Abstract
The isolation of a highly purified phosphoprotein, previously named protein A, from human parotid saliva is described. This protein has an unusually high amount of glycine, proline and dicarboxylic amino acids. Together these amino acids account for 80% of all residues. The protein contains 1.9mol of P/mol of protein, probably as phosphate in an ester linkage to serine, and about 0.5% carbohydrate, but no hexosamine. The N-terminal is blocked and the following C-terminal sequence is proposed: -Aal-Asp-Ser-Gln-Gly-Arg-Arg. The sioelectric point is 4.43. The molecular weight of the protein determined by ultracentrifugation is 9900 and from chemical analyses 11000. Circular-dichrosim and nuclea-magnetic-resonance spectra indicate the absence of polyproline and triple-helical-collagen-like structure for the protein. There is little restriction on the orientation of the single phenylalanine residue in the protein., but there is also an indication of conformational restraint in the protein.
Full text
PDF


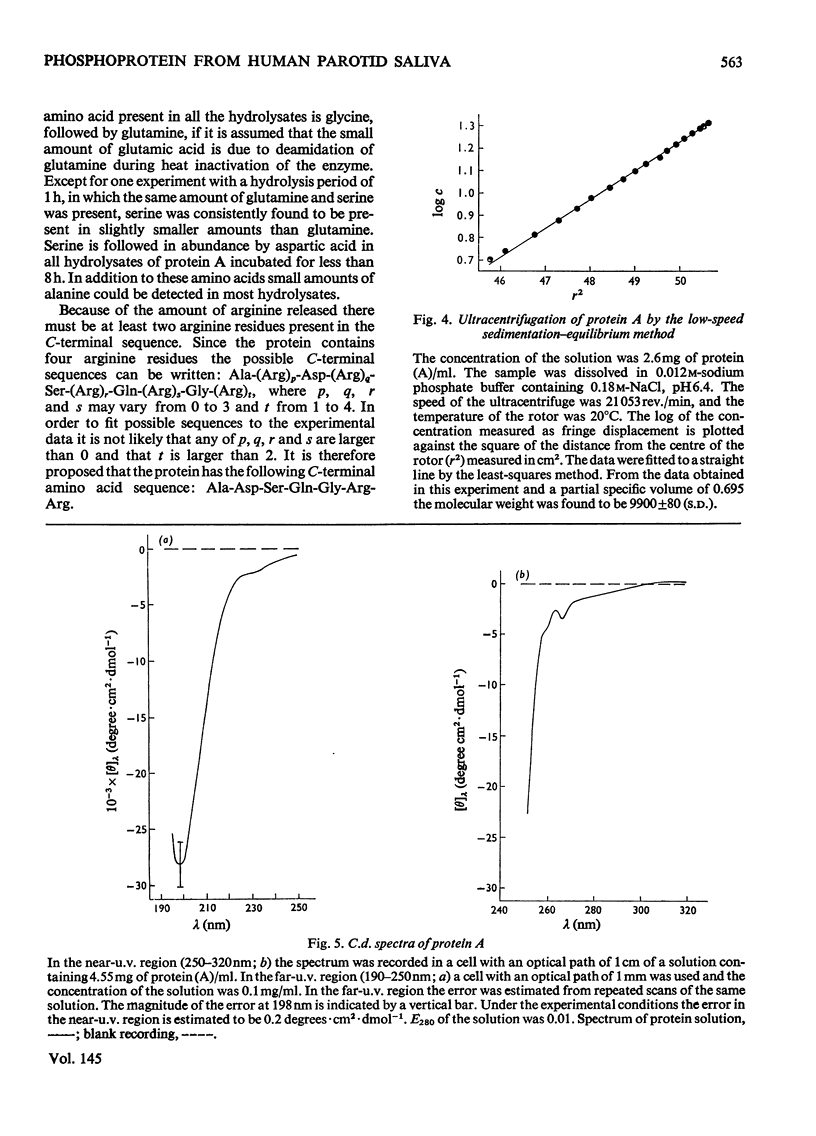
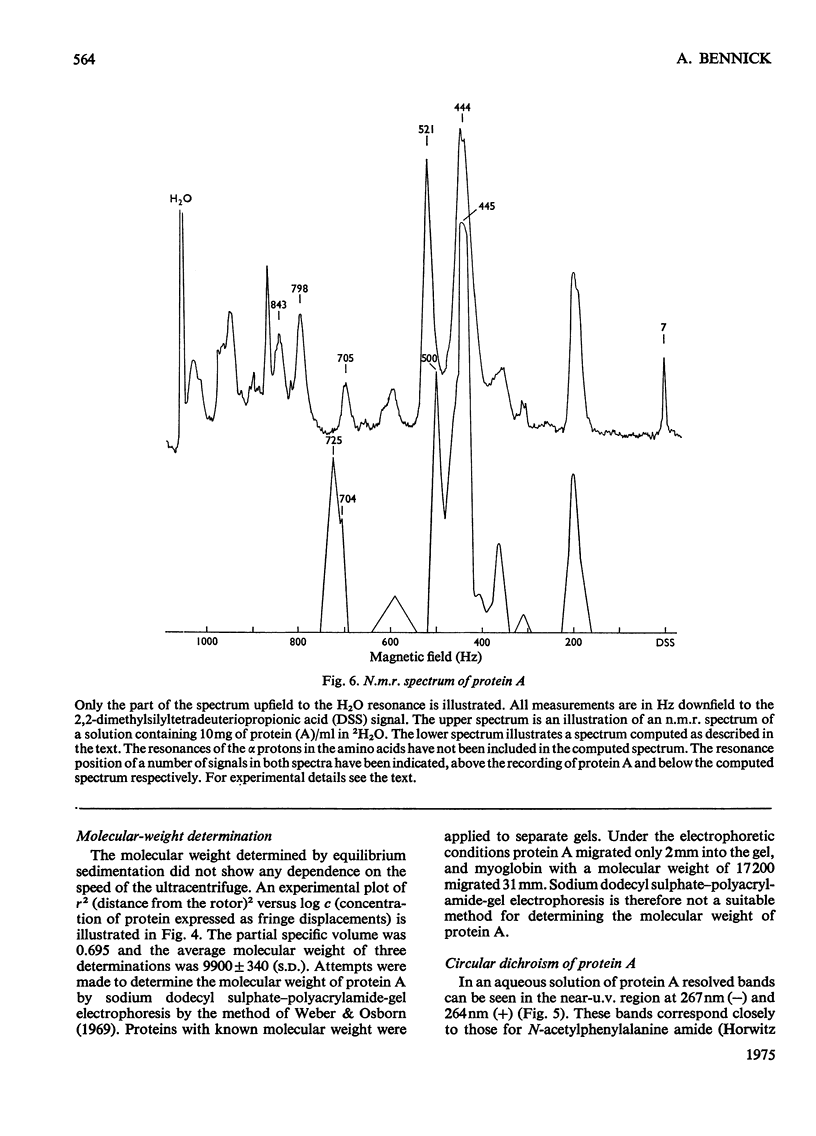



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anwar R. A. Comparison of elastins from various sources. Can J Biochem. 1966 Jun;44(6):725–734. doi: 10.1139/o66-090. [DOI] [PubMed] [Google Scholar]
- Armstrong W. G. Characterisation studies on the specific human salivary proteins adsorbed in vitro by hydroxyapatite. Caries Res. 1971;5(3):215–227. doi: 10.1159/000259749. [DOI] [PubMed] [Google Scholar]
- Azen E. A., Oppenheim F. G. Genetic polymorphism of proline-rich human salivary proteins. Science. 1973 Jun 8;180(4090):1067–1069. doi: 10.1126/science.180.4090.1067. [DOI] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Bennick A., Connell G. E. Purification and partial characterization of four proteins from human parotid saliva. Biochem J. 1971 Jul;123(3):455–464. doi: 10.1042/bj1230455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boat T. F., Wiesman U. N., Pallavicini J. C. Purification and properties of the calcium-precipitable protein in submaxillary saliva of normal and cystic fibrosis subjects. Pediatr Res. 1974 May;8(5):531–539. doi: 10.1203/00006450-197405000-00001. [DOI] [PubMed] [Google Scholar]
- Brown F. R., 3rd, Di Corato A., Lorenzi G. P., Blout E. R. Synthesis and structural studies of two collagen analogues: poly (L-prolyl-L-seryl-glycyl) and poly (L-prolyl-L-alanyl-glycyl). J Mol Biol. 1972 Jan 14;63(1):85–99. doi: 10.1016/0022-2836(72)90523-2. [DOI] [PubMed] [Google Scholar]
- Chrambach A., Reisfeld R. A., Wyckoff M., Zaccari J. A procedure for rapid and sensitive staining of protein fractionated by polyacrylamide gel electrophoresis. Anal Biochem. 1967 Jul;20(1):150–154. doi: 10.1016/0003-2697(67)90272-2. [DOI] [PubMed] [Google Scholar]
- Deber C. M., Bovey F. A., Carver J. P., Blout E. R. Nuclear magnetic resonance evidence for cis-peptide bonds in proline oligomers. J Am Chem Soc. 1970 Oct 21;92(21):6191–6198. doi: 10.1021/ja00724a016. [DOI] [PubMed] [Google Scholar]
- Dorrington K. J., Smith B. R. Conformational changes accompanying the dissociation and association of immunoglobulin-G subunits. Biochim Biophys Acta. 1972 Mar 15;263(1):70–81. doi: 10.1016/0005-2795(72)90160-2. [DOI] [PubMed] [Google Scholar]
- Eggert F. M., Allen G. A., Burgess R. C. Amelogenins. Purification and partial characterization of proteins from developing bovine dental enamel. Biochem J. 1973 Mar;131(3):471–484. doi: 10.1042/bj1310471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLEISCHMAJER R., FISHMAN L. AMINO-ACID COMPOSITION OF HUMAN DERMAL COLLAGEN. Nature. 1965 Jan 16;205:264–266. doi: 10.1038/205264a0. [DOI] [PubMed] [Google Scholar]
- Fasman G. D., Hoving H., Timasheff S. N. Circular dichroism of polypeptide and protein conformations. Film studies. Biochemistry. 1970 Aug 18;9(17):3316–3324. doi: 10.1021/bi00819a005. [DOI] [PubMed] [Google Scholar]
- Hay D. I. The interaction of human parotid salivary proteins with hydroxyapatite. Arch Oral Biol. 1973 Dec;18(12):1517–1529. doi: 10.1016/0003-9969(73)90127-1. [DOI] [PubMed] [Google Scholar]
- Hermodson M. A., Ericsson L. H., Titani K., Neurath H., Walsh K. A. Application of sequenator analyses to the study of proteins. Biochemistry. 1972 Nov 21;11(24):4493–4502. doi: 10.1021/bi00774a011. [DOI] [PubMed] [Google Scholar]
- Horwitz J., Strickland E. H., Billups C. Analysis of vibrational structure in the near-ultraviolet circular dichroism and absorption spectra of phenylalanine and its derivatives. J Am Chem Soc. 1969 Jan 1;91(1):184–190. doi: 10.1021/ja01029a034. [DOI] [PubMed] [Google Scholar]
- Jones S. R., Hofmann T. Penicillocarboxypeptidase-S, a nonspecific SH-dependent exopeptidase. Can J Biochem. 1972 Dec;50(12):1297–1310. doi: 10.1139/o72-175. [DOI] [PubMed] [Google Scholar]
- Kanaya S., Fujimoto D. Letter: Fragments of polyproline-like structure from a structural protein, cuticlin. J Mol Biol. 1973 Dec 15;81(3):415–418. doi: 10.1016/0022-2836(73)90151-4. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y., Kyogoku Y. Proton magnetic resonance of the triple helix of poly(prolylprolylglycyl)n with defined degree of polymerization. J Mol Biol. 1973 Dec 15;81(3):337–347. doi: 10.1016/0022-2836(73)90145-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levine M. J., Ellison S. A., Bahl O. P. The isolation from human parotid saliva and partial characterization of the protein core of a major parotid glycoprotein. Arch Oral Biol. 1973 Jul;18(7):827–837. doi: 10.1016/0003-9969(73)90053-8. [DOI] [PubMed] [Google Scholar]
- Madison V., Schellman J. Location of proline derivatives in conformational space. II. Theoretical optical activity. Biopolymers. 1970;9(5):569–588. doi: 10.1002/bip.1970.360090503. [DOI] [PubMed] [Google Scholar]
- Matsubara H., Sasaki R. M. High recovery of tryptophan from acid hydrolysates of proteins. Biochem Biophys Res Commun. 1969 Apr 29;35(2):175–181. doi: 10.1016/0006-291x(69)90263-0. [DOI] [PubMed] [Google Scholar]
- McDonald C. C., Phillips W. D. Proton magnetic resonance spectra of proteins in random-coil configurations. J Am Chem Soc. 1969 Mar 12;91(6):1513–1521. doi: 10.1021/ja01034a039. [DOI] [PubMed] [Google Scholar]
- Oppenheim F. G., Hay D. I., Franzblau C. Proline-rich proteins from human parotid saliva. I. Isolation and partial characterization. Biochemistry. 1971 Nov;10(23):4233–4238. doi: 10.1021/bi00799a013. [DOI] [PubMed] [Google Scholar]
- Percy M. E., Buchwald B. M. A manual method of sequential Edman degradation followed by dansylation for the determination of protein sequences. Anal Biochem. 1972 Jan;45(1):60–67. doi: 10.1016/0003-2697(72)90007-3. [DOI] [PubMed] [Google Scholar]
- SEIFTER S., DAYTON S. The estimation of glycogen with the anthrone reagent. Arch Biochem. 1950 Jan;25(1):191–200. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]