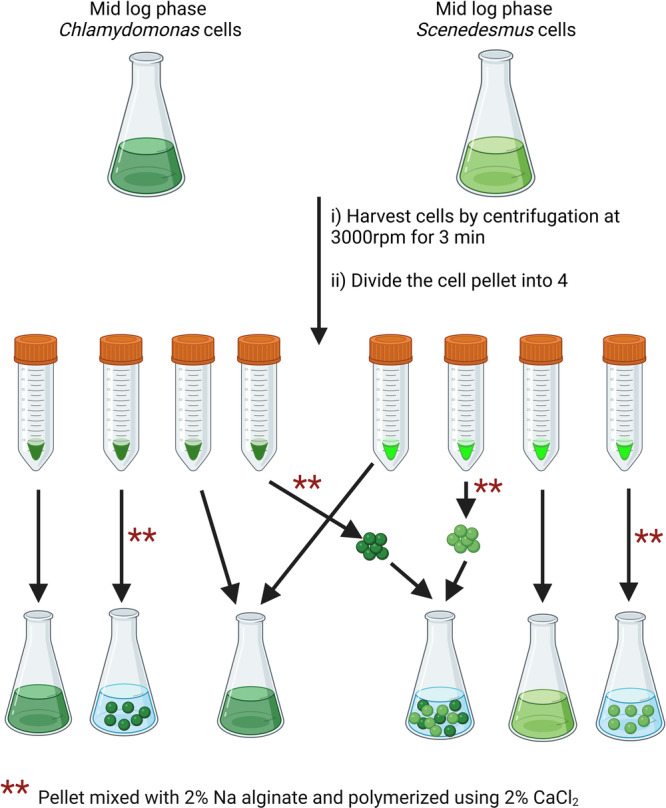
Schematic representation of the procedure used in the experimentation. Chlamydomonas and Scenedesmus were inoculated at 2 × 10⁵ cells/mL in 200 mL TAP media and grown under light with continuous shaking for 3 days until they reached mid‐log phase. Asynchronized cells were diluted to 4.5–4.7 × 10⁶ cells/mL and divided into four groups: two for liquid cultures and two for immobilization. For immobilization, pellets were resuspended in 2% Na‐alginate, and droplets were polymerized in 2% CaCl₂ to form ~4 mm beads (20 beads/culture). Beads were washed and introduced into DCF media. TAP media served as negative control, while TAP + 150 µM DCF without algae or with empty beads served as positive controls. “Created with BioRender.com.”
2.3. Measuring Algal Growth
For liquid cultures, 500 μL from each of the cultures were collected on Day 3 and Day 6 for cell counting and assessment of culture growth. For bead cultures, two beads per flask were collected and sacrificed and completely disintegrated in 10 mL of 4% NaHCO3 (Sigma‐Aldrich, CA) solution (w/v) (Mujtaba and Lee 2017) and the re‐suspended cells were used for counting. Cell counts for both liquid and disintegrated bead cultures were conducted using a hemocytometer chamber.
2.4. Measuring DCF Removal Efficiency
To measure DCF removal efficiency, 2 mL of each incubated culture was collected on Day 0, Day 3, and Day 6. All the samples were spun at 4200g for 5 min, and the supernatant was collected and filtered through 0.45 μm nitrocellulose filter before DCF measurements. Samples were analyzed by ISQ EM Single Quadrupole Mass Spectrometer instrument using positive ESI mode with an ion transfer tube temperature of 300°C and ion spray voltage of 3.0 kV. For DCF isolation, a C18 column (Hypersil ODS 50 × 4.6 mm 3‐Micron, Thermo Scientific) and mobile phase comprised of 90% acetonitrile and 10% water were used, run isocratically for 5 min with a flow rate of 1 mL/min at 40°C. The DCF peak area was used for calibration and % DCF removal efficiency in each sample was calculated using the following equation:
where d0 and dt represent the DCF concentration at Day 0 and Day 3/Day 6, respectively.
2.5. DCF Migration and Distribution
The amount removed (R, %), migration, and distribution of DCF was calculated using the following equation:
where B d, B a, and B s represent the biodegradation, bioaccumulation, and biosorption rates (%) of DCF via microalgae, respectively, and ΔR denotes abiotic removal (%) of DCF. These parameters were calculated following the methodology outlined by Song et al. (2019). To determine B d, B a, and B s on Day 6, the algae culture was centrifuged at 5000 rpm for 10 min and 1 mL of the supernatant was used to measure the residual amount of DCF. The resulting algae pellet was then shaken with 1 mL of methanol (5% v/v prepared in TAP, which is safe to use with cell wall‐less strains [Piasecki, Diller, and Brand 2009]) for 5 min, centrifuged again at 5000 rpm for 10 min, and the supernatant was collected to measure extracellular adsorption (B s). The remaining algae pellet was dissolved in a mixture of 1.5 mL dichloromethane and methanol (1:2 v/v), sonicated for 1 min, stored at −20°C overnight, and centrifuged at 5000 rpm for 10 min. The supernatant was collected to determine intracellular bioaccumulation content (B a). For the abiotically removed fraction (ΔR; primarily adsorption by alginate beads), the beads were removed from the solution, shaken in 2 mL of methanol, and centrifuged at 5000 rpm for 10 min. The supernatant was collected to measure the abiotic part (ΔR). The extraction of microalgae from the beads followed the procedure described for cell counting above (Mujtaba and Lee 2017).
2.6. Statistical Analysis
All assays were performed in at least three independent experiments. Data were expressed as mean ± SD. The GraphPad Prism 7 software (GraphPad Software, La Jolla, CA, USA) was used for statistical analysis. Two‐tailed Student's t‐tests were performed to evaluate the differences among groups. Differences among means were considered significant at p ≤ 0.05.
3. Results
3.1. Immobilization Does Not Impede Algae Growth
To assess whether immobilization affects algal growth, we compared the growth and survival rates of Chlamydomonas and Scenedesmus when immobilized in alginate beads versus their free‐floating counterparts in the presence of DCF (Figure 2A). We monitored cell density from Day 0 to Day 6 through cell counts. By Day 6, the total number of Chlamydomonas cells in liquid culture reached 1.33 × 108 cells, whereas the immobilized algae achieved 1.55 × 108 cells. Similarly, for Scenedesmus, the cell count in liquid cultures was 1.47 × 108 cells, while immobilized cultures exhibited a slightly higher count of 1.50 × 108 cells (Figure 2A,B). The slight variation in cell counts between immobilized and free‐floating cells was within the experimental margin of error (0.12 × 108 to 0.08 × 108 cells and p < 0.05), underscoring that immobilization in alginate beads maintains algal viability and proliferative capacity.
Figure 2.
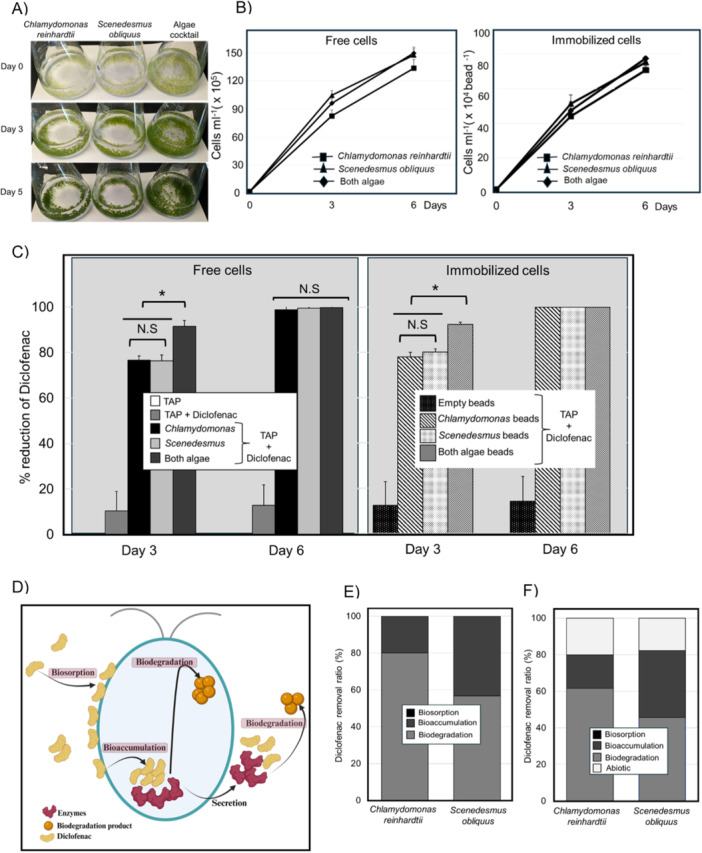
Growth and diclofenac removal in free and immobilized algae cultures. (A) alginate beads at the start and at the end of the experiment in Chlamydomonas reinhardtii, Scenedesmus obliquus, and algae cocktail (B) cell density of the free and immobilized cells at the start and end of the experiments. DCF removal efficiency in free and immobilized cells (C). By Day 3, Chlamydomonas and Scenedesmus immobilized counterparts achieved higher removal amounts than their free cells. Mixed cultures showed even higher efficiencies, compared to the monocultures. Complete removal of DCF was achieved in both free and immobilized cultures by Day 6. (D) An overview of three main mechanisms by which microalgae process organic pollutants. (E) Free and (F) immobilized Chlamydomonas and Scenedesmus removed DCF primarily via biodegradation with no biosorption in either culture. *p ≤ 0.05 in C. (D) “Created with BioRender.com”.
When examining mixed cultures, the total cell number in liquid cultures was 1.41 × 108 cells, and in immobilized algal beads, it was 1.48 × 108 cells. The combination of both algal species did not significantly enhance total cell numbers, suggesting potential competition for limited nutrients within the culture medium (Figure 2A,B). To substantiate this hypothesis, additional experimental data on nutrient uptake kinetics and medium composition over time would be valuable. Such information could confirm whether nutrient depletion occurs within the experimental timeframe, thereby explaining the observed cell number plateau in mixed cultures.
Overall, our observations indicate that the immobilization process does not impede algal growth. Both Chlamydomonas and Scenedesmus demonstrated growth rates comparable to their free‐floating counterparts, even under the stress of pharmaceutical contamination.
3.2. Immobilized C. reinhardtii, S. obliquus, and the Algae Cocktail Exhibit DCF Degradation Comparable to That of Free Cells
By Day 3, individual Chlamydomonas cultures in liquid media removed 76.6% of DCF, whereas immobilized Chlamydomonas achieved a slightly higher removal amount of 78.0%. Similarly, Scenedesmus in liquid culture removed 76.4% of DCF, while immobilized Scenedesmus demonstrated an improved removal efficiency of 80.1% (Figure 2C).
Despite the lack of a significant increase in cell numbers when combining the two algal species, the mixed algal cultures exhibited enhanced DCF degradation compared to individual cultures. The mixed liquid culture of Chlamydomonas and Scenedesmus achieved a DCF removal percentage of 91.4%, whereas the immobilized mixed culture demonstrated a slightly higher removal amount of 92.3%. These results suggest a synergistic effect in mixed cultures that enhances the degradation performance (Figure 2C).
By Day 6, we observed 100% removal of DCF in all tested cultures, including both free and immobilized, single and mixed algal cultures (Figure 2C). While prolonged incubation did not yield higher degradation rates, our data indicate that immobilized algae demonstrated slightly higher degradation rates during shorter incubation periods. This finding is particularly relevant for wastewater treatment applications, where the efficiency and speed of contaminant removal are crucial.
These findings indicate that immobilization does not compromise the algal ability to degrade DCF; in fact, it appears to enhance degradation efficiency, particularly in mixed cultures. The slight improvement in degradation rates for immobilized cells could be attributed to several factors, including more stable microenvironments within the alginate beads and potentially enhanced interactions between the algal cells and the contaminant discussed below under abiotic degradation.
3.3. DCF Migration and Distribution in Free and Immobilized Cells Are Comparable
Microalgae process organic pollutants through three main mechanisms: biodegradation, bioaccumulation, and biosorption (Dubey et al. 2023) (Figure 2D). To quantify DCF removal by biodegradation, biosorption, and bioaccumulation, we measured DCF concentrations on the outer surface and within microalgal cells at Day 6 samples (as described in Section 2.5). Given that there was 100% degradation of DCF by Day 6 in all samples (as determined by the residual DCF measurements), the amount removed (R) (equation from Section 2.5) was considered to be 100%. For free cell cultures, biodegradation was calculated by subtracting bioaccumulation and biosorption from R (Figure 2E). For immobilized samples, the abiotic removal was also subtracted from R when determining biodegradation (Figure 2F).
In free Chlamydomonas cultures, 80% (3.82 mg) of DCF was removed through biodegradation, while the remaining 20% (0.95 mg) was eliminated via bioaccumulation; notably, no significant contribution from biosorption was observed. Similarly, in free Scenedesmus cultures, approximately 56.8% (2.72 mg) of DCF underwent biodegradation, with 43.2% (2.06 mg) removed via bioaccumulation, and no discernible biosorption activity was calculated. Upon immobilization, the removal patterns shifted slightly: immobilized Chlamydomonas cells exhibited a distribution where 61.6% (2.94 mg) of DCF was degraded through biodegradation, 18.3% (0.87 mg) via bioaccumulation, and 20.1% (0.96 mg) via abiotic degradation. Conversely, in immobilized Scenedesmus cultures, biodegradation accounted for 45.6% (2.18 mg) of DCF removal, bioaccumulation for 36.6%, (1.74 mg), and abiotic degradation for 17.8% (0.85 mg). Notably, biosorption did not significantly contribute to DCF degradation in either free or immobilized algae cultures.
These findings underscore the varied mechanisms at play in DCF removal by algal species and highlight the potential efficacy of immobilization in altering removal pathways. The results (Figure 2E,F) underscore that biodegradation plays the key role in DCF removal in both free and immobilized groups which aligns with previous studies that have shown that biodegradation is the most effective way by which microalgae eliminate pharmaceuticals, including DCF (Norvill, Shilton, and Guieysse 2016; Cuellar‐Bermudez et al. 2017) and that immobilization does not hinder the biodegradation capabilities of microalgae. Instead, it may enhance degradation efficiency, particularly through abiotic removal effects facilitated by the alginate beads. The significant contribution of biodegradation and bioaccumulation to DCF removal highlights the metabolic versatility and potential of immobilized microalgae in wastewater treatment applications (further discussed in Section 4). Also, in combination with the negligible biosorption, appears to suggest that once internalized, DCF metabolism is quite fast. This means that the uptake of DCF, and not the intracellular metabolism, appears to be rate‐limiting to its overall degradation.
4. Discussion and Conclusions
While immobilized algae have been studied for bioremediation, there is a lack of direct comparisons with free algae cells and the use of algae cocktails, which limits the optimization of algal bioremediation techniques. A comparative analysis of free and immobilized algae, as well as algae combinations, will provide essential insights into their efficiencies and applications, improving algal‐based bioremediation strategies for various environmental contexts. Our study aimed to address this knowledge gap by investigating the efficacy of immobilized microalgae compared to their free‐floating counterparts individually and when combined. Specifically, we focused on C. reinhardtii and S. obliquus, two widely studied algal species known for their bioremediation potential and widespread use in wastewater treatment technologies (El‐Sheekh et al. 2023).
Our results show that immobilized algae exhibit comparable efficacy to free cells in removing pharmaceutical contaminants from wastewater. This is particularly noteworthy as it suggests that immobilization does not compromise the biodegradation capabilities of microalgae. Furthermore, we observed a synergistic effect when combining Chlamydomonas and Scenedesmus, resulting in enhanced removal efficiency compared to using each species individually. This highlights the potential for leveraging the inherently present microbiome diversity within immobilized systems to maximize remediation performance.
While previous research has explored the benefits of immobilization primarily in the context of nutrient absorption (Melnikova et al. 2022), our study contributes by focusing on pharmaceutical contaminant degradation. This expands the scope of understanding regarding the applicability of immobilized algae in wastewater treatment scenarios. We encapsulated the algae in alginate beads, a method that offers several advantages. Immobilized cells occupy less space, are easier to handle, and can achieve higher cell densities, allowing for repetitive use in product creation, and enhancing both the adsorption capacity and bioavailability of algal biomass (Carbone et al. 2020; Eroglu, Smith, and Raston 2015). Furthermore, immobilization has been shown to strengthen operational stability by preventing cell drift, increasing reaction rates due to higher cell densities, and facilitating growth and easy harvesting (Obaid, Salman, and Kadhim 2023). Additionally, immobilization offers protection against harsh environmental conditions such as metal toxicity, high salinity, pH fluctuations, and product inhibition (Han et al. 2022). This technique also safeguards aging cultures from photoinhibition, allows for increased biomass concentrations, and ensures less destructive cell recovery. Immobilized systems also protect microalgae from external threats, including predators and growth inhibitors (Nair, Senthilnathan, and Nagendra 2019; Lee et al. 2020). These benefits collectively underscore the potential of immobilization for enhancing the efficacy and sustainability of bioremediation processes.
Based on the previous study by Song et al. (2019) on the removal of Florfenicol (FF), a widely used veterinary antibiotic, by the microalgae, Chlorella sp. found that at a concentration of 46 mg/L, biodegradation in Chlorella sp. was the sole removal pathway, achieving 97% efficiency. However, as the concentration increased, FF began to show bioaccumulation and biosorption. At 159 mg/L, the total removal decreased to 74.7%, with biodegradation, bioaccumulation, and biosorption contributing 72.0%, 1.3%, and 1.4%, respectively. In our study, the efficiencies and fractions of DCF removal through biodegradation, bioaccumulation, biosorption, and abiotic removal were determined when cells were exposed to a DCF concentration of 150 µM. Nonetheless, variations in DCF concentrations can influence the metabolic activity of the microalgae, similar to the observations by Song et al. (2019), potentially altering the balance between biodegradation, bioaccumulation, and biosorption processes, which warrants further study. At lower DCF concentrations, bioaccumulation efficiency may increase as cells have a higher capacity to absorb and store the contaminant (Liakh et al. 2023). Conversely, at higher concentrations, the cells might experience toxicity, reducing their overall metabolic activity and thus decreasing biodegradation efficiency (Ben Ouada et al. 2019). Additionally, the abiotic removal might also vary with different DCF concentrations, as higher contaminant levels could enhance the adsorption capacity of the immobilization matrix, leading to greater abiotic degradation. Understanding these dynamics is crucial for optimizing bioremediation strategies in wastewater treatment. Tailoring the exposure concentrations of contaminants can potentially maximize the removal efficiencies of specific pathways (biodegradation, bioaccumulation, biosorption, and abiotic removal). Further research should systematically study the effects of various DCF concentrations on removal efficiencies to establish robust models for predicting microalgae performance in different environmental conditions. Hence, this approach will enhance the practical applicability of microalgae‐based treatment systems in diverse wastewater scenarios.
In conclusion, our study provides compelling evidence for the viability of immobilized algal systems as a means of efficiently removing pharmaceutical contaminants from wastewater. The observed synergistic effects and comparable performance between immobilized and free cells emphasize the potential of this approach for achieving more sustainable and effective bioremediation solutions. Moving forward, future research endeavors could delve deeper into elucidating the underlying mechanisms driving enhanced degradation efficiency and optimizing conditions for broader‐scale implementation in real‐world wastewater treatment. Overall, our study contributes to the advancement of sustainable wastewater treatment technologies and addresses environmental challenges associated with pharmaceutical contamination in aquatic ecosystems.
Author Contributions
Thamali Kariyawasam: conceptualization, investigation, writing–original draft, methodology, validation. Martin Petkovich: writing–review and editing, funding acquisition, supervision. Bas Vriens: funding acquisition, writing–review and editing, project administration, supervision.
Ethics Statement
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Supporting information
Supporting information.
Acknowledgements
We thank Dr. Mario Khalil for the technical support with HPLC measurements and The Queen's Mass Spectrometry Facility (Queens University, Kingston, ON). This research was supported by a New Frontiers in Research Fund ‐ Exploration 2020 (NFRFE‐2020‐00832), Canada.
1.
Table A1.
Chlamydomonas and Scenedesmus cell growth in TAP and TAP + 150 µM DCF.
| Media | Cell line | Cell counts (× 105 cells/mL) | |||
|---|---|---|---|---|---|
| Day 0 | Day 2 | Day 4 | Day 6 | ||
| TAP | Chlamydomonas | 23.3 ± 2.9 | 38. 3 ± 7.6 | 83.3 ± 7.6 | 131. 7 ± 10.4 |
| Scenedesmus | 21.67 ± 1.5 | 39.3 ± 2.1 | 85 ± 2.6 | 130.3 ± 2.5 | |
| TAP + DCF | Chlamydomonas | 26. 3 ± 1.5 | 39 ± 3.6 | 86. 7 ± 1.5 | 133. 3 ± 7.6 |
| Scenedesmus | 21.33 ± 3.1 | 36.3 ± 3.8 | 85.3 ± 2.5 | 120.3 ± 1.5 | |
Data Availability Statement
The data that supports the findings of this study are available in the supplementary material of this article.
References
- Abdelfattah, A. , Ali S. S., Ramadan H., et al. 2023. “Microalgae‐Based Wastewater Treatment: Mechanisms, Challenges, Recent Advances, and Future Prospects.” Environmental Science and Ecotechnology 13: 100205. 10.1016/j.ese.2022.100205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel‐Razek, M. A. , Abozeid A. M., Eltholth M. M., et al. 2019. “Bioremediation of a Pesticide and Selected Heavy Metals in Wastewater From Various Sources Using a Consortium of Microalgae and Cyanobacteria.” Slovenian Veterinary Research 56, no. Suppl 22: 61–73. 10.26873/SVR-744-2019. [DOI] [Google Scholar]
- Altman, R. , Bosch B., Brune K., Patrignani P., and Young C.. 2015. “Advances in NSAID Development: Evolution of Diclofenac Products Using Pharmaceutical Technology.” Drugs 75: 859–877. 10.1007/s40265-015-0392-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila, R. , Peris A., Eljarrat E., Vicent T., and Blánquez P.. 2021. “Biodegradation of Hydrophobic Pesticides by Microalgae: Transformation Products and Impact on Algae Biochemical Methane Potential.” Science of the Total Environment 754: 142114. 10.1016/j.scitotenv.2020.142114. [DOI] [PubMed] [Google Scholar]
- Bouly, L. , Courant F., Bonnafé E., et al. 2022. “Long‐Term Exposure to Environmental Diclofenac Concentrations Impairs Growth and Induces Molecular Changes in Lymnaea stagnalis Freshwater Snails.” Chemosphere 291: 133065. 10.1016/j.chemosphere.2021.133065. [DOI] [PubMed] [Google Scholar]
- Carbone, D. A. , Olivieri G., Pollio A., and Melkonian M.. 2020. “Comparison of Galdieria Growth and Photosynthetic Activity in Different Culture Systems.” AMB Express 10: 170. 10.1186/s13568-020-01110-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier, P. , and De la Noüe J.. 1985. “Efficiency of Immobilized Hyperconcentrated Algae for Ammonium and Orthophosphate Removal From Wastewaters.” Biotechnology Letters 7: 395–400. 10.1007/BF01166210. [DOI] [Google Scholar]
- Cuellar‐Bermudez, S. P. , Aleman‐Nava G. S., Chandra R., et al. 2017. “Nutrients Utilization and Contaminants Removal. A Review of Two Approaches of Algae and Cyanobacteria in Wastewater.” Algal Research 24: 438–449. 10.1016/j.algal.2016.08.018. [DOI] [Google Scholar]
- Dubey, S. , Chen C. W., Haldar D., et al. 2023. “Advancement in Algal Bioremediation for Organic, Inorganic, and Emerging Pollutants.” Environmental Pollution 317: 120840. 10.1016/j.envpol.2022.120840. [DOI] [PubMed] [Google Scholar]
- El‐Sheekh, M. M. , Galal H. R., Mousa A. S. H., and Farghl A. A. M.. 2023. “Coupling Wastewater Treatment, Biomass, Lipids, and Biodiesel Production of Some Green Microalgae.” Environmental Science and Pollution Research 30, no. 12: 35492–35504. 10.1007/s11356-022-21418-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Encarnação, T. , Palito C., Pais A. A. C. C., Valente A. J. M., and Burrows H. D.. 2020. “Removal of Pharmaceuticals From Water by Free and Imobilised Microalgae.” Molecules 25, no. 16: 3639. 10.3390/molecules25163639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eroglu, E. , Smith S. M., and Raston C. L.. 2015. “Application of Various Immobilization Techniques for Algal Bioprocesses.” Biomass and Biofuels From Microalgae: Advances in Engineering and Biology 2: 19–44. 10.1007/978-3-319-16640-7_2. [DOI] [Google Scholar]
- Escapa, C. , Coimbra R. N., Paniagua S., García A. I., and Otero M.. 2016. “Comparative Assessment of Diclofenac Removal From Water by Different Microalgae Strains.” Algal Research 18: 127–134. 10.1016/j.algal.2016.06.008. [DOI] [Google Scholar]
- Girijan, S. , and Kumar M.. 2019. “Immobilized Biomass Systems: An Approach for Trace Organics Removal From Wastewater and Environmental Remediation.” Current Opinion in Environmental Science & Health 12: 18–29. 10.1016/j.coesh.2019.08.005. [DOI] [Google Scholar]
- Goh, P. S. , Lau W. J., Ismail A. F., Samawati Z., Liang Y. Y., and Kanakaraju D.. 2022. “Microalgae‐Enabled Wastewater Treatment: A Sustainable Strategy for Bioremediation of Pesticides.” Water 15, no. 1: 70. 10.3390/w15010070. [DOI] [Google Scholar]
- Han, M. , Zhang C., Li F., and Ho S. H.. 2022. “Data‐Driven Analysis on Immobilized Microalgae System: New Upgrading Trends for Microalgal Wastewater Treatment.” Science of the Total Environment 852: 158514. 10.1016/j.scitotenv.2022.158514. [DOI] [PubMed] [Google Scholar]
- Harris, E. H. 1989. Chlamydomonas Sourcebook, vol. 2. San Diego: Academic Press. 10.1017/S0024282989000538. [DOI] [Google Scholar]
- Harshkova, D. , Liakh I., Bialevich V., Ondrejmišková K., Aksmann A., and Bišová K.. 2021. “Diclofenac Alters the Cell Cycle Progression of the Green Alga Chlamydomonas reinhardtii .” Cells 10, no. 8: 1936. 10.3390/cells10081936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hejna, M. , Kapuścińska D., and Aksmann A.. 2022. “Pharmaceuticals in the Aquatic Environment: A Review on Eco‐Toxicology and the Remediation Potential of Algae.” International Journal of Environmental Research and Public Health 19, no. 13: 7717. 10.3390/ijerph19137717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, H. , Jeong D., Im S., and Jang A.. 2020. “Optimization of Alginate Bead Size Immobilized With Chlorella vulgaris and Chlamydomonas reinhardtii for Nutrient Removal.” Bioresource Technology 302: 122891. 10.1016/j.biortech.2020.122891. [DOI] [PubMed] [Google Scholar]
- Liakh, I. , Harshkova D., Hrouzek P., Bišová K., Aksmann A., and Wielgomas B.. 2023. “Green Alga Chlamydomonas reinhardtii Can Effectively Remove Diclofenac From the Water Environment–A New Perspective on Biotransformation.” Journal of Hazardous Materials 455: 131570. 10.1016/j.jhazmat.2023.131570. [DOI] [PubMed] [Google Scholar]
- Ma, N. , Zhang N., Gao L., et al. 2020. “Removal of Diclofenac in Effluent of Sewage Treatment Plant by Photocatalytic Oxidation.” Water 12, no. 10: 2902. 10.3390/w12102902. [DOI] [Google Scholar]
- Melnikova, A. , Namsaraev Z., Komova A., et al. 2022. “Algaltextile—A New Biohybrid Material for Wastewater Treatment.” Biotechnology Reports 33: e00698. 10.1016/j.btre.2021.e00698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzaee, S. A. , Noorimotlagh Z., Ahmadi M., et al. 2021. “The Possible Oxidative Stress and DNA Damage Induced in Diclofenac‐Exposed Non‐Target Organisms in the Aquatic Environment: A Systematic Review.” Ecological Indicators 131: 108172. 10.1016/j.ecolind.2021.108172. [DOI] [Google Scholar]
- Mollamohammada, S. , Aly Hassan A., and Dahab M.. 2021. “Immobilized Algae‐Based Treatment of Herbicide‐Contaminated Groundwater.” Water Environment Research 93, no. 2: 263–273. 10.1002/wer.1405. [DOI] [PubMed] [Google Scholar]
- Mujtaba, G. , and Lee K.. 2017. “Treatment of Real Wastewater Using Co‐Culture of Immobilized Chlorella vulgaris and Suspended Activated Sludge.” Water Research 120: 174–184. 10.1016/j.watres.2017.04.078. [DOI] [PubMed] [Google Scholar]
- Mussa, Z. H. , Al‐Qaim F. F., Jawad A. H., Scholz M., and Yaseen Z. M.. 2022. “A Comprehensive Review for Removal of Non‐Steroidal Anti‐Inflammatory Drugs Attained From Wastewater Observations Using Carbon‐Based Anodic Oxidation Process.” Toxics 10, no. 10: 598. 10.3390/toxics10100598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair, A. T. , Senthilnathan J., and Nagendra S. M. S.. 2019. “Application of the Phycoremediation Process for Tertiary Treatment of Landfill Leachate and Carbon Dioxide Mitigation.” Journal of Water Process Engineering 28: 322–330. 10.1016/j.jwpe.2019.02.017. [DOI] [Google Scholar]
- Norvill, Z. N. , Shilton A., and Guieysse B.. 2016. “Emerging Contaminant Degradation and Removal in Algal Wastewater Treatment Ponds: Identifying the Research Gaps.” Journal of Hazardous Materials 313: 291–309. 10.1016/j.jhazmat.2016.03.085. [DOI] [PubMed] [Google Scholar]
- Numberger, D. , Ganzert L., Zoccarato L., et al. 2019. “Characterization of Bacterial Communities in Wastewater With Enhanced Taxonomic Resolution by Full‐Length 16S rRNA Sequencing.” Scientific Reports 9, no. 1: 9673. 10.1038/s41598-019-46015-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obaid, Z. H. , Salman J. M., and Kadhim N. F.. 2023. “Review on Toxicity and Removal of Pharmaceutical Pollutants Using Immobilised Microalgae.” Ecological Engineering & Environmental Technology 24, no. 6: 44–60. 10.12912/27197050/166013. [DOI] [Google Scholar]
- Oluwole, A. O. , Omotola E. O., and Olatunji O. S.. 2020. “Pharmaceuticals and Personal Care Products in Water and Wastewater: A Review of Treatment Processes and Use of Photocatalyst Immobilized on Functionalized Carbon in AOP Degradation.” BMC chemistry 14, no. 1: 62. 10.1186/s13065-020-00714-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Ouada, S. , Ben Ali R., Cimetiere N., Leboulanger C., Ben Ouada H., and Sayadi S.. 2019. “Biodegradation of Diclofenac by Two Green Microalgae: Picocystis sp. and Graesiella sp.” Ecotoxicology and Environmental Safety 186: 109769. 10.1016/j.ecoenv.2019.109769. [DOI] [PubMed] [Google Scholar]
- Pan, D. , Zhang C., Wang C. S., et al. 2024. “Unravelling Hidden Threats of Water Disinfection: Toxicity Evaluation and Toxic Products Identification During Diclofenac Degradation.” Environmental Pollution 345: 123424. 10.1016/j.envpol.2024.123424. [DOI] [PubMed] [Google Scholar]
- Patel, M. , Kumar R., Kishor K., Mlsna T., C. U. Pittman, Jr. , and Mohan D.. 2019. “Pharmaceuticals of Emerging Concern in Aquatic Systems: Chemistry, Occurrence, Effects, and Removal Methods.” Chemical Reviews 119, no. 6: 3510–3673. 10.1021/acs.chemrev.8b00299. [DOI] [PubMed] [Google Scholar]
- Pereira, M. V. , Dassoler A. F., Antunes P. W., Gonçalves R. F., and Cassini S. T.. 2020. “Indigenous Microalgae Biomass Cultivation in Continuous Reactor With Anaerobic Effluent: Effect of Dilution Rate on Productivity, Nutrient Removal and Bioindicators.” Environmental Technology 41: 1780–1792. 10.1080/09593330.2018.1549105. [DOI] [PubMed] [Google Scholar]
- Piasecki, B. P. , Diller K. R., and Brand J. J.. 2009. “Cryopreservation of Chlamydomonas reinhardtii: A Cause of Low Viability at High Cell Density.” Cryobiology 58, no. 1: 103–109. 10.1016/j.cryobiol.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Ruiz‐Marin, A. , Mendoza‐Espinosa L. G., and Stephenson T.. 2010. “Growth and Nutrient Removal in Free and Immobilized Green Algae in Batch and Semi‐Continuous Cultures Treating Real Wastewater.” Bioresource Technology 101, no. 1: 58–64. 10.1016/j.biortech.2009.02.076. [DOI] [PubMed] [Google Scholar]
- Sánchez‐Sandoval, D. S. , González‐Ortega O., Vazquez‐Martínez J., García de la Cruz R. F., and Soria‐Guerra R. E.. 2022. “Diclofenac Removal by the Microalgae Species Chlorella vulgaris, Nannochloropsis oculata, Scenedesmus acutus, and Scenedesmus obliquus .” 3 Biotech 12, no. 9: 210. 10.1007/s13205-022-03268-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathishkumar, P. , Meena R. A. A., Palanisami T., Ashokkumar V., Palvannan T., and Gu F. L.. 2020. “Occurrence, Interactive Effects and Ecological Risk of Diclofenac in Environmental Compartments and Biota—A Review.” Science of the Total Environment 698: 134057. 10.1016/j.scitotenv.2019.134057. [DOI] [PubMed] [Google Scholar]
- Sharma, J. , Mariam I., Suresh Kareya M., et al. 2023. “Metabolomic Response of Microalgae Towards Diclofenac Sodium During Its Removal From Water and Concomitant Recovery of Pigments and Lipids.” Bioresource Technology 371: 128617. 10.1016/j.biortech.2023.128617. [DOI] [PubMed] [Google Scholar]
- Song, C. , Wei Y., Qiu Y., Qi Y., Li Y., and Kitamura Y.. 2019. “Biodegradability and Mechanism of Florfenicol Via Chlorella sp. UTEX1602 and L38: Experimental Study.” Bioresource Technology 272: 529–534. 10.1016/j.biortech.2018.10.080. [DOI] [PubMed] [Google Scholar]
- Travieso, L. , Benitez F., Weiland P., Sánchez E., Dupeyrón R., and Dominguez A. R.. 1996. “Experiments on Immobilization of Microalgae for Nutrient Removal in Wastewater Treatments.” Bioresource Technology 55, no. 3: 181–186. 10.1016/0960-8524(95)00196-4. [DOI] [Google Scholar]
- Vieno, N. , and Sillanpää M.. 2014. “Fate of Diclofenac in Municipal Wastewater Treatment Plant—A Review.” Environment International 69: 28–39. 10.1016/j.envint.2014.03.021. [DOI] [PubMed] [Google Scholar]
- de Wilt, A. , Butkovskyi A., Tuantet K., et al. 2016. “Micropollutant Removal in an Algal Treatment System Fed With Source Separated Wastewater Streams.” Journal of Hazardous Materials 304: 84–92. 10.1016/j.jhazmat.2015.10.033. [DOI] [PubMed] [Google Scholar]
- Xie, N. , Zhong L., Ouyang L., et al. 2021. “Community Composition and Function of Bacteria in Activated Sludge of Municipal Wastewater Treatment Plants.” Water 13, no. 6: 852. 10.3390/w13060852. [DOI] [Google Scholar]
- Xie, P. , Chen C., Zhang C., Su G., Ren N., and Ho S. H.. 2020. “Revealing the Role of Adsorption in Ciprofloxacin and Sulfadiazine Elimination Routes in Microalgae.” Water Research 172: 115475. 10.1016/j.watres.2020.115475. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
The data that supports the findings of this study are available in the supplementary material of this article.


