Abstract
Escherichia coli strains that cause nonbloody diarrhea in infants are known to present three distinct patterns of adherence to epithelial cells, namely, localized (LA), diffuse (DA), and aggregative (AA) adherence. Strains with LA (typical Enteropathogenic Escherichia coli [EPEC]) are well recognized as a cause of secretory diarrhea, but the role of strains with DA (DAEC) is controversial, and strains with AA (EAEC) have been more frequently related to persistent diarrhea whereas its relationship with acute diarrhea is not well defined. To determine the relationship of the different types of E. coli adherence patterns with acute diarrhea (lasting less than 14 days) and persistent diarrhea (lasting more than 14 days) in São Paulo, Brazil, we studied stool specimens from 40 infants under 1 year of age with diarrhea and 40 age-matched control infants without any gastrointestinal symptoms. Twenty-eight (35.0%) of eighty cases yielded adherent E. coli (HEp-2 cells). Strains with localized and aggregative adherence were associated with acute and persistent diarrhea. A total of 11.2% of the adherent strains were typical EPEC serotypes and hybridized with the enteroadherence factor probe; 5.0% were EAEC and hybridized with the EAEC probe. DAEC strains were isolated from 10.0% of patients and 7.5% of controls and did not hybridize with the two probes used (daaC and AIDA-I). Strains with a localized adherence-like pattern (atypical EPEC) were found significantly more frequently (P = 0.028) in cultures from children with diarrhea (17.5%) than in controls (2.5%).
Escherichia coli strains that cause nonbloody diarrhea in infants are known to present three distinct patterns of adherence to epithelial cells: (i) localized adherence (LA), where microcolonies attach to one or two small areas on the cells (HeLa or HEp-2); (ii) diffuse adherence (DA), where bacteria cover the cells uniformly (39); and (iii) enteroadherent-aggregative adherence (AA), where the bacteria have a characteristic stacked-brick-like arrangement on the surface of the cells and on the glass slide free from the cells (35).
The LA pattern is significantly associated with diarrhea production (10, 30) and has been shown to be an important property of enteropathogenic E. coli (EPEC). A 50- to 60-MDa plasmid called the enteroadherence factor (EAF) is necessary for most EPEC strains to exhibit LA on tissue culture cells (2). A DNA probe to detect the EAF plasmid, called the EAF probe, has been used to identify localized adhering E. coli strains (12, 18, 34). The LA phenotype is associated with the induction of the attaching and effacing lesions (A/E) (33). A DNA probe derived from the chromosomal gene called eae (for E. coli attaching and effacing) has been developed and allows detection of EPEC strains (26).
The pathogenic role of E. coli showing a DA pattern (DAEC) in the etiology of diarrheal disease is controversial (3, 17, 22, 25). Two DNA probes specific for DAEC strains have been described. Bilge et al. (7) characterized a 14.3-kDa chromosomally encoded adhesin, termed F1845, and developed an intragenic 1-kb DNA probe (the daaC probe). Benz and Schmidt (5) described a plasmid-encoded outer membrane protein of 100 kDa, termed AIDA-I, which is involved in the DA phenotype. A cloned 6-kb DNA fragment from this plasmid, which was shown to be sufficient for the expression of DA phenotype, has been used as a specific DNA probe.
The role of E. coli giving an aggregative pattern of attachment to tissue culture cells (EAEC) in diarrhea has been more frequently related to persistent diarrhea (6, 9). EAEC strains possess a plasmid of ca. 60 MDa which is necessary for the expression of the aggregative phenotype (44). A 1-kb fragment from this plasmid has been used as a DNA probe to identify EAEC strains (4).
Recently, Scaletsky et al. (41) described a new adherence pattern called the localized adherence-like (LAL) pattern. This pattern is characterized by the presence of less-compact microcolonies or clusters of bacteria in a few cells observed only in tests with prolonged incubation periods (6 h). This pattern was observed in EAF-negative EPEC strains and was the same pattern named “poor LA” by Knutton et al. (28). A consensus definition was achieved at the Second International Symposium on EPEC: A/E, Shiga toxin-negative strains possessing the EAF plasmid would be called “typical EPEC,” while such strains that do not possess the EAF plasmid would be called “atypical EPEC” (27). The mechanism of this adherence pattern is unknown. The role of E. coli showing an LAL pattern as an agent of diarrhea is not established yet (37).
The purpose of the present study was to investigate the relationship of the different types of E. coli adherence patterns with acute and persistent diarrhea in São Paulo, Brazil.
MATERIALS AND METHODS
Patients.
From August 1995 to July 1996, 40 infants less than 12 months of age with diarrhea (28 acute and 12 persistent cases) were consecutively studied. Infants were admitted to São Paulo Hospital for medical treatment. Diarrhea was defined as the excretion of three or more liquid stools during the 24 h before admission with or without vomiting. Acute diarrhea was defined as diarrhea lasting less than 14 days at the moment of admission. Persistent diarrhea was defined as diarrhea of a presumably infectious etiology lasting more than 14 days.
Controls.
A control group comprised of 40 asymptomatic infants matched for age was randomly included from the well-baby outpatient clinic of the hospital. Control infants had had no gastrointestinal symptoms for at least 30 days prior to inclusion in the study.
Microbiological studies.
E. coli strains were isolated on MacConkey agar. Four separate lactose-fermenting colonies, presumed to be E. coli by colony morphology, and two non-lactose-fermenting colonies of each distinct morphologic type were cultivated in EPM and MILi (43) media for biochemical confirmation of the species or genus. All E. coli colonies were submitted to slide agglutination with polyvalent and monovalent antisera (PROBAC do Brasil, São Paulo, Brazil) against O antigens of EPEC serogroups and enterohemorrhagic E. coli. The serogroups considered were as follows: O26, O55, O86, O111, O114, O119, O125, O126, O127, O128ab, O142, O157, and O158. When two or more colonies of identical serotypes were isolated from the same infant, only one colony was kept. Shigella spp., Salmonella spp., Giardia lamblia, Yersinia enterocolitica, Campylobacter spp., cryptosporidia, and rotaviruses were searched for as described elsewhere (13, 14, 24). All strains were maintained in nutrient agar slants at room temperature.
Adhesion assay.
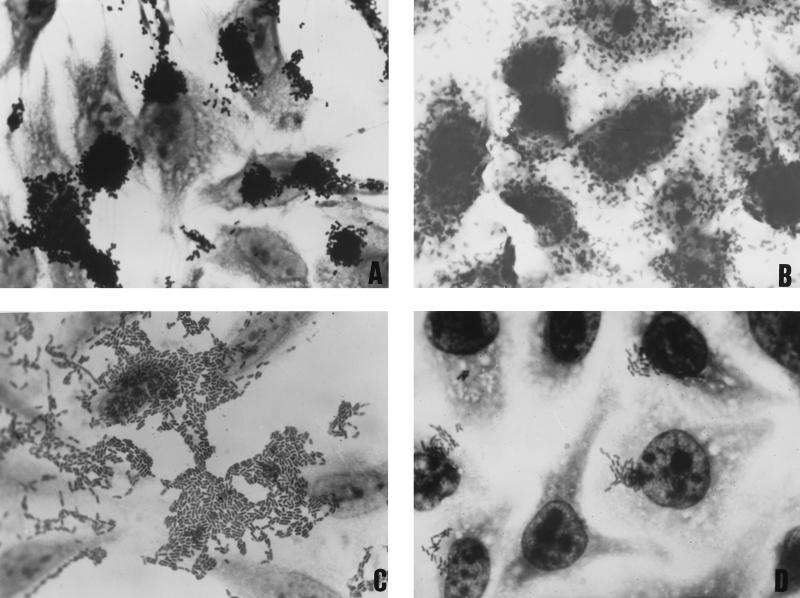
E. coli colonies were characterized by the pattern of adherence to HEp-2 cells in the presence of d-mannose as assayed by the method described by Scaletsky et al. (39). Monolayers were examined after 3 h of incubation. When the adherence pattern was negative, a new preparation was made and examined after a 6-h incubation period. Four distinct patterns of adherence were distinguished: LA occurred when the bacteria attached to localized areas of the HEp-2 cells in culture, forming distinct microcolonies after 3 h of incubation (Fig. 1A); DA occurred when bacteria adhered to the entire surface of the HEp-2 cells without formation of discrete microcolonies (Fig. 1B); AA was distinguished by prominent autoagglutination of the bacterial cells to each other on the surface of the cells, as well as on the glass coverslip free from the HEp-2 cells (Fig. 1C); and the LAL pattern, which was observed only in strains incubated for 6 h and was characterized by the formation of microcolonies or clusters less dense and compact than those displayed by typical LA-positive strains (Fig. 1D).
FIG. 1.
HEp-2 adherence patterns of enteroadherent E. coli. (A) LA pattern typical of EPEC. (B) DA pattern, which defines DAEC. (C) AA pattern, which defines EAEC. (D) LAL pattern, characterized by atypical EPEC.
DNA hybridization.
All E. coli strains with the LA, DA, AA, and LAL types of adherence were tested by colony DNA hybridization (32) with EPEC adherence factor EAF, E. coli attaching and effacing gene A encoding intimin (eaeA), DA factors (daaC, AIDA-I), and enteroaggregative E. coli adherence factor (EAEC) probes. The EAF probe was a 1-kb SalI-BamHI fragment from plasmid pMAR2 (34), the eaeA probe was a 1-kb SalI-KpnI fragment from plasmid pCVD434 (26), the daaC probe was a 350-bp PstI fragment of pSLM852 (7), the AIDA-I probe was a 450-bp EcoRI fragment of pIB6 (5), and the EAEC probe was a 1-kb EcoRI-PstI fragment of pCVD432 (4). These probes were labeled with the BioPrime primer extension kit and detected by using the Blue-Gene system (both from Gibco-BRL, Gaithersburg, Md.). Probe hybridization was performed overnight at 42°C in a hybridization solution containing 45% formamide. After hybridization, filters were washed in 0.16× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–0.01% sodium dodecyl sulfate solution at 50°C as described by Gicquelais et al. (16).
RESULTS
Symptoms, such as vomiting, fever, the presence of blood in the stools, and the degree of dehydration, were recorded, and their frequencies were similar in children with both acute and persistent diarrheas (Table 1).
TABLE 1.
Clinical characteristics of children with acute and persistent diarrheaa
| Symptom | No. of children with:
|
|
|---|---|---|
| Acute diarrhea (n = 28) | Persistent diarrhea (n = 12) | |
| Vomiting | 22 | 11 |
| Fever | 18 | 10 |
| Blood in stool | 2 | 1 |
| Dehydration | ||
| Mild | 5 | 1 |
| Moderate | 22 | 11 |
| Severe | 1 | 0 |
The mean durations of acute and persistent diarrhea were 5.3 ± 3.3 and 22.1 ± 4.4 days, respectively.
The prevalence of enteropathogenic agents identified in the stool cultures of patients and controls is presented in Table 2. EPEC was the organism most commonly detected in diarrheal stools, followed by Shigella spp. Salmonella spp., Y. enterocolitica, and Campylobacter spp. were not found. No differences regarding the presence of rotavirus were observed between the diarrheic and control infants. Cryptosporidium spp. and G. lamblia were found only in infants with diarrhea.
TABLE 2.
Prevalence of enteropathogens in infants with diarrhea (acute and persistent) and controls in São Paulo
| Enteropathogen | No. of positive infants
|
||
|---|---|---|---|
| Acute (n = 28) | Persistent (n = 12) | Controls (n = 40) | |
| EPEC serogroup | |||
| O26 | 1 | 0 | 0 |
| O55 | 0 | 2 | 0 |
| O86 | 1 | 0 | 0 |
| O111 | 1 | 1 | 1 |
| O119 | 2 | 2 | 0 |
| O126 | 1 | 0 | 0 |
| O127 | 1 | 1 | 0 |
| O128ab | 1 | 1 | 0 |
| O142 | 1 | 0 | 0 |
| O158 | 0 | 1 | 1 |
| Shigella spp. | 3 | 0 | 0 |
| Salmonella spp. | 0 | 0 | 0 |
| Y. enterocolitica | 0 | 0 | 0 |
| Campylobacter spp. | 0 | 0 | 0 |
| Rotavirus | 3 | 1 | 3 |
| G. lamblia | 2 | 1 | 0 |
| Cryptosporidium spp. | 2 | 0 | 0 |
Only the E. coli strains isolated from the stools as a single potential enteropathogenic agent were studied. Six isolated colonies of E. coli from 65 cases (390 strains) were tested for adherence to HEp-2 cells. Four E. coli isolates were lost during storage. Thus, a total of 386 E. coli strains were independently assayed for adherence to HEp-2 cells.
Two infants with acute diarrhea had simultaneous infections due to E. coli isolates with different adherence patterns: one patient was infected with LA and AA pattern strains, and one was infected with LA and LAL pattern strains. One infant with persistent diarrhea was infected with AA and DA pattern strains. These three patients were excluded from the analysis of the association with diarrhea.
Twenty-eight (35%) of eighty fecal specimens yielded E. coli adherent to HEp-2 cells. LA was the most frequent pattern among isolates (11.2%), followed by LAL (10.0%), DA (8.7%), and AA (5.0%) (Table 3).
TABLE 3.
Adhesion pattern of E. coli strains isolated from patients and controls
| E. coli adhesion pattern | Total (n = 80) | No. (%) of cases:
|
||
|---|---|---|---|---|
| With diarrhea (n = 40) | Controls (n = 40) | Pa | ||
| LA | 9 (11.2) | 9 (22.5) | 0 | 0.001 |
| LAL | 8 (10.0) | 7 (17.5) | 1 (2.5) | 0.028 |
| DA | 7 (8.7) | 4 (10.0) | 3 (7.5) | 0.50 |
| AA | 4 (5.0) | 4 (10.0) | 0 | 0.057 |
Fishers exact test.
Strains with LA were significantly associated with diarrhea (9 of 40 [22.5%] versus 0 of 40 [0.0%]; P = 0.001). The prevalence of these strains was similar in children with acute and children with persistent diarrhea (Table 4). E. coli that showed an AA to HEp-2 cells were only identified in stool cultures from children with diarrhea (4 of 40 [10.0%]; P = 0.057). These strains were found both in children with acute and persistent diarrhea. The proportion of strains with DA in culture cells from children with diarrhea was similar to that in children without diarrhea (P = 0.50). Strains with the LAL pattern were found with significantly higher frequency (P = 0.028) in cultures from children with diarrhea (7 of 40 [17.5%]) than in controls (1 of 40 [2.5%]). The number of strains in children with acute diarrhea was similar to that in children with persistent diarrhea.
TABLE 4.
O:H types and adherence patterns of E. coli isolated from infants with acute or persistent diarrhea and from controls
| Adherence pattern | O:H typea | No. of positive infants
|
||
|---|---|---|---|---|
| Acute | Persistent | Control | ||
| LA | O55:H− | 2 | ||
| O86:H34 | 1 | |||
| O111:H− | 1 | |||
| O119:H6 | 2 | 2 | ||
| OND:H45 | 1 | |||
| LAL | O26:H− | 1 | ||
| O111:H− | 1 | 1 | ||
| O127:HND | 1 | 1 | ||
| O128ab:H− | 1 | |||
| O128ab:H35 | 1 | |||
| OND:H4 | 1 | |||
| AA | O44:H18 | 1 | ||
| O78:H2 | 1 | |||
| OND:H26 | 1 | |||
| OND:H16 | 1 | |||
| DA | O158:H4 | 1 | ||
| OND:H2 | 1 | |||
| OND:H7 | 1 | |||
| OND:HND | 2 | 1 | 1 | |
ND, not determined.
Table 4 presents the O:H types of the E. coli adherence patterns isolated from the patients and controls. An association between classical serotypes and the LA pattern was observed, since 50.0% (9 of 18) of the classical serogroups presented in Table 2 showed the LA pattern. The O:H EPEC serotypes were O55:H−, O86:H34, O111:H−, and O119:H6. Only one strain with the LA pattern did not belong to the classical serogroup, namely, OND:H45. The LAL pattern was found in strains of the following serotypes: O26:H−, O111:H−, O127:HND, O128ab:H−, O128ab:H35, and OND:H4. These serotypes, which (except for OND:H4) belong to the classic serogroups, were detected in patients and in one control. On the other hand, there was no association between classic O serotypes and AA or DA. The AA pattern was associated with serotypes O44:H18, O78:H2, OND:H26, and OND:H16, and the DA pattern was associated with serotypes O158:H4, OND:H2, OND:H7, and OND:HND.
All E. coli strains were tested for hybridization with the eaeA, EAF, EAEC, and DAEC DNA probes (Table 5).
TABLE 5.
Relationship between hybridization with DNA probes for adherence-related sequences and patterns of adherence to HEp-2 cells of E. coli isolated from patients and controls
| Adherence pattern (no. of strains)a | No. of isolates that hybridized with DNA probe:
|
||||
|---|---|---|---|---|---|
| eaeA | EAF | EAEC | daaC | AIDA-I | |
| LA (39) | 39 | 39 | 0 | 0 | 0 |
| LAL (23) | 23 | 0 | 0 | 0 | 0 |
| AA (15) | 0 | 0 | 15 | 0 | 0 |
| DA (19) | 0 | 0 | 0 | 0 | 0 |
| NA (290) | 0 | 0 | 0 | 0 | 0 |
Total of 386 strains. NA, nonadherent.
EPEC was analyzed on the basis of EAF and eaeA probe positives. By using the eaeA DNA probe, which is specific for both atypical and typical EPEC, 62 strains were found to be positive. Further characterization of these strains was achieved by hybridization with the EAF DNA probe. Only 39 of these hybridized with this probe. These typical EPEC strains showed an LA pattern. The other 23 strains that hybridized with the eaeA and were EAF negative showed an LAL pattern.
Detection of EAEC was performed by hybridization with the EAEC DNA probe. All isolates that showed an aggregative phenotype reacted with the EAEC DNA probe.
Two DNA probes, daaC and AIDA-I, were used to detect DAEC isolates. None of the 19 DAEC strains isolated reacted with these probes.
DISCUSSION
This study prospectively examined the association of E. coli HEp-2 adherence patterns with the duration of diarrhea. Several epidemiological studies examining the role of the different enteroadherent E. coli in children with diarrhea have been performed, mostly in developing countries (8, 15, 21). However, few investigations that have been published on this topic indicated the type and duration of diarrhea caused by these strains (1, 9).
The present study was conducted in the emergency room of the São Paulo Hospital and was designed to isolate and characterize E. coli HEp-2 adherence patterns in children with acute or persistent diarrhea.
Our results support the evidence from prospective case-control studies showing an association between strains with the LA and AA patterns and diarrhea (9, 21). We also found a strong association between strains with LAL pattern and diarrhea. There was no association between strains with DA and diarrhea.
We have also confirmed that the LA pattern is characteristically associated with classical O serotypes of EPEC and diarrhea. A total of 88.9% of the LA EPEC strains isolated from patients belonged to classic O serogroups. The most common serogroups found in our study were O55 and O119, which are also common in Mexico (9) and Chile (31). LAL pattern was also found in strains belonging to classical serotypes. This pattern was not observed in tests with two incubation periods (30 min and 3 h). This could explain why some strains of EPEC serogroups isolated from patients with diarrhea did not show any pattern of adherence (40). On the other hand, EAEC and DAEC strains belonging to classic O serogroups were infrequently isolated. One EAEC strain was O44:H18, a proven pathogen (36, 44). This strain was isolated from a child with persistent diarrhea.
There were no differences in the clinical presentations of children infected with the different enteroadherent E. coli. Also, the 16 children who did not show any adherence pattern did not differ in their clinical presentations from children who harbored enteroadherent E. coli.
The typical EPEC strain continues to be an important cause of diarrhea in children up to 1 year (29, 38). In our study, nine strains adhered with an LA pattern and hybridized with the EAF probe. These strains were isolated in patients with acute or persistent diarrhea.
Several outbreaks have implicated atypical EPEC as the causative agent (23, 42, 45), while other studies have not recovered atypical EPEC more frequently from diarrheal patients than from controls (11, 19, 20, 31). In the present study these strains showed an LAL pattern and were significantly associated with diarrhea. The mechanism by which E. coli LAL bacteria cause diarrhea is not known yet. These strains present only the eaeA genes and do not possess the EAF factor. However, volunteer-based studies performed by Levine et al. (30) have shown that JPN15 (a plasmid-cured EPEC strain) caused diarrhea but did so less severely than the wild-type strain. Thus, it is possible that atypical EPEC lacking plasmid-encoded determinants also has the ability to cause diarrhea.
Four E. coli strains characterized as EAEC were detected in this study. EAEC strains have been implicated as etiologic agents of infantile diarrhea in developing countries (3, 6, 18), most commonly among children with persistent diarrhea. In this study, EAEC strains were detected in children with acute or persistent diarrhea. These strains were equally detected by HEp-2 cell adherence pattern and by hybridization with the EAEC probe. Despite the fact that our results did not reach statistical significance (P = 0.058), we believe that this tendency indicates an effective enteropathogenic role of EAEC in diarrhea in our patients.
Recent studies have implicated DAEC strains as an agent of diarrhea (17, 22, 25), while other studies have not recovered DAEC strains more frequently from patients than from controls (9, 15). In the present study, there was no correlation between DAEC strains and diarrhea. All seven DAEC strains isolated in this study were defined only phenotypically by determination of their pattern of adherence to HEp-2 cells. These strains did not hybridize with the daaC and AIDA-I probes.
In conclusion, this study reafirms the importance of the typical EPEC as the most prevalent bacterial etiologic agent in infants under 1 year of age in developing countries. Moreover, we could also report a potential new enteropathogenic group of E. coli as a cause of both acute and persistent diarrhea. The existence of the LAL adherence pattern observed in patients with diarrhea may indicate a new mechanism of production of the disease.
Further studies are necessary to confirm the present observation as well as to elucidate the pathogenesis of this new type of enteric infection.
ACKNOWLEDGMENTS
This work was supported by Financiadora de Estudos e Projetos (FINEP), the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP).
REFERENCES
- 1.Albert M J, Faruque S M, Faruque A S, Neogi P K, Ansaruzzaman M, Bhuiyan N A, Alam K, Akbar M S. Controled study of Escherichia coli diarrheal infections in Bangladesh children. J Clin Microbiol. 1995;33:973–977. doi: 10.1128/jcm.33.4.973-977.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baldini M M, Kaper J B, Levine M M, Candy D C A, Moon H W. Plasmid-mediated adhesion of enteropathogenic Escherichia coli. J Pediatr Gastroenterol Nutr. 1983;2:534–538. doi: 10.1097/00005176-198302030-00023. [DOI] [PubMed] [Google Scholar]
- 3.Baqui A H, Sack R B, Black R E, Haider K, Hossain A, Alim A R M A, Yunus M, Chowdhury H R, Siddique A K. Enteropathogens associated with acute and persistent diarrhea in Bangladesh children <5 years of age. J Infect Dis. 1992;166:792–796. doi: 10.1093/infdis/166.4.792. [DOI] [PubMed] [Google Scholar]
- 4.Baudry B, Savarino S J, Vial P, Kaper J B, Levine M M. A sensitive and specific DNA probe to identify enteroaggregative E. coli, a recently discovered diarrheal pathogen. J Infect Dis. 1990;161:1249–1251. doi: 10.1093/infdis/161.6.1249. [DOI] [PubMed] [Google Scholar]
- 5.Benz I, Schmidt M A. Cloning and expression of an adhesin (AIDA-I) involved in diffuse adherence of enteropathogenic Escherichia coli. Infect Immun. 1989;57:1506–1511. doi: 10.1128/iai.57.5.1506-1511.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhan M K, Raj P, Levine M M, Kaper J B, Bhandari N, Srivastava R, Kumar R, Sazawal S. Enteroaggregative Escherichia coli associated with persistent diarrhea in a cohort of rural children in India. J Infect Dis. 1989;159:1061–1064. doi: 10.1093/infdis/159.6.1061. [DOI] [PubMed] [Google Scholar]
- 7.Bilge S S, Clausen C R, Lau W, Moseley S L. Molecular characterization of a fimbrial adhesin, F1845, mediating diffuse adherence of diarrhea-associated Escherichia coli to HEp-2 cells. J Bacteriol. 1989;171:4281–4289. doi: 10.1128/jb.171.8.4281-4289.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biswas R, Nelson E A S, Lewindon P J, Lyon D J, Sullivan P B, Echeverria P. Molecular epidemiology of Escherichia coli diarrhea in children in Hong Kong. J Clin Microbiol. 1996;34:3233–3234. doi: 10.1128/jcm.34.12.3233-3234.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cravioto A, Tello A, Navarro A, Ruiz J, Villafan H, Uribe F, Eslava C. Association of Escherichia coli HEp-2 adherence patterns with type and duration of diarrhea. Lancet. 1991;337:262–264. doi: 10.1016/0140-6736(91)90868-p. [DOI] [PubMed] [Google Scholar]
- 10.Echeverria P, Taylor D N, Bettelheim K A, Chatkacomorakot A, Changchawalit S, Thongcharoen A, Leksomboon U. HeLa cell-adherent enteropathogenic Escherichia coli in children under 1 year of age in Thailand. J Clin Microbiol. 1987;25:1472–1475. doi: 10.1128/jcm.25.8.1472-1475.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Echeverria P, Orskov F, Orskov I, Knutton S, Scheutz F, Brown J E, Lexomboon U. Attaching and effacing enteropathogenic Escherichia coli as a cause of infantile diarrhea in Bangkok. J Infect Dis. 1991;164:550–554. doi: 10.1093/infdis/164.3.550. [DOI] [PubMed] [Google Scholar]
- 12.Echeverria P, Hoge C W, Bodhidatta L, Tungtaem C, Hermann J, Imlarp S, Tamura K. Etiology of diarrhea in a rural community in Western Thailand: importance of enteric viruses and enterovirulent Escherichia coli. J Infect Dis. 1994;169:916–919. doi: 10.1093/infdis/169.4.916. [DOI] [PubMed] [Google Scholar]
- 13.Edwards P R, Ewing W H. Identification of Enterobacteriaceae. 3rd ed. Minneapolis, Minn: Burgess Publishing Co.; 1972. [Google Scholar]
- 14.Flewett T H, Arias C F, Venecas A. Comparative evaluation of the WHO and DAKOPATTS enzyme-linked immunoassay kits for rotavirus detection. Bull W H O. 1989;67:369–374. [PMC free article] [PubMed] [Google Scholar]
- 15.Forestier C, Meyer M, Favre-Bonte S, Rich C, Malpuech G, Le Bouguenec C, Sirot J, Joly B, De Champs C. Enteroadherent Escherichia coli and diarrhea in children: a prospective case-control study. J Clin Microbiol. 1996;34:2897–2903. doi: 10.1128/jcm.34.12.2897-2903.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gicquelais K G, Baldini M M, Martinez J, Maggi L, Martin W C, Prado V, Kaper J B, Levine M M. Practical and economical method for using biotinylated DNA probes with bacterial colony blots to identify diarrhea-causing Escherichia coli. J Clin Microbiol. 1990;28:2485–2490. doi: 10.1128/jcm.28.11.2485-2490.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Girón J A, Jones T, Millán-Velasco F, Castro-Munoz E, Zarate L, Fry J, Frankel G, Moseley S L, Baudry B, Kaper J B, Schoolnik G K, Riley L W. Diffuse-adhering Escherichia coli (DAEC) as a putative cause of diarrhea in Mayan children in Mexico. J Infect Dis. 1991;163:507–513. doi: 10.1093/infdis/163.3.507. [DOI] [PubMed] [Google Scholar]
- 18.Gomes T A T, Blake P A, Trabulsi L R. Prevalence of Escherichia coli strains with localized, diffuse, and aggregative adherence to HeLa cells in infants with diarrhea and matched controls. J Clin Microbiol. 1989;27:266–269. doi: 10.1128/jcm.27.2.266-269.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gomes T A T, Vieira M A M, Wachsmuth I K, Blake P A, Trabulsi L R. Serotype-specific prevalence of Escherichia coli strains with EPEC adherence factor genes in infants with and without diarrhea in São Paulo, Brazil. J Infect Dis. 1989;160:131–135. doi: 10.1093/infdis/160.1.131. [DOI] [PubMed] [Google Scholar]
- 20.Gomes T A T, Rassi V, Macdonald K L, Ramos S R T S, Trabulsi L R, Vieira M A M, Guth B E C, Candeias J A N, Ivey C, Toledo M R F, Blake P A. Enteropathogens associated with acute diarrheal disease in urban infants in São Paulo, Brazil. J Infect Dis. 1991;164:331–337. doi: 10.1093/infdis/164.2.331. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez R, Marino M, Cloralt R, Pequeneze M, Schael I P. Age-specific prevalence of Escherichia coli with localized and aggregative adherence in Venezuelan infants with acute diarrhea. J Clin Microbiol. 1997;35:1103–1107. doi: 10.1128/jcm.35.5.1103-1107.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gunzburg S T, Chang B J, Elliot S J, Burke V, Gracey M. Diffuse and enteroaggregative patterns of adherence of enteric Escherichia coli isolated from aboriginal children from the Kimberley region of Western Australia. Infect Immun. 1993;167:755–758. doi: 10.1093/infdis/167.3.755. [DOI] [PubMed] [Google Scholar]
- 23.Hedberg C W, Savarino S J, Besser J M, Paulus C J, Thelen V M, Myers L J, Cameron D N, Barrett T J, Kaper J B, Osterholm M T. An outbreak of foodborne illness caused by Escherichia coli O39:NM, an agent that does not fit into the existing scheme for classifying diarrheogenic E. coli. J Infect Dis. 1997;176:1625–1628. doi: 10.1086/517342. [DOI] [PubMed] [Google Scholar]
- 24.Henricksens S A, Pohlens J F L. Staining of cryptosporidia by a modified Ziehl-Neelsen tecnique. Acta Vet Scand. 1981;22:594–596. doi: 10.1186/BF03548684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jallat C, Livrelli V, Darfeuille-Michaud A, Rich C, Joly B. Escherichia coli strains involved in diarrhea in France: high prevalence and heterogeneity of diffusely adhering strains. J Clin Microbiol. 1993;31:2031–2037. doi: 10.1128/jcm.31.8.2031-2037.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jerse A E, Jun Y, Tall B D, Kaper J B. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc Natl Acad Sci USA. 1990;87:7839–7843. doi: 10.1073/pnas.87.20.7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaper J B. Defining EPEC. Rev Microbiol. 1996;27(Suppl. 1):130–133. [Google Scholar]
- 28.Knutton S, Phillips A D, Smith H R, Gross R J, Shaw R, Warson P, Price E. Screening for enteropathogenic Escherichia coli in infants with diarrhea by the fluorescent-actin staining test. Infect Immun. 1991;59:365–371. doi: 10.1128/iai.59.1.365-371.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levine M M, Edelman R. Enteropathogenic Escherichia coli of classic serotypes associated with infant diarrhea: epidemiology and pathogenesis. Epidemiol Rev. 1984;6:31–51. doi: 10.1093/oxfordjournals.epirev.a036274. [DOI] [PubMed] [Google Scholar]
- 30.Levine M M, Nataro J P, Karch H, Baldini M M, Kaper J B, Black R E, Clements M L, O’Brien A D. The diarrheal response of humans to some classic serotypes of enteropathogenic Escherichia coli is dependent on a plasmid encoding an enteroadhesiveness factor. J Infect Dis. 1985;152:550–559. doi: 10.1093/infdis/152.3.550. [DOI] [PubMed] [Google Scholar]
- 31.Levine M M, Prado V, Robins-Browne R M, Lior H, Kaper J B, Moseley S L, Gicquelais K, Nataro J P, Vial P, Tall B. Use of DNA probes and HEp-2 cell adherence assay to detect diarrheagenic Escherichia coli. J Infect Dis. 1988;158:224–228. doi: 10.1093/infdis/158.1.224. [DOI] [PubMed] [Google Scholar]
- 32.Maas R. An improved colony hybridization method with significantly increased sensitivity for detection of single genes. Plasmid. 1983;10:296–298. doi: 10.1016/0147-619x(83)90045-8. [DOI] [PubMed] [Google Scholar]
- 33.Moon H W, Whipp S C, Argenzio R A, Levine M M, Giannella R A. Attaching and effacing of rabbit and human enteropathogenic Escherichia coli in pig and rabbit intestines. Infect Immun. 1983;41:1340–1351. doi: 10.1128/iai.41.3.1340-1351.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nataro J P, Baldini M M, Kaper J B, Black R E, Bravo N, Levine M M. Detection of an adherence factor of enteropathogenic Escherichia coli with a DNA probe. J Infect Dis. 1985;152:560–565. doi: 10.1093/infdis/152.3.560. [DOI] [PubMed] [Google Scholar]
- 35.Nataro J P, Kaper J B, Robins-Browne R, Prado V, Vial P, Levine M M. Patterns of adherence of diarrheagenic Escherichia coli to HEp-2 cells. Paediatr Infect Dis J. 1987;6:829–831. doi: 10.1097/00006454-198709000-00008. [DOI] [PubMed] [Google Scholar]
- 36.Nataro J P, Deng Y, Cookson S, Cravioto A, Savarino S J, Guers L D, Levine M M, Tacket C O. Heterogeneity of enteroaggregative Escherichia coli virulence demonstrated in volunteers. J Infect Dis. 1995;171:465–468. doi: 10.1093/infdis/171.2.465. [DOI] [PubMed] [Google Scholar]
- 37.Nataro J P, Kaper J B. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998;11:142–201. doi: 10.1128/cmr.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robins-Browne R M. Traditional enteropathogenic Escherichia coli of infantile diarrhea. Rev Infect Dis. 1987;9:28–53. doi: 10.1093/clinids/9.1.28. [DOI] [PubMed] [Google Scholar]
- 39.Scaletsky I C A, Silva M L M, Trabulsi L R. Distinctive patterns of adherence of enteropathogenic Escherichia coli to HeLa cells. Infect Immun. 1984;45:534–536. doi: 10.1128/iai.45.2.534-536.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scaletsky I C A, Silva M L M, Toledo M R F, Davis B R, Blake P A, Trabulsi L R. Correlation between adherence to HeLa cells and serogroups, serotypes, and bioserotypes of Escherichia coli. Infect Immun. 1985;49:528–532. doi: 10.1128/iai.49.3.528-532.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scaletsky I C A, Pelayo J S, Giraldi R, Rodrigues J, Pedroso M Z, Trabulsi L R. EPEC adherence to HEp-2 cells. Rev Microbiol. 1996;27(Suppl. 1):58–62. [Google Scholar]
- 42.Scotland S M, Willshaw G A, Smith H R, Said B, Stokes N, Rowe B. Virulence properties of Escherichia coli strains belonging to serogroups O26, O55, O111 and O128 isolated in the United Kingdom in 1991 from patients with diarrhea. Epidemiol Infect. 1993;111:429–438. doi: 10.1017/s0950268800057150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toledo M R F, Fontes C F, Trabulsi L R. MILi-um meio para realização dos testes de motilidade, indol e lisina descarboxilase. Rev Microbiol. 1982;13:230–235. [Google Scholar]
- 44.Vial P A, Robins-Browne R, Lior H, Prado V, Kaper J B, Nataro J P, Maneval D, Elsayed A, Levine M M. Characterization of enteroadherent-aggregative E. coli, a putative agent of diarrheal disease. J Infect Dis. 1988;158:70–79. doi: 10.1093/infdis/158.1.70. [DOI] [PubMed] [Google Scholar]
- 45.Viljanen M K, Peltola T, Junnila S Y T, Olkkonen L, Jarvinen H, Kuistila M, Huovinen P. Outbreak of diarrhea due to Escherichia coli O111:B4 in school children and adults: association of Vi antigen-like reactivity. Lancet. 1990;336:831–834. doi: 10.1016/0140-6736(90)92337-h. [DOI] [PubMed] [Google Scholar]