Abstract
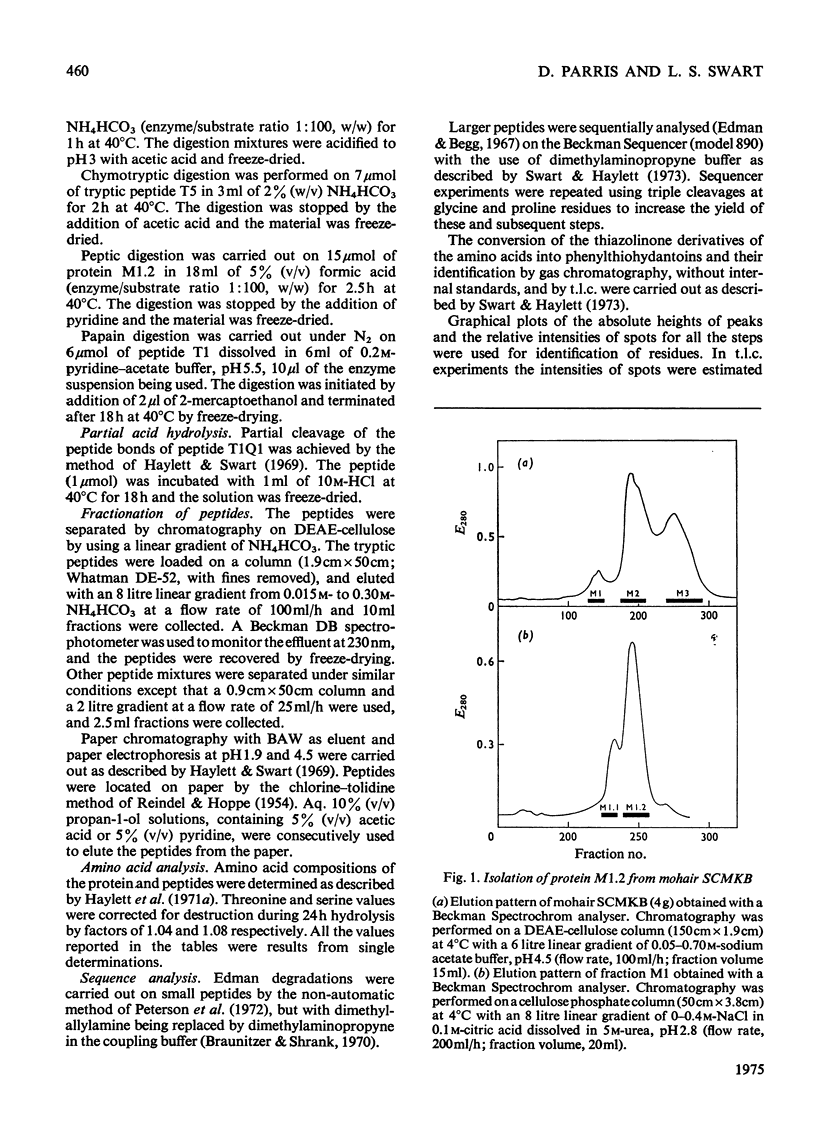
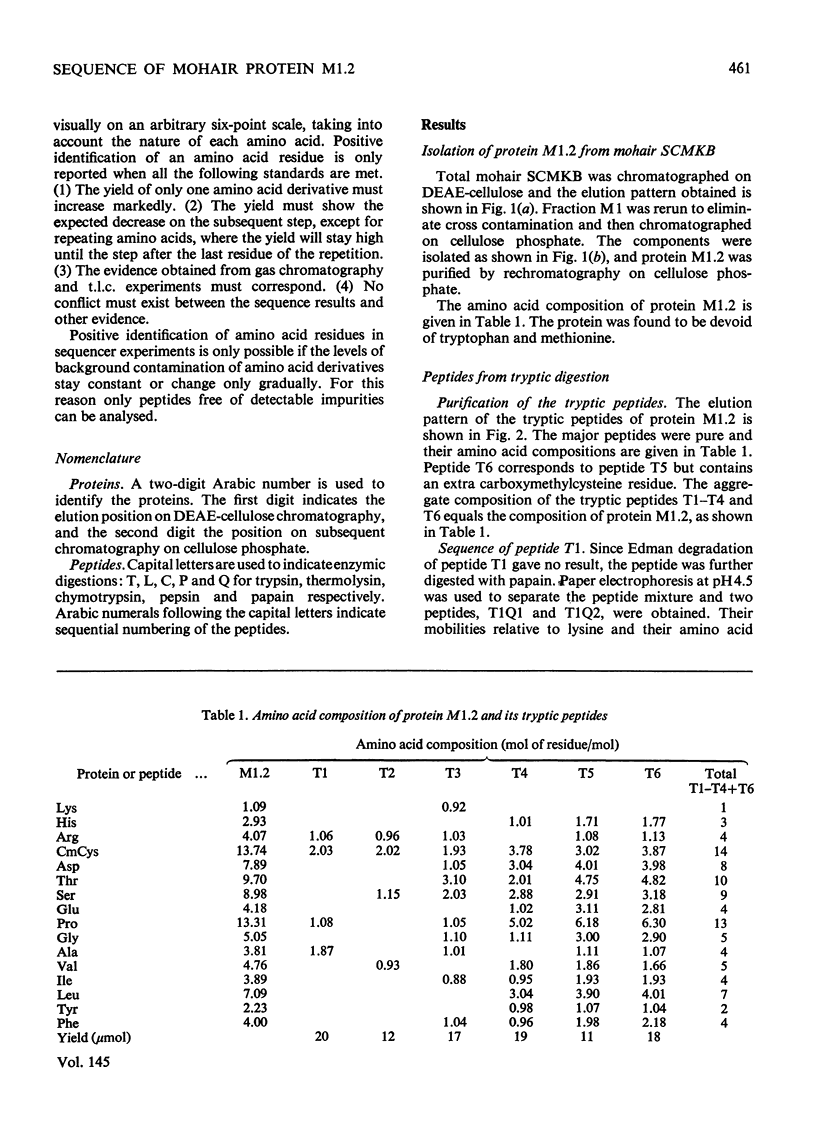
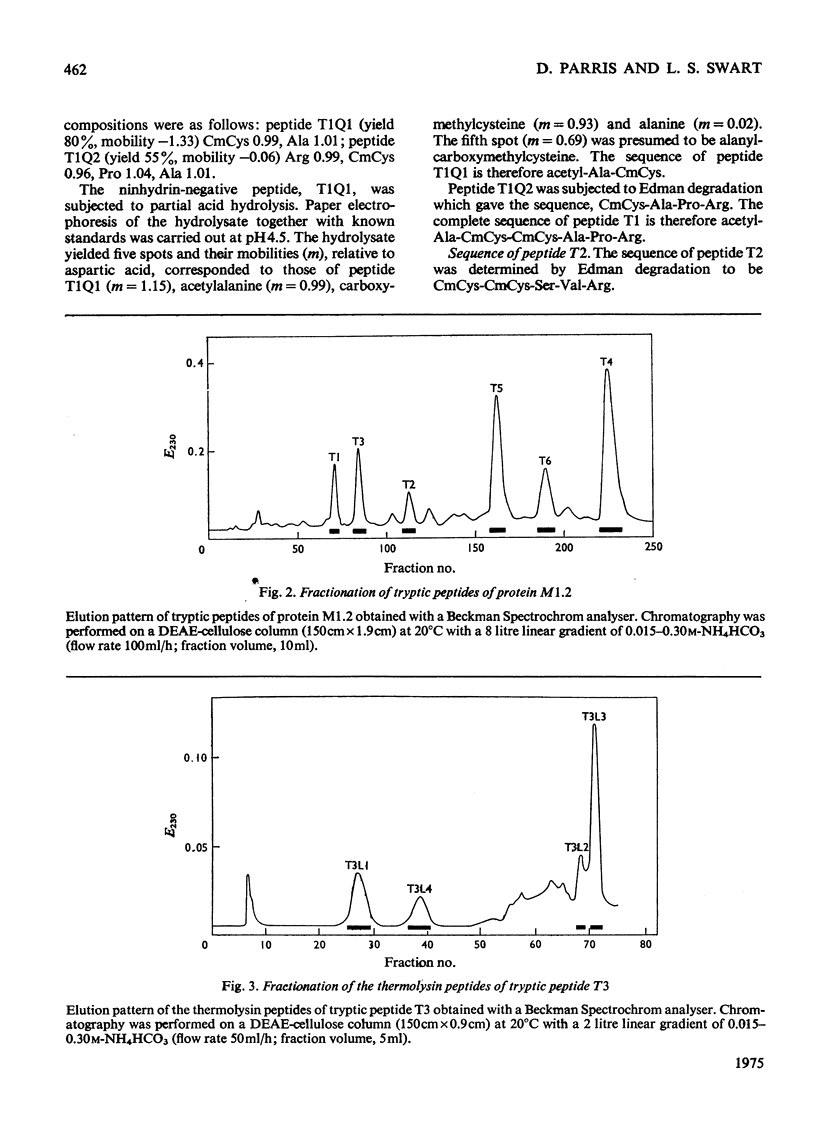
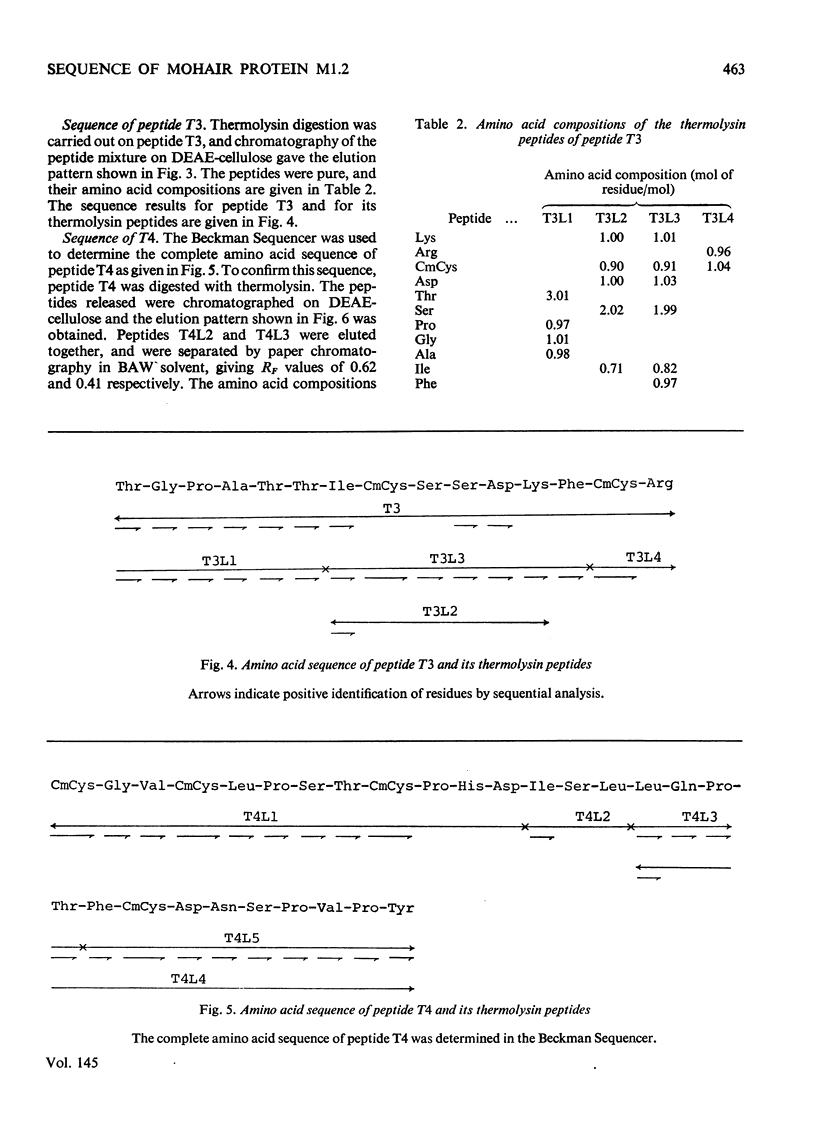
The complete amino acid sequence of mohair protein, SCMKB-M1.2 (97 residues), was determined. The protein was isolated from reduced and carboxymethylated mohair by chromatography on DEAE-cellulose phosphate. Peptides for sequence determination were obtained by digestion with trypsin, pepsin, chymotrypsin, thermolysin and papain, and were fractionated by DEAE-cellulose chromatography, paper chromatography and electrophoresis. The sequence of the peptides were determined by the Edman degradation method (by use of both the Beckman Sequence and a non-automatic procedure), and by partial acid hydrolysis. The protein is closely homologous to wool protein SCMKB-IIIB2, and also contains acetylated alanine as N-terminal amino acid.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradbury J. H. The structure and chemistry of keratin fibers. Adv Protein Chem. 1973;27:111–211. doi: 10.1016/s0065-3233(08)60447-7. [DOI] [PubMed] [Google Scholar]
- Braunitzer G., Schrank B. 3-Dimethylamino-1-propin als Puffersubstanz. Hoppe Seylers Z Physiol Chem. 1970 Mar;351(3):417–417. [PubMed] [Google Scholar]
- Edman P., Begg G. A protein sequenator. Eur J Biochem. 1967 Mar;1(1):80–91. doi: 10.1007/978-3-662-25813-2_14. [DOI] [PubMed] [Google Scholar]
- Elleman T. C. The amino acid sequence of protein SCMK-B2A from the high-sulphur fraction of wool keratin. Biochem J. 1972 Dec;130(3):833–845. doi: 10.1042/bj1300833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haylett T., Swart L. S., Parris D. Studies on the high-sulphur proteins of reduced merino wool. Amino acid sequence of protein SCMKB-3B 3 . Biochem J. 1971 Jun;123(2):191–200. doi: 10.1042/bj1230191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindley H., Elleman T. C. The preparation and properties of a group of proteins from the high-sulphur fraction of wool. Biochem J. 1972 Jul;128(4):859–867. doi: 10.1042/bj1280859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson J. D., Nehrlich S., Oyer P. E., Steiner D. F. Determination of the amino acid sequence of the monkey, sheep, and dog proinsulin C-peptides by a semi-micro Edman degradation procedure. J Biol Chem. 1972 Aug 10;247(15):4866–4871. [PubMed] [Google Scholar]
- Swart L. S., Haylett T. Studies on the high-sulphur proteins of reduced Merino wool. Amino acid sequence of protein SCMKB-3A3. Biochem J. 1973 Aug;133(4):641–654. doi: 10.1042/bj1330641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swart L. S., Haylett T. Studies on the high-sulphur proteins of reduced merino wool. Amino acid sequence of protein SCMKB-3B 4 . Biochem J. 1971 Jun;123(2):201–210. doi: 10.1042/bj1230201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swart L. S. Homology in the amino-acid sequences of the high-sulphur proteins from wool. Nat New Biol. 1973 May 2;243(122):27–29. [PubMed] [Google Scholar]


