Abstract
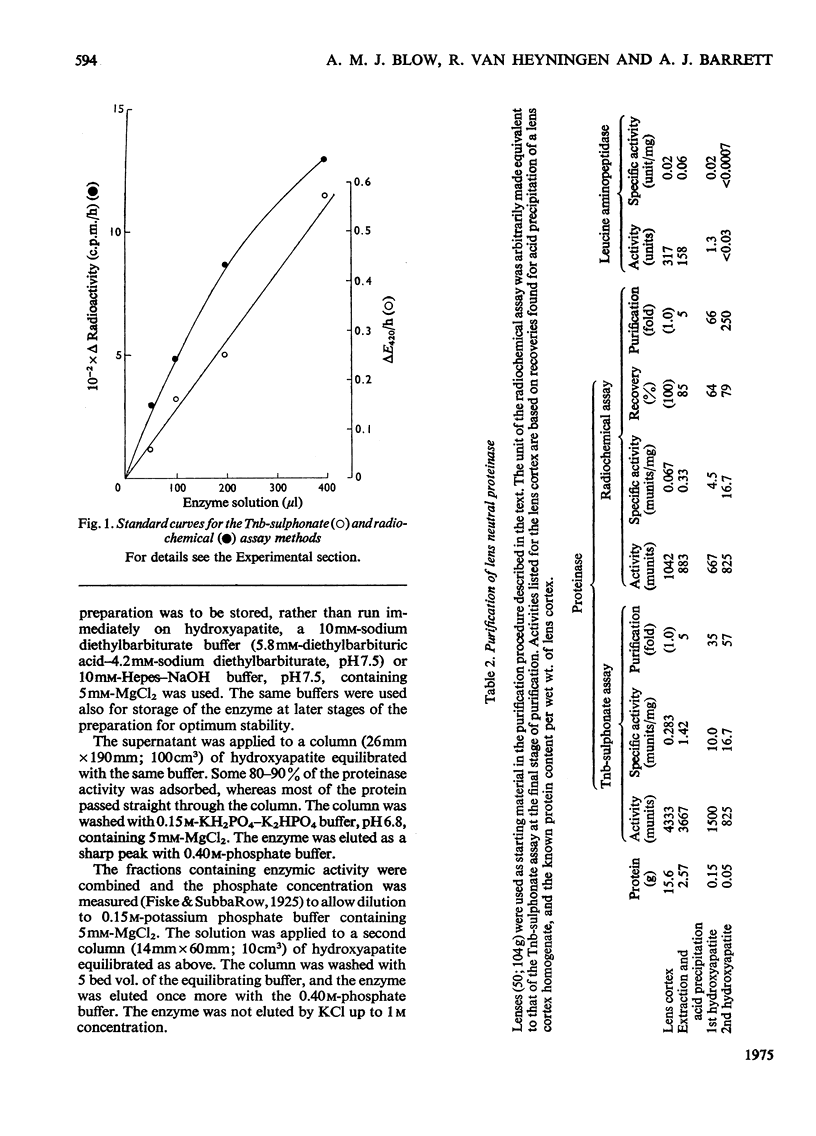
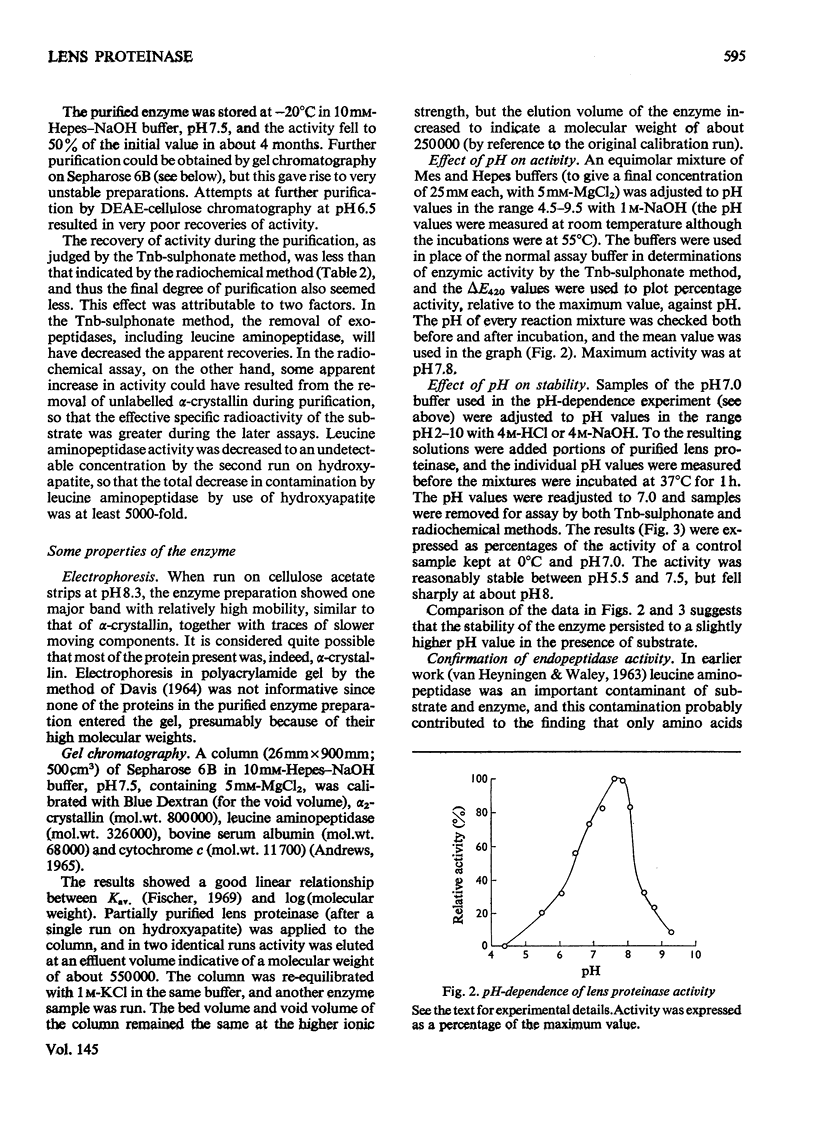
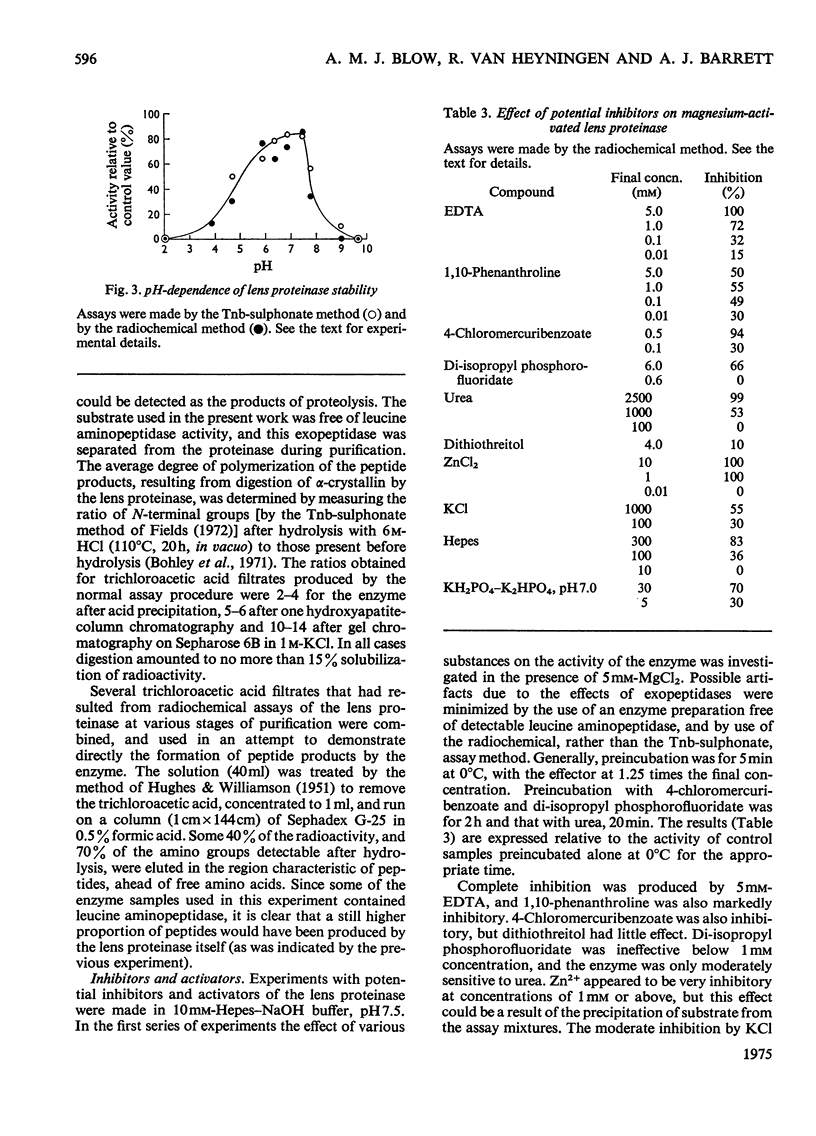
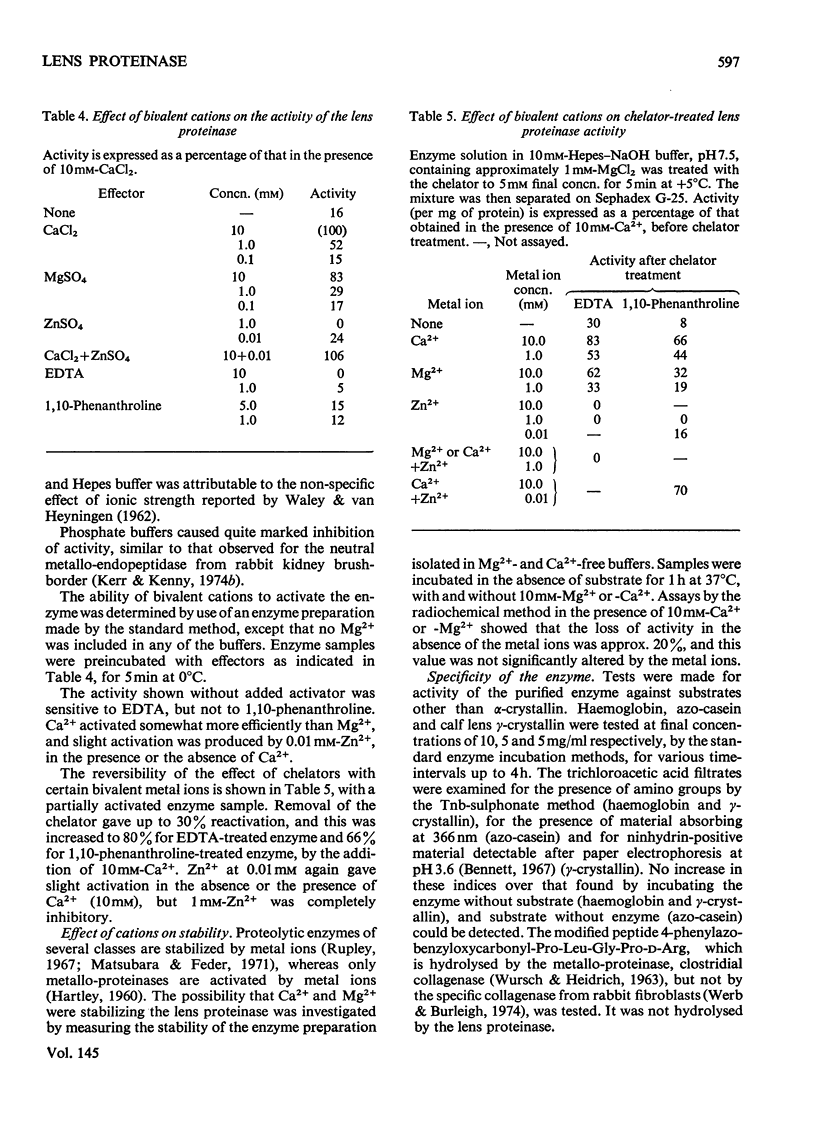
1. Two new assay methods were developed for the lens proteinase. In both, the substrate was alpha2-crystallin (a major lens protein); in the first method, the products were detected by reaction with trinitrobenzenesulphonate in the presence of SO32-, whereas in the second method, 3H-labelled substrate was used, and the products were detected as radioactivity soluble in trichloroacetic acid. 2. The neutral proteinase from bovine lens was partially purified by extraction of the lens at pH5.0 and column chromatography on hydroxyapatite and Sepharose 6B gel. 3. The purified enzyme had no detectable activity against haemoglobin, azo-casein or gamma-crystallin under optimum conditions for alpha2-crystallin. 4. The enzyme showed greatest activity and stability at pH7.5. It was reversibly inhibited by EDTA and 1,10-phenanthroline, and activated by Ca2+ and Mg2+. 5. Molecular weights obtained for the enzyme by chromatography on Sepharose 6B were approx. 500,000 in buffer of I = 0.02, and 250,000 at I = 1.02. 6. The properties of the purified lens proteinase are such as to suggest that this enzyme could account for the entire endopeptidase activity of the lens.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J. Cathepsin D. Purification of isoenzymes from human and chicken liver. Biochem J. 1970 Apr;117(3):601–607. doi: 10.1042/bj1170601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- FRANCOIS J., RABAEY M., WIEME R. J. New method for fractionation of lens proteins. AMA Arch Ophthalmol. 1954 Apr;53(4):481–486. doi: 10.1001/archopht.1955.00930010483004. [DOI] [PubMed] [Google Scholar]
- Fürst P., Jonsson A., Josephson B., Philipson B. Recovery of 15N in liver, muscle tissue, and in the lens of the eye after administration of 15N-ammonium acetate to rats and rabbits. Scand J Clin Lab Invest. 1973 Mar;31(2):213–217. doi: 10.3109/00365517309084312. [DOI] [PubMed] [Google Scholar]
- HANSON H. Proteolytic enzymes. Exp Eye Res. 1962 Jun;1:468–479. doi: 10.1016/s0014-4835(62)80040-2. [DOI] [PubMed] [Google Scholar]
- HARTLEY B. S. Proteolytic enzymes. Annu Rev Biochem. 1960;29:45–72. doi: 10.1146/annurev.bi.29.070160.000401. [DOI] [PubMed] [Google Scholar]
- HUGHES D. E., WILLIAMSON D. H. Removal of acid by trioctylamine from samples for microbiological assay. Biochem J. 1951 Apr;48(4):487–490. doi: 10.1042/bj0480487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall T. C., Cocking E. C. High-efficiency liquid-scintillation counting of 14C-labelled material in aqueous solution and determination of specific activity of labelled proteins. Biochem J. 1965 Sep;96(3):626–633. doi: 10.1042/bj0960626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E. D., Jr, Krane S. M. Collagenases (first of three parts). N Engl J Med. 1974 Sep 12;291(11):557–563. doi: 10.1056/NEJM197409122911105. [DOI] [PubMed] [Google Scholar]
- Harris E. D., Jr, Krane S. M. Collagenases (second of three parts). N Engl J Med. 1974 Sep 19;291(12):605–609. doi: 10.1056/NEJM197409192911205. [DOI] [PubMed] [Google Scholar]
- Harris E. D., Jr, Krane S. M. Collagenases (third of three parts). N Engl J Med. 1974 Sep 26;291(13):652–661. doi: 10.1056/NEJM197409262911305. [DOI] [PubMed] [Google Scholar]
- Kaplan A. The determination of urea, ammonia, and urease. Methods Biochem Anal. 1969;17:311–324. doi: 10.1002/9780470110355.ch7. [DOI] [PubMed] [Google Scholar]
- Kerr M. A., Kenny A. J. The molecular weight and properties of a neutral metallo-endopeptidase from rabbit kidney brush border. Biochem J. 1974 Mar;137(3):489–495. doi: 10.1042/bj1370489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr M. A., Kenny A. J. The purification and specificity of a neutral endopeptidase from rabbit kidney brush border. Biochem J. 1974 Mar;137(3):477–488. doi: 10.1042/bj1370477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Swanson A. A., Nichols J. T. Human senile cataractous lens protease. Isolation and some chemical characteristics. Biochem J. 1971 Nov;125(2):575–584. doi: 10.1042/bj1250575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN HEYNINGEN R., WALEY S. G. Neutral proteinases in the lens. 2. Partial purification and properties. Biochem J. 1963 Jan;86:92–101. doi: 10.1042/bj0860092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALEY S. G., VAN HEYNINGEN R. Neutral proteinases in the lens. Biochem J. 1962 May;83:274–283. doi: 10.1042/bj0830274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WUENSCH E., HEIDRICH H. G. ZUR QUANTITATIVEN BESTIMMUNG DER KOLLAGENASE. Hoppe Seylers Z Physiol Chem. 1963;333:149–151. doi: 10.1515/bchm2.1963.333.1.149. [DOI] [PubMed] [Google Scholar]
- Werb Z., Burleigh M. C. A specific collagenase from rabbit fibroblasts in monolayer culture. Biochem J. 1974 Feb;137(2):373–385. doi: 10.1042/bj1370373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. W., Fulhorst H. W. Regional differences in protein synthesis within the lens of the rat. Invest Ophthalmol. 1966 Jun;5(3):288–297. [PubMed] [Google Scholar]



