Abstract
Purpose of the review
There have been tremendous modifications to the humeral component since Paul Grammont first introduced the reverse total shoulder arthroplasty in 1985. The purpose of this article is to review historical design features and their drawbacks and to summarize the clinical outcomes of modern designs.
Recent findings
Decreasing the neck-shaft angle and increasing humeral lateralization have helped address problems of scapular notching and limited internal and external rotation that were common with traditional designs. Advancements in proximal porous coatings have also facilitated the development of short-stem and stemless implants, which decreases the need for cement fixation and allows preservation of bone stock. Moreover, a reduction in stem length with smaller metaphyseal and diaphyseal filling ratios may limit stress shielding. Current humeral implants have an aseptic loosening rate less than 1%. Despite promising results, many of these new humeral design features do not have long-term data and continued surveillance of their performance is necessary.
Summary
The humeral stem design significantly influences clinical and radiographic outcomes. Surgeons should be mindful of these design variables to increase impingement-free range of motion, minimize scapular notching, reduce stress shielding, and improve implant survivorship.
Keywords: Reverse total shoulder arthroplasty, Humeral stem, Stem design, Stem fixation
Introduction
Paul Grammont introduced the modern reverse total shoulder arthroplasty (RTSA) in 1985 as a novel solution for treating patients with rotator cuff arthropathy [1]. His first design, called the Trompette prosthesis, featured a cemented two-thirds glenosphere and a 155° neck-shaft angle monoblock all polyethylene humeral component. In his series of 8 patients, only 5 patients achieved forward elevation over 100°[2]. Since then, there has been tremendous modifications to both the glenoid and humeral components to improve clinical outcomes and mitigate complications in patients. Although much of the attention has been directed towards the glenosphere, understanding past flaws and successes in the reverse humeral stem is critical as RTSA utilization becomes increasingly common worldwide.
The design features of a reverse humeral component are based on Grammont’s principles of a semi-constrained implant that would increase the deltoid moment arm to compensate for the rotator cuff insufficiency. Initially, all humeral components were cemented long stemmed implants with a 155° neck-shaft angle. Despite being an incredibly successful design, the drawbacks of the traditional Grammont-style prosthesis included poor restoration of internal and external rotation as well as excessive impingement of the humeral polyethylene with the medial scapular pillar, leading to polyethylene wear and scapular notching [3]. Mark Frankle modified the reverse principles to optimize the impingement-free range of motion by utilizing a more lateralized glenosphere and a more vertical neck-shaft angle [4]. Over the past two decades, additional modifications to the reverse humeral component follow Frankle’s design and include shorter humeral stems and cementless fixation.
In this review, we will explore historical design features of the reverse humeral component. The shortcomings of these designs and impetus behind newer modifications will be reviewed. Lastly, the outcomes of modern stems will be summarized and compared to traditional stems.
Neck-Shaft Angle
The original Grammont prosthesis featured a non-anatomic humeral inclination of 155°. Despite many surgeons considering this design as the gold standard, an increased neck-shaft angle placed the polyethylene cup in a more horizontal orientation, resulting in progressive scapular impingement and adduction deficit [5]. Manufacturers addressed this problem by reducing the neck-shaft angle to as low as 135°, which also effectively lateralizes the humerus. Biomechanical and computational studies have shown that reducing the neck-shaft angle improved impingement-free adduction by up to 28°, but decreased abduction by up to 9°[5–7]. Another potential concern with decreasing the neck-shaft angle is that less force may be required to dislocate the shoulder due to decreased articular contact with the inferior portion of the glenosphere in adduction [7].
In a meta-analyses of 3,134 shoulder arthroplasties, the Grammont-style prosthesis has been clinically shown to have greater improvement in abduction by 8.2° but less improvement in external rotation by 8.5° compared to designs with less humeral head inclination [8]. No difference was observed for the other planes of motion and patient-reported outcomes, such as Constant-Murley and American Shoulder and Elbow Society (ASES) scores [8]. At a mean follow-up of 3.8 years, scapular notching was significantly more common in the Grammont-style prosthesis than the designs with lower neck-shaft angles (40% vs. 17%) [8]. Interestingly, some reports have found that the dislocation rate of the Grammont-style prosthesis to be higher, while others have found no such association [8, 9]. It is important to note that solely attributing these differences to a lower neck shaft angle would be misleading because important glenosphere factors, such as lateralization, offset, and tilt, are not controlled for. Moreover, the overall neck-shaft angle is also dependent on the stem alignment (neutral, varus, or valgus) with respect to the humeral shaft.
Humeral Version
The humeral version of the RTSA was initially recommended to be between 0° and 30° based on expert opinion [10]. However, an increasing number of biomechanical studies have explored the effect of increasing humeral retroversion on stability and external rotation in different shoulder positions [11, 12]. In a cadaveric biomechanical model, Stephenson et al. recommended that the optimal humeral version was between 20° and 40° of retroversion, giving a potential for impingement-free range of motion from 28° to 44° in external rotation with the arm adducted [12]. Another cadaveric study suggested that retroversion did not affect the muscle force requirements required for scaption, but found that implanting the humeral component in 0° to 20° of retroversion allowed for maximal internal rotation, which is a movement that is required for daily activities [11]. Subsequent cadaveric studies have corroborated earlier biomechanical evidence that increased humeral retroversion was associated with a decrease in internal rotation and an increase in external rotation [13]. The authors found that the best balance in rotational motion was obtained with the native retroversion of the humeral head.
Clinically, the effect of humeral component version on patient outcomes and motion is also unclear. Several studies have compared humeral components placed in 0° and 20° of retroversion and found no difference in shoulder range of motion, strength, or functional outcomes [14, 15]. However, one study found that external rotation, internal rotation, functional scores, and pain relief were significantly better in patients with individualized humeral retroversion compared to patients with a fixed retroversion of 20°[16].
Stem Length
The stem length of a reverse humeral component has largely been adapted from the anatomic stem. The length of most traditional stems was arbitrarily set to occupy the proximal third to half of the humerus (~ 100 – 150 mm) to achieve early implant stability either through cementation or press fit fixation [17]. This was particularly advantageous in patients with compromised proximal bone quality due to osteoporosis or fracture. However, perceived drawbacks of conventional long-stemmed diaphyseal-fitting humeral components has led to the development of multiple humeral implants with shorter stems (< 100 mm) [18]. These shortcomings include difficulty with stem extraction at the time of revision surgery [19], management of periprosthetic fractures [20], and proximal bone resorption from stress shielding [21, 22]. Furthermore, implantation of standard length implants may not be possible in patients with proximal humeral deformity, existing hardware, or extreme anatomic variance [23].
Recently, there has been an emergence of stemless reverse humeral implants (< 50 mm) in Europe. These implants are not currently approved for use in the United States as not enough large-scale data has been collected to determine how they compare to standard or short-length implants. Like short-stemmed implants, the design rationale behind stemless implants was to preserve bone stock and maximize proximal humeral loading, thereby minimizing stress shielding. This, however, comes at the cost of initial implant fixation. Reports on early displacement and subsidence of stemless implants are likely attributed to overestimating bone quality, and in the cases of osteoporotic bone, stemmed cemented prosthesis should be used [24].
The early clinical outcomes of short-stem and stemless implants are comparable to that of standard length stems [25–27]. In a systematic review of 10 studies with short-stem implants, patients achieved a mean flexion of 134°, abduction of 119°, and external rotation of 32° with a Constant score of 69.3 [25]. Similarly, in a recent systematic review of stemless implants, patients achieved a mean flexion of 136°, abduction of 125°, and external rotation of 47° with a Constant score of 62.7 [26]. The aseptic humeral loosening rate at short-term follow-up was 0% and 0.2% short-stem and stemless implants, respectively.
Radiographic Adaptations
Radiographic changes, such as stress shielding and radiolucent lines, are common around the reverse humeral component, though the clinical relevance of these bony adaptions remains unclear (Fig. 1) [17]. In the literature, the rates of stress shielding are highly variable due to inconsistent definitions and different follow-up periods. Tuberosity resorption, cortical thinning, or medial calcar osteolysis are all terms that radiographically describe stress shielding and are manifestations of the same concept [17]. At 8—12 year follow-up, Melis et al. assessed 34 shoulders after a standard length Grammont-style RTSA and found that 100% of patients had greater tuberosity resorption, 76% had lesser tuberosity resorption, and 47% had cortical thinning [28]. Harmsen et al. similarly found signs of internal stress shielding (osteopenia) in 97% of shoulders with standard length Grammont-style RTSA at 2-year follow-up, but no signs of external stress shielding (cortical thinning) or osteolysis [29]. More recently, radiographic comparative studies between long-stemmed and short-stemmed implants have demonstrated that short stemmed implants have a lower rate of stress shielding [27, 30, 31]. In a retrospective study of 275 patients at 1-year follow-up, Erickson et al. found a calcar osteolysis rate of 2.2% in short-stemmed implants compared to 12.9% in long-stemmed implants [27]. Similarly, Merolla et al. found that a significantly lower rate of cortical thinning (26% vs 58%), spot welds (11% vs 0%), and tuberosity resorption (33% vs 10%), in short-stemmed curved implants compared to long-stemmed Grammont-style implants at 2-year follow-up [30]. Moroder et al. also found fewer radiolucent lines and visible bone density loss in patients with stemless components compared to standard length component (13% vs 29%) [32]. These clinical findings are corroborated by computational studies, which show that a reduction in stem length may decrease proximal bone resorption by producing humeral stresses that more closely match the stress distribution in native bone [33].
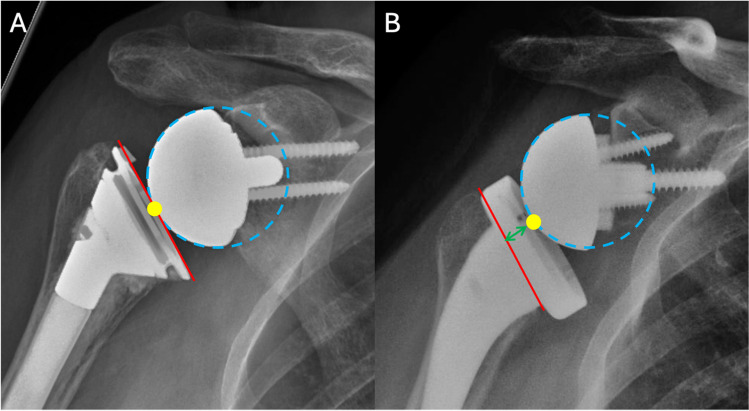
Fig. 1.
Radiographs of a cementless short-stem reverse total shoulder arthroplasty (A) immediately after surgery and at (B) 6-year follow-up. There are signs of stress shielding, such as calcar osteolysis (red star), lateral cortical thinning (green triangle), and spot welds (yellow arrow)
In addition to stem length, the canal filling ratio of the humeral implant has become an increasingly important radiographic indicator in predicting stress shielding and bony adaptations (Fig. 2). A higher metaphyseal and diaphyseal filling ratio were found to be associated with stress shielding and proximal bone resorption [22, 27, 34]. Based on these studies, the ideal metaphyseal and diaphyseal filling ratio is estimated to be between 0.60 – 0.70 and 0.57 – 0.80, respectively [27, 34]. Of note, humeral bone remodeling due to stress shielding was not associated with worse clinical outcomes, but may compromise the fixation of the implant in the long-term [35].
Fig. 2.
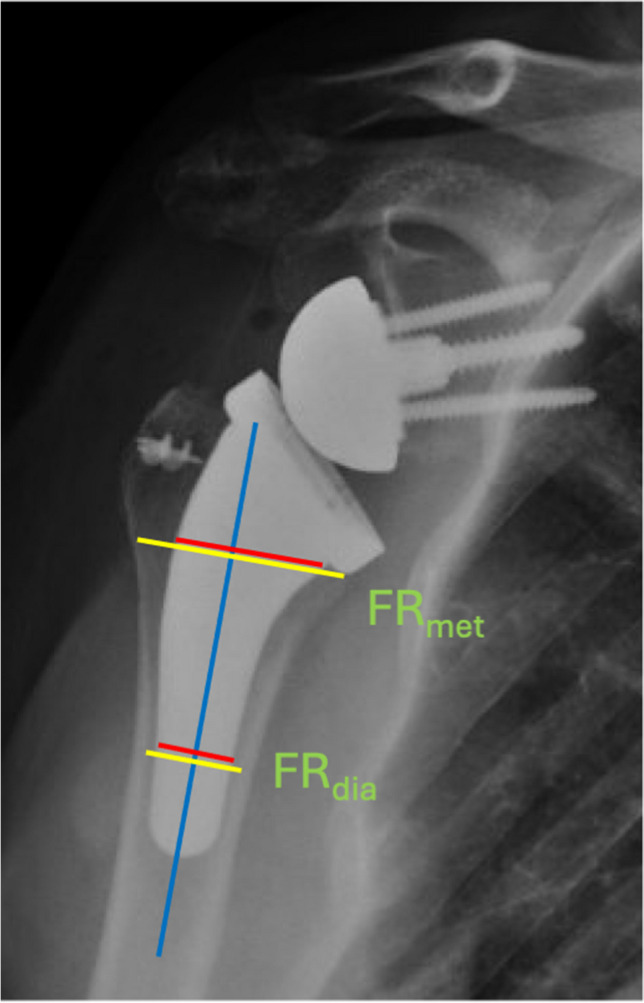
Filling ratios are measured at the metaphysis (FRmet) with a perpendicular line to the humeral shaft axis (blue line) starting at the medial-inferior border of the humeral tray – or at the diaphysis (FRdia) with a perpendicular line to the shaft axis intersecting at the distal third of the humeral stem. The ratio between the red and yellow lines is the filling ratio at each level
Humeral Stem Alignment
One benefit of longer stems is that a tight fit into the endosteal canal facilitates adequate stem alignment. Short stemmed or stemless implants that do not engage the cylindrical portion of the canal can easily be malaligned. Studies have shown that standard length implants are placed in neutral alignment 89%—98% of the time compared with 53%—96% in short-stemmed cases [21, 27, 34, 36, 37]. Valgus alignment of a short stemmed implant can lead to a reverse polyethylene that is excessively horizontal, which increases the risk of scapular notching [27]. Conversely, varus alignment of a short stemmed implant can lead to distal contact with the lateral endosteal cortex, which has been shown to increase the risk of stress shielding and bony adaptations [34, 38]. In addition to stem length, smaller stem sizes with decreased metaphyseal and diaphyseal filling ratios were also more likely to be malaligned [38]. Despite this, humeral stem malalignment has not been shown to adversely affect functional and clinical outcomes at short-term follow-up [27].
Implant Fixation
Most early RTSAs used a cemented humeral component to achieve adequate immediate fixation due to a concern that the semiconstrained nature of the articulation will impart greater shear stresses at the stem-bone interface. However, cementing increases surgical time and can lead to thromboembolism. Moreover, in the case of revision, removal of a well-fixed cemented stem is difficult, often requiring humeral osteotomy, and the risk of iatrogenic fracture and proximal humeral bone loss is increased [19]. Because of these issues, cementless humeral components were introduced. Early generation cementless implants were designed without a proximal porous coating. Schnetzke et al. analyzed these designs in a case series of 52 patients with mean follow-up of 32 months [39]. In their series, cortical thinning was seen in 52% of patients, osteopenia in 83%, and spot welds in 79%. Similarly, Casagrande et al. found radiolucent lines to be present in 71% of patients at short-term follow-up, of which 8% of these stems were determined to be at risk for radiographic loosening [40]. In an effort to reduce the risk of humeral loosening, subsequent modifications include a proximal titanium plasma spray to promote metaphyseal ingrowth. In a comparative study of 68 patients, Morwood et al. found that uncoated stems had a significantly higher risk of loosening (21% vs 3%) and radiolucencies (44% vs 21%) compared with proximally coated designs [41].
Few retrospective studies have compared the incidence of aseptic loosening between cemented and cementless stems in patients with rotator cuff arthropathy [42–44]. In a series of 292 patients, Gilot et al. found a 1.2% and 0% rate of humeral loosening in the cemented and cementless groups, respectively [42]. Wiater et al. similarly found no difference in radiolucent lines (2.7% vs 3.1%) and patient-reported outcome scores between cemented and cementless groups at 3-year follow-up [44]. A recent systematic review of reverse shoulder arthroplasties for all indications found an aseptic loosening rate of 1.2% and 0.8% for cemented and cementless stems [45]. Interestingly, humeral radiolucent lines were significantly more common among cemented stems compared to cementless stems (15.9% vs 9.5%). Subgroup analyses by indication for reverse shoulder arthroplasty also revealed that the highest rates of stem loosening were for tumor and revision after failed arthroplasty at 8.1% and 1.2%, respectively. These results confirm that aseptic loosening following primary reverse shoulder arthroplasty is uncommon and that clinical outcomes between cemented and cementless stems are similar at midterm follow-up. The decision to cement a humeral stem should be based on the patient age, surgical indication, proximal bone quality and availability.
Inlay Versus Onlay
The traditional Grammont prosthesis featured an inlay humeral design in which the pivot point of the polyethylene is below or at the level of the humeral osteotomy. The proposed advantages of the inlay design was that it increased bony metaphyseal contact and ingrowth, which can theoretically improve stem fixation [46]. Furthermore, the inlay design may restore the humerus into a more anatomic position when used in combination with a lateralized glenosphere [47]. In recent years, an onlay humeral tray was developed to facilitate the conversion of an anatomic total shoulder arthroplasty to a reverse shoulder arthroplasty in the setting of a revision (Fig. 3). An onlay humeral stem has a convertible platform that rests on the cut humeral surface, thereby shifting the polyethylene pivot point above the level of the humeral osteotomy. The onlay design has a curved metaphyseal stem that preserves more tuberosity bone stock, potentially reducing the risk of greater tuberosity fracture. Another benefit of the onlay stem is that it lateralizes the humerus. This increases compressive stability of the implant through the deltoid wrapping effect [48]. Other biomechanical studies have shown that the moment arms of the rotator cuff muscles are increased with onlay compared to inlay designs, which may improve shoulder motion and strength [49].
Fig. 3.
Radiographs illustrating the difference between a (A) Grammont design with an inlay metaphyseal component and (B) lateralized design with an onlay tray. The pivot point (yellow) of the glenosphere in an inlay design is at or below the neck osteotomy (red), whereas in an onlay design it is above the neck osteotomy
It should be highlighted that the distinction between an inlay and onlay humeral stem is not solely determined by the stem design. An inlay humeral stem design may functionally serve as an onlay design if a humeral spacer is utilized or the polyethylene thickness is increased so that the pivot point is above the level of the humeral osteotomy. This may occur when the surgeon makes a low humeral neck cut or when additional polyethylene is needed to adequately tension the deltoid. Comparative studies between the two designs may not necessarily take this into consideration, thereby making interpretation of these results difficult [50]. Reproducible measurements that can be used to estimate the lateralization and distalization of the humerus after reverse shoulder arthroplasty, such as the lateralization and distalization shoulder angles, may help characterize the effect of having an inlay or onlay humeral stem more accurately [51].
In a retrospective study comparing 36 inlay stems and 38 onlay stems with medialized glenospheres, Merolla et al. found that the onlay group had significantly greater external rotation and lower rates of scapular notching [30]. However, Meshram et al. found no significant difference in range of motion, scapular notching, acromial stress fractures, and revision rates between inlay designs with lateralized glenospheres and onlay designs with medialized glenospheres [46]. Another study comparing outcomes of inlay and onlay designs with lateralized glenospheres demonstrated improved external rotation and forward flexion in the onlay group at 2-year follow-up [52]. There were also no differences in acromial fractures and scapular notching. These findings suggest that increased glenohumeral lateralization, whether it is achieved on the glenoid or humeral side, can improve shoulder range of motion and decrease scapular notching.
Although the previously mentioned small comparative studies showed no difference in the rate of acromial stress fractures, a large series of 485 reverse shoulder arthroplasties with onlay designs reported a prevalence of 4.3%, raising a potential concern about the use onlay designs [53]. This was almost fourfold higher than the fracture rate reported with the classic Grammont inlay design [54]. Conversely, in a multicenter study of 3,995 patients treated with an onlay humeral stem with a medialized glenosphere, the rate of acromial stress fracture was 1.8% [55]. A recent systematic review by the ASES Multicenter Taskforce also found no difference between onlay and inlay designs [56].
Conclusion
The reverse shoulder prosthesis has demonstrated excellent long-term clinical outcomes and implant survivorship for an increasing number of indications. Advancements in humeral stem design have addressed problems of scapular notching and limited shoulder internal and external rotation that were common with the traditional Grammont design. Moreover, the addition of proximal porous coatings has promoted the development of short-stem and stemless implants, which preserves bone stock and potentially decreases the amount of stress shielding. Despite promising results, many of these new humeral design features do not have long-term data and continued surveillance of their performance is necessary. New innovations, such as patient-specific instrumentation and navigation, may be the next step to improve component positioning and soft tissue tensioning.
Key References
- Gutierrez S, Levy JC, Frankle MA, Cuff D, Keller TS, Pupello DR et al. Evaluation of abduction range of motion and avoidance of inferior scapular impingement in a reverse shoulder model. J Shoulder Elbow Surg. 2008;17(4):608–15. 10.1016/j.jse.2007.11.010.
- Biomechanical Sawbones study that demonstrated that neck-shaft angle had the largest effect on inferior scapular impingement, followed by inferior glenosphere position.
- Stephenson DR, Oh JH, McGarry MH, Rick Hatch GF, 3rd, Lee TQ. Effect of humeral component version on impingement in reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(4):652–8. 10.1016/j.jse.2010.08.020.
- Early biomechanical cadaveric study that found that increased retroversion increases external rotation, leading to the recommendation of placing the humeral stem between 20° and 40° of retroversion.
- Oh JH, Sharma N, Rhee SM, Park JH. Do individualized humeral retroversion and subscapularis repair affect the clinical outcomes of reverse total shoulder arthroplasty? J Shoulder Elbow Surg. 2020;29(4):821–9. 10.1016/j.jse.2019.08.016.
- Retrospective study that found that improved pain relief, internal and external rotation, and functional scores in patients with humeral components implanted in their native retroversion compared to patients with components implanted in 20° of retroversion.
- Erickson BJ, Denard PJ, Griffin JW, Gobezie R, Lederman E, Werner BC. Initial and 1-Year Radiographic Comparison of Reverse Total Shoulder Arthroplasty With a Short Versus Standard Length Stem. J Am Acad Orthop Surg. 2022;30(14):e968-e78. 10.5435/JAAOS-D-21-01032.
- At 1-year follow-up, there was no significant differences in clinical outcomes between standard-length and short-length stems. Short-length stems had significantly less calcar osteolysis (2.2% vs 12.9%) and fewer radiographic changes (tuberosity resorption, lucencies, and subsidence) (0.7% vs 5.0%) than standard-length stems.
- Melis B, DeFranco M, Ladermann A, Mole D, Favard L, Nerot C et al. An evaluation of the radiological changes around the Grammont reverse geometry shoulder arthroplasty after eight to 12 years. J Bone Joint Surg Br. 2011;93(9):1240–6. 10.1302/0301-620X.93B9.25926.
- At 8 to 12-year follow-up, 88% of Grammont-style prostheses had scapular notching. Humeral subsidence was observed in 8.8% and 2.9% of cemented and cementless components, respectively. Stress shielding was significantly more frequent in uncemented components.
- Raiss P, Schnetzke M, Wittmann T, Kilian CM, Edwards TB, Denard PJ et al. Postoperative radiographic findings of an uncemented convertible short stem for anatomic and reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2019;28(4):715–23. 10.1016/j.jse.2018.08.037.
- Case series that demonstrated that larger stems with diaphyseal filling ratios ≥ 0.80 were at an increased risk of high bone adaptations.
- Morwood MP, Johnston PS, Garrigues GE. Proximal ingrowth coating decreases risk of loosening following uncemented shoulder arthroplasty using mini-stem humeral components and lesser tuberosity osteotomy. J Shoulder Elbow Surg. 2017;26(7):1246–52. 10.1016/j.jse.2016.11.041.
- At 2-year follow-up, proximally porous coated stems had fewer radiolucencies compared to uncoated stems (21% vs 44%), though there were no significant difference in final range of motion or outcome scores.
- Grey B, Rodseth RN, Roche SJ. Humeral Stem Loosening Following Reverse Shoulder Arthroplasty: A Systematic Review and Meta-Analysis. JBJS Rev. 2018;6(5):e5. 10.2106/JBJS.RVW.17.00129.
- A meta-analysis of 75 articles that found an aseptic loosening rate of 1.2% and 0.8% for cemented and cementless stems, respectively. However, humeral radiolucent lines were more common with cemented compared with uncemented stems (15.9% vs 9.5%).
Authors’ Contributions
A.C.L and F.S. performed the literature search, data collection, and writing of the original manuscript text. F.S. prepared the figures. B.T.F. conceptualized this review and edited the manuscript.
Funding
No funds, grants, or other support was received.
Data Availability
No datasets were generated or analysed during the current study.
Declarations
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Flatow EL, Harrison AK. A history of reverse total shoulder arthroplasty. Clin Orthop Relat Res. 2011;469(9):2432–9. 10.1007/s11999-010-1733-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baulot E, Sirveaux F, Boileau P. Grammont’s idea: The story of Paul Grammont’s functional surgery concept and the development of the reverse principle. Clin Orthop Relat Res. 2011;469(9):2425–31. 10.1007/s11999-010-1757-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naveed MA, Kitson J, Bunker TD. The Delta III reverse shoulder replacement for cuff tear arthropathy: a single-centre study of 50 consecutive procedures. J Bone Joint Surg Br. 2011;93(1):57–61. 10.1302/0301-620X.93B1.24218. [DOI] [PubMed] [Google Scholar]
- 4.Levy JC, Virani N, Pupello D, Frankle M. Use of the reverse shoulder prosthesis for the treatment of failed hemiarthroplasty in patients with glenohumeral arthritis and rotator cuff deficiency. J Bone Joint Surg Br. 2007;89(2):189–95. 10.1302/0301-620X.89B2.18161. [DOI] [PubMed] [Google Scholar]
- 5.Gutierrez S, Levy JC, Frankle MA, Cuff D, Keller TS, Pupello DR, et al. Evaluation of abduction range of motion and avoidance of inferior scapular impingement in a reverse shoulder model. J Shoulder Elbow Surg. 2008;17(4):608–15. 10.1016/j.jse.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Ladermann A, Denard PJ, Boileau P, Farron A, Deransart P, Terrier A, et al. Effect of humeral stem design on humeral position and range of motion in reverse shoulder arthroplasty. Int Orthop. 2015;39(11):2205–13. 10.1007/s00264-015-2984-3. [DOI] [PubMed] [Google Scholar]
- 7.Oh JH, Shin SJ, McGarry MH, Scott JH, Heckmann N, Lee TQ. Biomechanical effects of humeral neck-shaft angle and subscapularis integrity in reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(8):1091–8. 10.1016/j.jse.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Holsters L, Sadeghi N, Gendera H, Groen V, Bruls V, Heerspink OL. Influence of humeral stem inclination in reverse shoulder arthroplasty on range of motion: a meta-analysis. JSES Rev Rep Tech. 2021;1:102–12. 10.1016/j.xrrt.2021.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erickson BJ, Frank RM, Harris JD, Mall N, Romeo AA. The influence of humeral head inclination in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2015;24(6):988–93. 10.1016/j.jse.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Jassim SS, Ernstbrunner L, Ek ET. Does humeral component version affect range of motion and clinical outcomes in reverse total shoulder arthroplasty? A systematic review. J Clin Med. 2021;10(24). 10.3390/jcm10245745. [DOI] [PMC free article] [PubMed]
- 11.Gulotta LV, Choi D, Marinello P, Knutson Z, Lipman J, Wright T, et al. Humeral component retroversion in reverse total shoulder arthroplasty: a biomechanical study. J Shoulder Elbow Surg. 2012;21(9):1121–7. 10.1016/j.jse.2011.07.027. [DOI] [PubMed] [Google Scholar]
- 12.Stephenson DR, Oh JH, McGarry MH, Rick Hatch GF, Lee TQ. Effect of humeral component version on impingement in reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(4):652–8. 10.1016/j.jse.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 13.Berhouet J, Garaud P, Favard L. Influence of glenoid component design and humeral component retroversion on internal and external rotation in reverse shoulder arthroplasty: a cadaver study. Orthop Traumatol Surg Res. 2013;99(8):887–94. 10.1016/j.otsr.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Aleem AW, Feeley BT, Austin LS, Ma CB, Krupp RJ, Ramsey ML, Getz CL. Effect of humeral component version on outcomes in reverse shoulder arthroplasty. Orthopedics (Thorofare, NJ). 2017;40(3):179–86. [DOI] [PubMed] [Google Scholar]
- 15.Rhee YG, Cho NS, Moon SC. Effects of humeral component retroversion on functional outcomes in reverse total shoulder arthroplasty for cuff tear arthropathy. J Shoulder Elbow Surg. 2015;24(10):1574–81. 10.1016/j.jse.2015.03.026. [DOI] [PubMed] [Google Scholar]
- 16.Oh JH, Sharma N, Rhee SM, Park JH. Do individualized humeral retroversion and subscapularis repair affect the clinical outcomes of reverse total shoulder arthroplasty? J Shoulder Elbow Surg. 2020;29(4):821–9. 10.1016/j.jse.2019.08.016. [DOI] [PubMed] [Google Scholar]
- 17.Denard PJ, Raiss P, Gobezie R, Edwards TB, Lederman E. Stress shielding of the humerus in press-fit anatomic shoulder arthroplasty: review and recommendations for evaluation. J Shoulder Elbow Surg. 2018;27(6):1139–47. 10.1016/j.jse.2017.12.020. [DOI] [PubMed] [Google Scholar]
- 18.Romeo AA, Thorsness RJ, Sumner SA, Gobezie R, Lederman ES, Denard PJ. Short-term clinical outcome of an anatomic short-stem humeral component in total shoulder arthroplasty. J Shoulder Elbow Surg. 2018;27(1):70–4. 10.1016/j.jse.2017.05.026. [DOI] [PubMed] [Google Scholar]
- 19.Van Thiel GS, Halloran JP, Twigg S, Romeo AA, Nicholson GP. The vertical humeral osteotomy for stem removal in revision shoulder arthroplasty: results and technique. J Shoulder Elbow Surg. 2011;20(8):1248–54. 10.1016/j.jse.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 20.Athwal GS, Sperling JW, Rispoli DM, Cofield RH. Periprosthetic humeral fractures during shoulder arthroplasty. J Bone Joint Surg Am. 2009;91(3):594–603. 10.2106/JBJS.H.00439. [DOI] [PubMed] [Google Scholar]
- 21.Denard PJ, Noyes MP, Walker JB, Shishani Y, Gobezie R, Romeo AA, et al. Proximal stress shielding is decreased with a short stem compared with a traditional-length stem in total shoulder arthroplasty. J Shoulder Elbow Surg. 2018;27(1):53–8. 10.1016/j.jse.2017.06.042. [DOI] [PubMed] [Google Scholar]
- 22.Kramer M, Olach M, Zdravkovic V, Manser M, Raiss P, Jost B, et al. The effects of length and width of the stem on proximal humerus stress shielding in uncemented primary reverse total shoulder arthroplasty. Arch Orthop Trauma Surg. 2024;144(2):663–72. 10.1007/s00402-023-05129-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keener JD, Chalmers PN, Yamaguchi K. The humeral implant in shoulder arthroplasty. J Am Acad Orthop Surg. 2017;25(6):427–38. 10.5435/JAAOS-D-15-00682. [DOI] [PubMed] [Google Scholar]
- 24.Kadum B, Mafi N, Norberg S, Sayed-Noor AS. Results of the Total Evolutive Shoulder System (TESS): a single-centre study of 56 consecutive patients. Arch Orthop Trauma Surg. 2011;131(12):1623–9. 10.1007/s00402-011-1368-4. [DOI] [PubMed] [Google Scholar]
- 25.Tross AK, Woolson TE, Nolte PC, Schnetzke M, Loew M, Millett PJ. Primary reverse shoulder replacement with a short stem: A systematic literature review. JSES Rev Rep Tech. 2021;1(1):7–16. 10.1016/j.xrrt.2020.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ajibade DA, Yin CX, Hamid HS, Wiater BP, Martusiewicz A, Wiater JM. Stemless reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2022;31(5):1083–95. 10.1016/j.jse.2021.12.017. [DOI] [PubMed] [Google Scholar]
- 27.Erickson BJ, Denard PJ, Griffin JW, Gobezie R, Lederman E, Werner BC. Initial and 1-year radiographic comparison of reverse total shoulder arthroplasty with a short versus standard length stem. J Am Acad Orthop Surg. 2022;30(14):e968–78. 10.5435/JAAOS-D-21-01032. [DOI] [PubMed] [Google Scholar]
- 28.Melis B, DeFranco M, Ladermann A, Mole D, Favard L, Nerot C, et al. An evaluation of the radiological changes around the Grammont reverse geometry shoulder arthroplasty after eight to 12 years. J Bone Joint Surg Br. 2011;93(9):1240–6. 10.1302/0301-620X.93B9.25926. [DOI] [PubMed] [Google Scholar]
- 29.Harmsen SM, Norris TR. Radiographic changes and clinical outcomes associated with an adjustable diaphyseal press-fit humeral stem in primary reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2017;26(9):1589–97. 10.1016/j.jse.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 30.Merolla G, Walch G, Ascione F, Paladini P, Fabbri E, Padolino A, et al. Grammont humeral design versus onlay curved-stem reverse shoulder arthroplasty: comparison of clinical and radiographic outcomes with minimum 2-year follow-up. J Shoulder Elbow Surg. 2018;27(4):701–10. 10.1016/j.jse.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 31.Erickson BJ, Chalmers PN, Denard PJ, Gobezie R, Romeo AA, Lederman ES. Current state of short-stem implants in total shoulder arthroplasty: a systematic review of the literature. JSES Int. 2020;4(1):114–9. 10.1016/j.jses.2019.10.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moroder P, Ernstbrunner L, Zweiger C, Schatz M, Seitlinger G, Skursky R, et al. Short to mid-term results of stemless reverse shoulder arthroplasty in a selected patient population compared to a matched control group with stem. Int Orthop. 2016;40(10):2115–20. 10.1007/s00264-016-3249-5. [DOI] [PubMed] [Google Scholar]
- 33.Razfar N, Reeves JM, Langohr DG, Willing R, Athwal GS, Johnson JA. Comparison of proximal humeral bone stresses between stemless, short stem, and standard stem length: a finite element analysis. J Shoulder Elbow Surg. 2016;25(7):1076–83. 10.1016/j.jse.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 34.Raiss P, Schnetzke M, Wittmann T, Kilian CM, Edwards TB, Denard PJ, et al. Postoperative radiographic findings of an uncemented convertible short stem for anatomic and reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2019;28(4):715–23. 10.1016/j.jse.2018.08.037. [DOI] [PubMed] [Google Scholar]
- 35.Raiss P, Edwards TB, Deutsch A, Shah A, Bruckner T, Loew M, et al. Radiographic changes around humeral components in shoulder arthroplasty. J Bone Joint Surg Am. 2014;96(7):e54. 10.2106/JBJS.M.00378. [DOI] [PubMed] [Google Scholar]
- 36.Ladermann A, Chiu JC, Cunningham G, Herve A, Piotton S, Bothorel H, et al. Do short stems influence the cervico-diaphyseal angle and the medullary filling after reverse shoulder arthroplasties? Orthop Traumatol Surg Res. 2020;106(2):241–6. 10.1016/j.otsr.2019.12.010. [DOI] [PubMed] [Google Scholar]
- 37.Giuseffi SA, Streubel P, Sperling J, Sanchez-Sotelo J. Short-stem uncemented primary reverse shoulder arthroplasty: clinical and radiological outcomes. Bone Joint J. 2014;96-B(4):526–9. 10.1302/0301-620X.96B3.32702. [DOI] [PubMed] [Google Scholar]
- 38.Abdic S, Athwal GS, Wittmann T, Walch G, Raiss P. Short stem humeral components in reverse shoulder arthroplasty: stem alignment influences the neck-shaft angle. Arch Orthop Trauma Surg. 2021;141(2):183–8. 10.1007/s00402-020-03424-4. [DOI] [PubMed] [Google Scholar]
- 39.Schnetzke M, Coda S, Raiss P, Walch G, Loew M. Radiologic bone adaptations on a cementless short-stem shoulder prosthesis. J Shoulder Elbow Surg. 2016;25(4):650–7. 10.1016/j.jse.2015.08.044. [DOI] [PubMed] [Google Scholar]
- 40.Casagrande DJ, Parks DL, Torngren T, Schrumpf MA, Harmsen SM, Norris TR, et al. Radiographic evaluation of short-stem press-fit total shoulder arthroplasty: short-term follow-up. J Shoulder Elbow Surg. 2016;25(7):1163–9. 10.1016/j.jse.2015.11.067. [DOI] [PubMed] [Google Scholar]
- 41.Morwood MP, Johnston PS, Garrigues GE. Proximal ingrowth coating decreases risk of loosening following uncemented shoulder arthroplasty using mini-stem humeral components and lesser tuberosity osteotomy. J Shoulder Elbow Surg. 2017;26(7):1246–52. 10.1016/j.jse.2016.11.041. [DOI] [PubMed] [Google Scholar]
- 42.Gilot G, Alvarez-Pinzon AM, Wright TW, Flurin PH, Krill M, Routman HD, et al. The incidence of radiographic aseptic loosening of the humeral component in reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24(10):1555–9. 10.1016/j.jse.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 43.King JJ, Farmer KW, Struk AM, Wright TW. Uncemented versus cemented humeral stem fixation in reverse shoulder arthroplasty. Int Orthop. 2015;39(2):291–8. 10.1007/s00264-014-2593-6. [DOI] [PubMed] [Google Scholar]
- 44.Wiater JM, Moravek JE Jr, Budge MD, Koueiter DM, Marcantonio D, Wiater BP. Clinical and radiographic results of cementless reverse total shoulder arthroplasty: a comparative study with 2 to 5 years of follow-up. J Shoulder Elbow Surg. 2014;23(8):1208–14. 10.1016/j.jse.2013.11.032. [DOI] [PubMed] [Google Scholar]
- 45.Grey B, Rodseth RN, Roche SJ. Humeral stem loosening following reverse shoulder arthroplasty: a systematic review and meta-analysis. JBJS Rev. 2018;6(5):e5. 10.2106/JBJS.RVW.17.00129. [DOI] [PubMed] [Google Scholar]
- 46.Meshram P, Joseph J, Zhou Y, Srikumaran U, McFarland EG. Lateralized glenosphere reverse shoulder arthroplasty: inlay and onlay designs have similar clinical outcomes in patients with glenohumeral osteoarthritis. J Shoulder Elbow Surg. 2022;31(4):747–54. 10.1016/j.jse.2021.08.016. [DOI] [PubMed] [Google Scholar]
- 47.Cogan CJ, Ho JC, Entezari V, Iannotti JP, Ricchetti ET. The influence of reverse total shoulder arthroplasty implant design on biomechanics. Curr Rev Musculoskelet Med. 2023;16(3):95–102. 10.1007/s12178-023-09820-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beltrame A, Di Benedetto P, Cicuto C, Cainero V, Chisoni R, Causero A. Onlay versus Inlay humeral steam in Reverse Shoulder Arthroplasty (RSA): clinical and biomechanical study. Acta Biomed. 2019;90(12-S):54–63. 10.23750/abm.v90i12-S.8983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hamilton MA, Roche CP, Diep P, Flurin PH, Routman HD. Effect of prosthesis design on muscle length and moment arms in reverse total shoulder arthroplasty. Bull Hosp Jt Dis. 2013;2013(71 Suppl 2):S31–5. [PubMed] [Google Scholar]
- 50.Larose G, Fisher ND, Gambhir N, Alben MG, Zuckerman JD, Virk MS, et al. Inlay versus onlay humeral design for reverse shoulder arthroplasty: a systematic review and meta-analysis. J Shoulder Elbow Surg. 2022;31(11):2410–20. 10.1016/j.jse.2022.05.002. [DOI] [PubMed] [Google Scholar]
- 51.Boutsiadis A, Lenoir H, Denard PJ, Panisset JC, Brossard P, Delsol P, et al. The lateralization and distalization shoulder angles are important determinants of clinical outcomes in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2018;27(7):1226–34. 10.1016/j.jse.2018.02.036. [DOI] [PubMed] [Google Scholar]
- 52.Polisetty TSBA, Levy JC, Badman BL. Onlay versus inlay reverse total shoulder arthroplasty: a retrospective comparison of radiographic and clinical outcomes. Semin Arthroplast J Should Elbow Surg. 2021;31:202–8. [Google Scholar]
- 53.Ascione F, Kilian CM, Laughlin MS, Bugelli G, Domos P, Neyton L, et al. Increased scapular spine fractures after reverse shoulder arthroplasty with a humeral onlay short stem: an analysis of 485 consecutive cases. J Shoulder Elbow Surg. 2018;27(12):2183–90. 10.1016/j.jse.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 54.Neyton L, Erickson J, Ascione F, Bugelli G, Lunini E, Walch G. Grammont Award 2018: Scapular fractures in reverse shoulder arthroplasty (Grammont style): prevalence, functional, and radiographic results with minimum 5-year follow-up. J Shoulder Elbow Surg. 2019;28(2):260–7. 10.1016/j.jse.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 55.Routman HD, Simovitch RW, Wright TW, Flurin PH, Zuckerman JD, Roche CP. Acromial and scapular fractures after reverse total shoulder arthroplasty with a medialized glenoid and lateralized humeral implant: an analysis of outcomes and risk factors. J Bone Joint Surg Am. 2020;102(19):1724–33. 10.2106/JBJS.19.00724. [DOI] [PubMed] [Google Scholar]
- 56.Shah SS, Roche AM, Sullivan SW, Gaal BT, Dalton S, Sharma A, et al. The modern reverse shoulder arthroplasty and an updated systematic review for each complication: part II. JSES Int. 2021;5(1):121–37. 10.1016/j.jseint.2020.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.