Abstract
The design of an effective vaccine against Plasmodium falciparum, the most deadly malaria parasite of humans, requires a careful definition of the epitopes and the immune responses involved in protection. Liver-stage antigen 1 (LSA-1) is specifically expressed during the hepatic stage of P. falciparum and elicits cellular and humoral immune responses in naturally exposed individuals. We report here that interleukin-10 (IL-10) production in response to LSA-1 predicts resistance to P. falciparum after eradication therapy. Resistance was not related to gamma interferon or tumor necrosis factor alpha production. This is the first report that human IL-10 responses are associated with resistance after eradication therapy, and our findings support the inclusion of LSA-1 in a vaccine against malaria.
Falciparum malaria kills over 1 million individuals in sub-Saharan Africa each year, and the development of an effective malaria vaccine remains a public health priority. Plasmodium falciparum is inoculated by mosquitoes into the human bloodstream as a motile sporozoite that requires development within hepatocytes prior to infecting erythrocytes. Although severe disease occurs during the erythrocytic phase of infection, a protective vaccine could limit parasite growth at any stage of development.
Because cytotoxic T lymphocytes (CTLs) and gamma interferon (IFN-γ) can kill liver-stage malaria parasites in animal models (26, 28, 31, 32, 34), human vaccines designed to control the liver-stage of P. falciparum have focused on eliciting similar responses. Liver-stage antigen 1 (LSA-1) (15), a 200-kDa antigen that accumulates as flocculent material in the parasitophorous vacuole of infected hepatocytes (17), is a leading candidate for inclusion in a P. falciparum vaccine. LSA-1 contains several B- and T-cell epitopes that are immunogenic during the course of natural infection (12), and it stimulates CTLs and IFN-γ in naturally exposed individuals (12, 16). A specific LSA-1 peptide that associates with HLA-B53 elicits CTL responses (16), and HLA-B53 has been associated with naturally acquired resistance to severe malaria in some but not all studies (9, 16).
Human residents of areas where malaria is holoendemic develop naturally acquired resistance to malarial infection, and this resistance serves as a model for vaccine development. Because P. falciparum can cause frequent infections, and ongoing infection can bias measurements of immune responses (23), cross-sectional studies have a limited ability to define responses that predict protection. Therefore, we conducted a prospective study, defining the reappearance of parasitemia after eradication therapy in a cohort of naturally exposed volunteers, to examine the protective role of naturally acquired anti-LSA-1 responses. Understanding the relationship between these responses and resistance to parasitemia can guide the rational design of an LSA-1-based vaccine.
During two consecutive transmission seasons, we eradicated detectable parasitemia in volunteers, measured cellular immune responses against LSA-1 recombinant proteins, and analyzed how well these responses predicted subsequent parasitemia. In both seasons, interleukin-10 (IL-10) responses to LSA-1, but not IFN-γ or tumor necrosis factor alpha (TNF-α) responses, were significantly associated with reduced measures of parasitemia. This is the first study to suggest IL-10 is involved in resistance after eradication therapy, and our results support efforts to develop a malaria vaccine based on LSA-1.
MATERIALS AND METHODS
Study site and cohort description.
The study site in western Kenya was 10 km north of Lake Victoria, in the adjoining villages of Wangarot, Riwa Ojelo, and Waringa, Rarieda Division, Nyanza Province. The entomological inoculation rate in this area can exceed 300 infectious bites per person per year (2, 3). Details of this study site and the measures of parasitemia in this cohort in consecutive transmission seasons will be presented elsewhere (20). This study was conducted according to a protocol approved by ethical review boards of both the Walter Reed Army Institute of Research and the Kenya Medical Research Institute. All volunteers gave signed informed consent prior to entry into the study.
After the exclusion of individuals with abnormal hemograms or evidence of chronic disease on physical examination, 178 males aged 12 to 35 years entered the study at the beginning of the low-transmission season in August 1996. To initiate the study, volunteers were simultaneously treated with 3 days of quinine sulfate (10 mg/kg of body weight twice daily) and 7 days of doxycycline (100 mg twice daily) to eradicate current malaria infections. Five volunteers were removed from first-season analysis because their parasitemias persisted during the week following treatment with quinine and doxycycline.
Thick and thin blood smears were obtained weekly from each volunteer for 4 months after initial treatment with quinine and doxycycline. Each smear was interpreted by two microscopists to quantify parasitemia, and the mean value of the two reads was used to calculate malaria endpoints for the season. These endpoints included time to reappearance of parasitemia, mean parasitemia on all blood smears taken during the season, and frequency of detectable parasitemia.
To minimize unreported antimalarial use, trained field workers visited volunteers each day to assess their well-being. Sick volunteers were transported to the study clinic for complete physical exam. Volunteers with positive blood films and clinical syndromes suggestive of acute malaria were treated with three tabs of Fansidar (Hoffman-La Roche).
In April 1997, 144 of the 178 volunteers who participated in the first season were reenrolled at the start of the high-transmission season, again treated with quinine and doxycycline, and then monitored in the same manner as in the first season. One volunteer was removed from second-season analysis because his parasitemia persisted during the week following treatment with quinine and doxycycline.
Blood collection and processing.
In each season, volunteers donated 10 ml of blood into heparinized tubes 2 weeks after treatment with quinine and doxycycline. Peripheral blood mononuclear cells (PBMCs) were separated by Ficoll-Hypaque (Sigma) density centrifugation, resuspended in 10% dimethyl sulfoxide in fetal bovine serum, and cryopreserved in temperature-controlled freezing boxes, followed by long-term storage in liquid nitrogen. Thawed PBMCs had an average viability of greater than 90% as determined by trypan blue exclusion, and 96% of samples in the first season responded to phytohemagglutinin stimulation with a stimulation index of >5. Sufficient PBMCs for analysis were obtained on 141 of 173 volunteers in the first season and 120 of 143 volunteers in the second season.
Clinical laboratory.
Hemograms were performed on heparinized blood, using a Coulter cell counter model T-890 (Coulter Corp., Hialeah, Fla.). ABO blood group and hemoglobin phenotype were determined on 154 volunteers with commercially available reagents (Sigma, St. Louis, Mo.).
Antigens.
Two recombinant LSA-1 polypeptides were expressed with a thioredoxin fusion partner. The LSA-1 N-terminal polypeptide (LSA N) contained amino acids 28 to 150, and the C-terminal polypeptide (LSA C) contained amino acids 1630 to 1909, based on the sequence of LSA-1 from parasite strain NF-54 (36). These recombinant proteins flank the central repeat region of LSA-1. Fusion proteins were purified by metal chelate chromatography. Thioredoxin alone was purified under identical conditions and used as the negative control for all assays. LSA N and LSA C were used at a concentration of 10 μg/ml, and thioredoxin was used at an equivalent molar concentration. All assays were performed with a single lot of each recombinant protein. Recombinant proteins were greater than 95% pure as determined by Coomassie blue staining of sodium dodecyl sulfate-polyacrylamide gels. Lipopolysaccharide in the LSA C preparation was measured to be less than 100 endotoxin units/mg of protein by the gel clot Limulus amebocyte lysate method.
Lymphocyte assays.
PBMCs were thawed, washed twice in RPMI 1640, and resuspended in RPMI 1640 supplemented with 10% human AB serum at 0.5 × 106/ml. PBMCs were used at 50,000 cells per well in round-bottom microtiter plates. Stimulants were added to a final volume of 200 μl. Cells were incubated for 5 days in a humidified incubator at 37°C and 5% CO2. On day 5, 125-μl samples of cell-free culture supernatant were harvested from each well and stored at −70°C for subsequent cytokine analyses.
Cytokine assays.
Cytokine analyses on culture supernatants were performed in duplicate on 50-μl samples by sandwich enzyme-linked immunosorbent assay (ELISA). Paired antibodies and standards for IFN-γ (MabTech, Nacka, Sweden), TNF-α (Genzyme, Cambridge, Mass.), and IL-10 (PharMingen, San Diego, Calif.) were used as previously described (13). Standard curves for each cytokine were linear to at least 10 pg/ml. To calculate the LSA-1-specific cytokine response, the background cytokine concentration measured in thioredoxin-stimulated wells was subtracted from the cytokine concentration measured in wells stimulated with LSA N or LSA C.
Statistical analyses.
We examined relationships between anti-LSA-1 cytokine responses and resistance to parasitemia. Measures of parasitemia included time to reappearance of parasitemia, mean parasitemia, and frequency of detectable parasitemia. Time to reappearance of parasitemia was examined with Kaplan-Meier models (group differences evaluated with log rank test) and Cox proportional hazards models. Mean parasitemia and frequency of parasitemia were evaluated with Pearson’s correlation analysis and Student’s two-tailed t test.
Immunologic data were analyzed dichotomously (Kaplan-Meier and Student’s two-tailed t test) or continuously (Cox and Pearson’s analyses), as appropriate for each statistical test. Cytokine responses were dichotomized as detectable (≥10 pg/ml) or undetectable (<10 pg/ml), based on the sensitivity of our ELISA. When analyzed as continuous data, cytokine responses, mean parasitemia, and frequency of parasitemia required loge transformation [ln(value+1)] to obtain normal distributions. Potential confounding of immunologic variables by age, ABO blood group, or hemoglobin phenotype was explored with contingency table analyses, analysis of variance and multivariate linear regression where appropriate.
Analyses were performed with blood smears taken over the entire season of follow-up (raw data) or with only those blood smears taken before first treatment with Fansidar (adjusted data). Analyses of both adjusted and raw data yielded similar results; therefore, only results for the adjusted data are presented here.
All analyses were performed with StatView version 5.0 on Macintosh computers.
RESULTS
Parasitemia reappeared after eradication treatment in most volunteers during both seasons.
In the first season, 33% of volunteers had P. falciparum present on the blood smear prior to eradication treatment. Of the 173 volunteers included for analysis during the first season, parasitemia reappeared in 50% within 13 weeks (Kaplan-Meier estimate) and reappeared in a total of 101 volunteers by the end of the season.
In the second season, 53% of volunteers had P. falciparum present on the blood smear prior to eradication treatment. Of the 143 volunteers included for analysis, parasitemia reappeared in 50% within 6 weeks (Kaplan-Meier estimate) and reappeared in a total of 135 volunteers by the end of the season. The absence of parasitemia during the first season did not predict the absence of parasitemia during the second season (contingency table analysis, P = NS [not significant]).
IL-10 responses to LSA C predicted resistance to malaria in the first season.
LSA-1 recombinant proteins frequently elicited cytokine responses from naturally exposed individuals (Table 1). IFN-γ, TNF-α, and IL-10 were detected in 60, 62, and 61%, respectively, of samples stimulated with LSA N and in 57, 71, and 24%, respectively, of samples stimulated with LSA C. All assays were performed on PBMCs collected 2 weeks after eradication of malaria. TNF-α responses were significantly lower among donors who were infected prior to eradication treatment (Student’s t test, P = 0.02); no other cytokine measurements were related to the presence or absence of parasitemia prior to eradication (P = NS). By Pearson’s analysis, IFN-γ levels correlated mildly with TNF-α levels after stimulation with both LSA N (r = 0.273, P = 0.001) and LSA C (r = 0.366, P < 0.001). IL-10 levels correlated weakly with IFN-γ levels after stimulation with LSA N (r = 0.180, P = 0.03) but not after stimulation with LSA C (P = NS).
TABLE 1.
Cytokine production in response to LSA-1 polypeptides in consecutive transmission seasons
| Cytokine (stimulant) | Low-transmission season
|
High-transmission season
|
||||||
|---|---|---|---|---|---|---|---|---|
| Mean (±SEM) cytokine concn (pg/ml) | n | Range | % Responders (≥10 pg of cytokine/ml) | Mean (±SEM) cytokine concn (pg/ml) | n | Range | % Responders (≥10 pg of cytokine/ml) | |
| IFN-γ (LSA N) | 122 (29) | 141 | 0–2,637 | 60 | 17.7 (7.5) | 120 | 0–636 | 19 |
| IFN-γ (LSA C) | 212 (50) | 141 | 0–3,818 | 57 | 13.5 (6) | 120 | 0–551 | 14 |
| TNF-α (LSA N) | 77 (15) | 141 | 0–1,627 | 62 | 282 (88) | 120 | 0–8,694 | 31 |
| TNF-α (LSA C) | 147 (29) | 141 | 0–2,708 | 71 | 624 (114) | 120 | 0–6,998 | 63 |
| IL-10 (LSA N) | 33 (4) | 141 | 0–464 | 61 | 88 (21) | 118 | 0–1,324 | 43 |
| IL-10 (LSA C) | 8 (1.4) | 140 | 0–109 | 24 | 33 (9.7) | 113 | 0–752 | 27 |
Higher levels of IL-10 in response to LSA C were associated with significantly longer time to reappearance of parasitemia (Cox analysis, P = 0.03), lower mean parasitemia (Pearson’s analysis, r = −0.211, P = 0.01), and lower frequency of parasitemia (Pearson’s analysis, r = −0.176, P = 0.04). These relationships remained statistically significant after accounting for age, ABO blood group and hemoglobin phenotype. No other cytokine measurements, including IL-10 responses to LSA N, were related to resistance (P = NS).
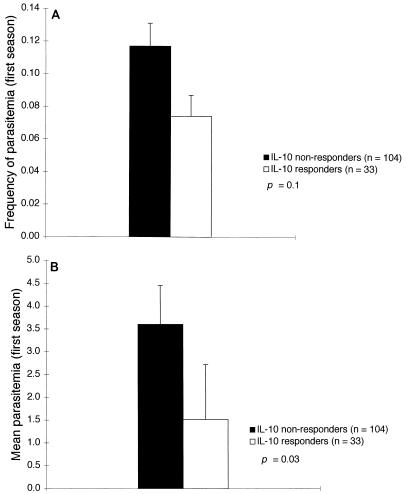
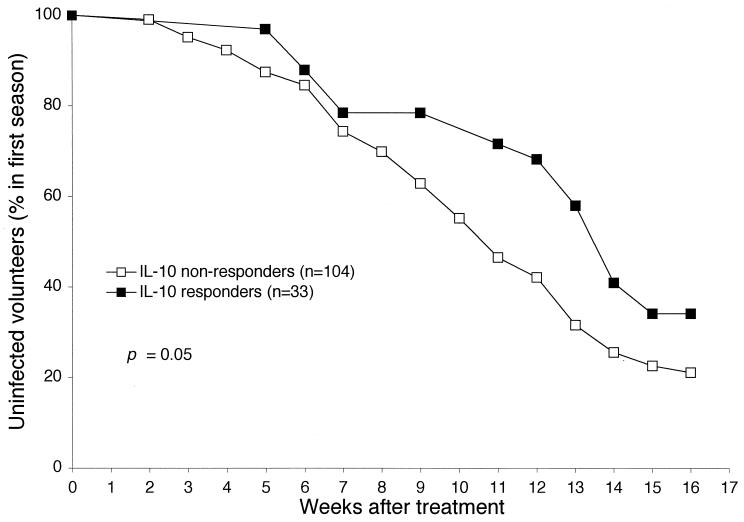
Similar relationships were observed when IL-10 responses to LSA C were analyzed as a dichotomous variable (detectable versus undetectable) in Kaplan-Meier analysis of time to reappearance of parasitemia and Student’s t tests on mean parasitemia and frequency of parasitemia. Individuals who produced detectable IL-10 in response to LSA C had significantly longer times to reappearance of parasitemia (P = 0.05), 58% lower mean parasitemia (P = 0.03), and 37% lower frequency of parasitemia (P = 0.1) (Fig. 1 and 2). When individuals were dichotomized on the basis of other cytokine measurements, measures of parasitemia did not significantly differ between groups.
FIG. 1.
IL-10 responses to LSA C measured before the low-transmission (first) season are associated with a reduced frequency of parasitemia (A) and a reduced density of parasitemia (B). Parasitemia on blood smears was quantified microscopically as the number of parasites per 200 leukocytes. Bars represent standard errors of the means.
FIG. 2.
IL-10 responses to LSA C measured before the low-transmission (first) season are associated with increased time to reappearance of parasitemia, as determined by Kaplan-Meier analysis.
IL-10 responses to LSA N predicted resistance to malaria in the second season.
During the second season, IFN-γ, TNF-α, and IL-10 were detected in 19, 31, and 43%, respectively, of samples stimulated with LSA N and in 14, 63, and 27%, respectively, of samples stimulated with LSA C (Table 1). None of the cytokine responses measured during the first season significantly correlated with the same responses during the second season (Pearson’s analysis, P = NS). The proportion of volunteers who produced detectable IL-10 to LSA C remained stable from the first to the second season, while the frequency of other cytokine responses decreased substantially (Table 1). Assays were performed on PBMCs collected 2 weeks after eradication of malaria; none of the cytokine measurements were associated with the presence or absence of parasitemia prior to eradication (Student’s t test, P = NS for all cytokine measurements). By Pearson’s analysis, IFN-γ levels correlated mildly with IL-10 levels after stimulation with LSA C (r = 0.249, P = 0.008); no other correlations between cytokine levels were observed (P = NS).
IL-10 production in response to LSA N was significantly associated with frequency of parasitemia (r = −0.233, P = 0.01) and mean parasitemia (r = −0.252, P = 0.007). Higher levels of IL-10 in response to LSA N correlated with lower mean parasitemia and lower frequency of parasitemia. IL-10 responses to LSA C were not related to measures of parasitemia during the second season (in contrast to the first season), nor were other cytokine measurements related (P = NS).
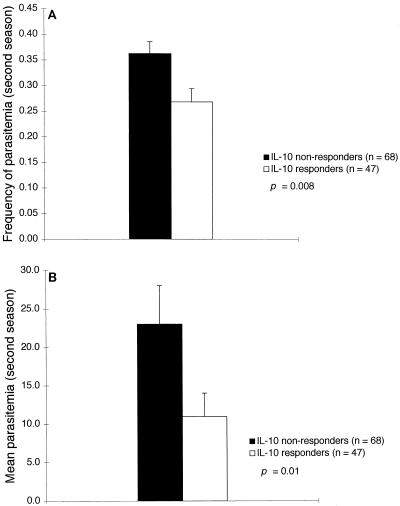
Similar results were obtained when IL-10 responses to LSA N were analyzed as a dichotomous variable (detectable versus undetectable) in Student’s t tests on mean parasitemia and frequency of parasitemia. Individuals who produced detectable IL-10 in response to LSA N had 52% lower mean parasitemia (P = 0.01) and 26% lower frequency of parasitemia (P = 0.008) (Fig. 3). Measures of parasitemia did not differ when individuals were dichotomized based on any of the other cytokine measurements (P = NS).
FIG. 3.
IL-10 responses to LSA N measured before the high-transmission (second) season are associated with a reduced frequency of parasitemia (A) and a reduced density of parasitemia (B). Parasitemia on blood smears was quantified microscopically as the number of parasites per 200 leukocytes. Bars represent standard errors of the means.
DISCUSSION
We report that measures of parasitemia after eradication therapy are significantly reduced in individuals who produce IL-10 in response to a liver-stage-specific antigen. Individuals who produced IL-10 in response to LSA C in the first season (low transmission) had a 58% reduction in mean parasitemia and a 37% reduction in frequency of detectable parasitemia compared to nonresponders. In the second (high-transmission) season, IL-10 production in response to LSA N was associated with similar reductions in parasitemia. Because IL-10 responses to LSA-1 are associated with naturally acquired resistance, a vaccine that elicits these responses may enhance protection in humans.
Earlier studies have examined the development of immune responses that could arrest parasite development in the liver. Human studies have identified low levels of CTL activity in naturally exposed populations (1, 10, 16, 22) and in irradiated sporozoite-immunized volunteers (24, 33). Other studies indicate that antibody, proliferative, cytokine, and CTL responses to LSA-1 peptides are detectable in naturally exposed individuals (8, 11, 12). Connelly et al. reported that in an area of Papua New Guinea where malaria is holoendemic, 10 of 11 individuals with two negative blood smears (obtained 6 months apart) produced detectable IFN-γ in response to an LSA-1 peptide, while only 9 of 27 individuals with one or two positive blood smears produced detectable IFN-γ in response to this peptide (8). No association was found between blood smear status and IFN-γ responses to two other LSA-1 peptides.
We did not observe a relationship between IFN-γ or TNF-α responses and measures of parasitemia. Previous animal data support a paradigm whereby CTL and IFN-γ responses are principal mediators of pre-erythrocytic immunity (26, 28, 31, 32, 34), and Connelly et al. have reported that IFN-γ production in response to an LSA-1 peptide is associated with the absence of parasitemia (8). IFN-γ and TNF-α responses were high in our cohort but failed to predict reductions in parasitemia; we did not examine CTL responses. Possibly, CTL and IFN-γ responses are essential components of pre-erythrocytic immunity in humans but are ubiquitous at the site of infected hepatocytes in chronically exposed individuals and therefore no longer differentiate susceptible and resistant phenotypes.
IL-10, TNF-α, and IFN-γ are cross-regulatory cytokines. Whereas TNF-α can contribute to the induction of IL-10 release in vivo (30), IL-10 inhibits the release of proinflammatory cytokines, including TNF-α, by human monocytes and neutrophils, and inhibits IFN-γ secretion by Th1 lymphocytes (reviewed in reference 21). Levels of TNF-α are elevated in patients with malaria, and these elevations are associated with severe disease (14). IL-10 levels are also high during symptomatic malaria (25) but may be only slightly elevated in individuals with asymptomatic parasitemia (18). We did not identify significant relationships between IL-10 and TNF-α responses in the same season. However, IL-10 and TNF-α responses were all greater during the high-transmission season than during the low-transmission season (Table 1), possibly reflecting the increased exposure to malaria.
Assessing immunity against liver-stage parasites is problematic. Hepatic tissue is generally inaccessible to direct evaluation, relatively few antigens specific to this stage have been defined, and PBMCs can only indirectly reflect the responses occurring at the site of the infected hepatocyte. Given these limitations, we believe that the design of our study has distinct advantages to identify immune responses associated with resistance. Cytokine measurements were determined after infection was eradicated, avoiding confounding of immune responses by active infection, and IL-10 responses were unrelated to pretreatment parasitemia in both seasons. Cytokine measurements were made immediately before the collection of parasitemia data, temporally supporting a cause (immune response)-and-effect (reduced parasitemia) relationship. Thus, our cytokine measurements were performed in the absence of ongoing infection, were unrelated to recent infection, and were timed to support a causal relationship with resistance.
In both a high- and a low-transmission season, IL-10 production (measured 2 weeks after malaria eradication) was associated with resistance to subsequent parasitemia. Measures of parasitemia (time to reappearance of parasitemia, mean parasitemia, and frequency of parasitemia) are related and can be influenced by both pre-erythrocytic and erythrocytic immunity, making it difficult to define the point at which IL-10 responses may be involved in protection. LSA-1 is not expressed during the erythrocytic stages of the parasite and has not been shown to share cross-reactive epitopes with blood-stage parasites, suggesting that anti-LSA-1 responses are elicited during liver-stage parasite development.
We did not determine the cellular source of cytokines in our samples, and therefore deducing a mechanism by which IL-10 augments resistance is largely speculative. IL-10 has several immunostimulatory effects that could participate in antiparasite effector pathways. For example, CD8+ CTLs mediate killing of liver-stage parasites in animal models of malaria (26, 32), and IL-10 can enhance CTL activity. In mice, IL-10 augments IL-2-induced differentiation, proliferation, and cytotoxicity of CD4− CD8+ cells (7). In humans, IL-10 is a chemoattractant for CD8+ lymphocytes (19) and could recruit CD8+ CTLs to the site of infected hepatocytes.
IL-10 could also enhance antibody-dependent cellular inhibition (ADCI) activity against P. falciparum. ADCI is mediated by the cooperative activity of antibodies and monocytes, resulting in arrested parasite development (4, 5). Human IL-10 induces activated B cells to secrete immunoglobulin (Ig) (27), particularly the cytophilic IgG1 and IgG3 isotypes (6), and supports the retention of Fcγ receptors on the surface of monocytes (29). Thus, IL-10 could enhance an ADCI effect triggered as merozoites emerge from the ruptured hepatocyte. Separately, elevated antibody levels could inhibit parasite growth by agglutinating the flocculent mass of LSA-1 around merozoites emerging from ruptured hepatocytes, as first suggested by Zhu and Hollingdale (36).
IL-10 production in response to LSA C predicted resistance in the first (low-transmission) season, while IL-10 production in response to LSA N predicted resistance in the second (high-transmission) season. Among other possibilities, the change in apparently protective epitopes may be due to naturally occurring sequence variation of LSA-1. Sequence variation in both the N and C termini of LSA-1 has been reported in parasites from this study site (35). As a consequence, the epitopes shared by recombinant LSA-1 polypeptides (used as in vitro stimulants) and naturally occurring LSA-1 (expressed by circulating parasites) can change between seasons.
Our study supports a protective role for IL-10 production in response to LSA-1. Additional investigation is required to define specific mechanisms, including antibody, CTL activity, and enhanced ADCI, involved in LSA-1-mediated protection during acute and chronic human infection. In summary, through direct association of anti-LSA-1 responses with resistance in a naturally exposed population, our study supports the inclusion of LSA-1 in multicomponent vaccines and suggests that IL-10 responses may augment resistance to P. falciparum in chronically exposed humans.
ACKNOWLEDGMENTS
J.D.K. was a National Research Council fellow and an American Society of Tropical Medicine and Hygiene/Becton Dickinson fellow.
This work is published with the kind permission of the director of the Kenya Medical Research Institute.
We thank Raphael Onyango, Samuel Oduor Wangowe, and Frederick Onyango for excellent supervision of the field studies and the volunteers for their participation. Stephen Hoffman provided thoughtful discussions of pre-erythrocytic immunity, and W. Ripley Ballou, David Fidock, and Michal Fried reviewed the manuscript.
REFERENCES
- 1.Aidoo M, Lalvani A, Allsopp C E, Plebanski M, Meisner S J, Krausa P, Browning M, Morris J S, Gotch F, Fidock D A, Takiguchi M, Robson K J H, Greenwood B M, Druilhe P, Whittle H C, Hill A V S. Identification of conserved antigenic components for a cytotoxic T lymphocyte-inducing vaccine against malaria. Lancet. 1995;345:1003–1007. doi: 10.1016/s0140-6736(95)90754-8. [DOI] [PubMed] [Google Scholar]
- 2.Beier J C, Oster C N, Onyango F K, Bales J D, Sherwood J A, Perkins P V, Chumo D K, Koech D V, Whitmire R E, Roberts C R, Diggs C L, Hoffman S L. Plasmodium falciparum incidence relative to entomologic inoculation rates at a site proposed for testing malaria vaccines in western Kenya. Am J Trop Med Hyg. 1994;50:529–536. doi: 10.4269/ajtmh.1994.50.529. [DOI] [PubMed] [Google Scholar]
- 3.Beier J C, Perkins P V, Onyango F K, Gargan T P, Oster C N, Whitmire R E, Koech D K, Roberts C R. Characterization of malaria transmission by Anopheles (Diptera: Culicidae) in western Kenya in preparation for malaria vaccine trials. J Med Entomol. 1990;27:570–577. doi: 10.1093/jmedent/27.4.570. [DOI] [PubMed] [Google Scholar]
- 4.Bouharoun-Tayoun H, Attanath P, Sabchareon A, Chongsuphajaisiddhi T, Druilhe P. Antibodies that protect humans against Plasmodium falciparum blood stages do not on their own inhibit parasite growth and invasion in vitro, but act in cooperation with monocytes. J Exp Med. 1990;172:1633–1641. doi: 10.1084/jem.172.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouharoun-Tayoun H, Oeuvray C, Lunel F, Druilhe P. Mechanisms underlying the monocyte-mediated antibody-dependent killing of Plasmodium falciparum asexual blood stages. J Exp Med. 1995;182:409–418. doi: 10.1084/jem.182.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Briere F, Servet-Delprat C, Bridon J M, Saint-Remy J M, Banchereau J. Human interleukin 10 induces naive surface immunoglobulin D+ (sIgD+) B cells to secrete IgG1 and IgG3. J Exp Med. 1994;179:757–762. doi: 10.1084/jem.179.2.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen W F, Zlotnik A. IL-10: a novel cytotoxic T cell differentiation factor. J Immunol. 1991;147:528–534. [PubMed] [Google Scholar]
- 8.Connelly M, King C L, Bucci K, Walters S, Genton B, Alpers M P, Hollingdale M, Kazura J W. T-cell immunity to peptide epitopes of liver-stage antigen 1 in an area of Papua New Guinea in which malaria is holoendemic. Infect Immun. 1997;65:5082–5087. doi: 10.1128/iai.65.12.5082-5087.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dieye A, Rogier C, Trape J F, Sarthou J L, Druilhe P. HLA class I-associated resistance to severe malaria: a parasitological re-assessment. Parasitol Today. 1997;13:48–49. doi: 10.1016/s0169-4758(96)20062-6. [DOI] [PubMed] [Google Scholar]
- 10.Doolan D L, Khamboonruang C, Beck H P, Houghten R A, Good M F. Cytotoxic T lymphocyte (CTL) low-responsiveness to the Plasmodium falciparum circumsporozoite protein in naturally-exposed endemic populations: analysis of human CTL response to most known variants. Int Immunol. 1993;5:37–46. doi: 10.1093/intimm/5.1.37. [DOI] [PubMed] [Google Scholar]
- 11.Ferreira da Cruz M F, Deslandes D C, Oliveira F J, Montenegro J S, Tartar A, Druilhe P, Daniel R C. Antibody responses to Plasmodium falciparum sporozoite-, liver- and blood-stage synthetic peptides in migrant and autochthonous populations in malaria endemic areas. Parasite. 1995;2:23–29. doi: 10.1051/parasite/1995021023. [DOI] [PubMed] [Google Scholar]
- 12.Fidock D A, Gras-Masse H, Lepers J P, Brahimi K, Benmohamed L, Mellouk S, Guerin-Marchand C, Londono A, Raharimalala L, Meis J F, Langsley G, Roussilhon C, Tartar A, Druilhe P. Plasmodium falciparum liver stage antigen-1 is well conserved and contains potent B and T cell determinants. J Immunol. 1994;153:190–204. [PubMed] [Google Scholar]
- 13.Fried M, Muga R O, Misore A O, Duffy P E. Malaria elicits type 1 cytokines in the human placenta: IFN-gamma and TNF-alpha associated with pregnancy outcomes. J Immunol. 1998;160:2523–2530. [PubMed] [Google Scholar]
- 14.Grau G E, Taylor T E, Molyneux M E, Wirima J J, Vassalli P, Hommel M, Lambert P H. Tumor necrosis factor and disease severity in children with falciparum malaria. N Engl J Med. 1989;320:1586–1591. doi: 10.1056/NEJM198906153202404. [DOI] [PubMed] [Google Scholar]
- 15.Guerin-Marchand C, Druilhe P, Galey B, Londono A, Patarapotikul J, Beaudoin R L, Dubeaux C, Tartar A, Mercereau-Puijalon O, Langsley G. A liver-stage-specific antigen of Plasmodium falciparum characterized by gene cloning. Nature. 1987;329:164–167. doi: 10.1038/329164a0. [DOI] [PubMed] [Google Scholar]
- 16.Hill A V, Elvin J, Willis A C, Aidoo M, Allsopp C E, Gotch F M, Gao X M, Takiguchi M, Greenwood B M, Townsend A R, McMichael A J, Whittle H C. Molecular analysis of the association of HLA-B53 and resistance to severe malaria. Nature. 1992;360:434–439. doi: 10.1038/360434a0. [DOI] [PubMed] [Google Scholar]
- 17.Hollingdale M R, Aikawa M, Atkinson C T, Ballou W R, Chen G, Li J, Meis J, Sina B, Wright C, Zhu J. Non-CS pre-erythrocytic protective antigens. Immunol Lett. 1990;25:71–76. doi: 10.1016/0165-2478(90)90094-7. [DOI] [PubMed] [Google Scholar]
- 18.Jakobsen P H, McKay V, N’Jie R, Olaleye B O, D’Alessandro U, Bendtzen K, Schousboe I, Greenwood B M. Soluble products of inflammatory reactions are not induced in children with asymptomatic Plasmodium falciparum infections. Clin Exp Immunol. 1996;105:69–73. doi: 10.1046/j.1365-2249.1996.d01-718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jinquan T, Larsen C G, Gesser B, Matsushima K, Thestrup-Pedersen K. Human IL-10 is a chemoattractant for CD8+ T lymphocytes and an inhibitor of IL-8-induced CD4+ T lymphocyte migration. J Immunol. 1993;151:4545–4551. [PubMed] [Google Scholar]
- 20.Kurtis, J. D., and P. E. Duffy. 1997. Unpublished data.
- 21.Lalani I, Bhol K, Ahmed A R. Interleukin-10: biology, role in inflammation and autoimmunity. Ann Allergy Asthma Immunol. 1997;79:469–483. doi: 10.1016/S1081-1206(10)63052-9. [DOI] [PubMed] [Google Scholar]
- 22.Lalvani A, Hurt N, Aidoo M, Kibatala P, Tanner M, Hill A V. Cytotoxic T lymphocytes to Plasmodium falciparum epitopes in an area of intense and perennial transmission in Tanzania. Eur J Immunol. 1996;26:773–779. doi: 10.1002/eji.1830260408. [DOI] [PubMed] [Google Scholar]
- 23.Luty A J, Mayombo J, Lekoulou F, Mshana R. Immunologic responses to soluble exoantigens of Plasmodium falciparum in Gabonese children exposed to continuous intense infection. Am J Trop Med Hyg. 1994;51:720–729. doi: 10.4269/ajtmh.1994.51.720. [DOI] [PubMed] [Google Scholar]
- 24.Malik A, Egan J E, Houghten R A, Sadoff J C, Hoffman S L. Human cytotoxic T lymphocytes against the Plasmodium falciparum circumsporozoite protein. Proc Natl Acad Sci USA. 1991;88:3300–3304. doi: 10.1073/pnas.88.8.3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peyron F, Burdin N, Ringwald P, Vuillez J P, Rousset F, Banchereau J. High levels of circulating IL-10 in human malaria. Clin Exp Immunol. 1994;95:300–303. doi: 10.1111/j.1365-2249.1994.tb06527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodrigues M M, Cordey A S, Arreaza G, Corradin G, Romero P, Maryanski J L, Nussenzweig R S, Zavala F. CD8+ cytolytic T cell clones derived against the Plasmodium yoelii circumsporozoite protein protect against malaria. Int Immunol. 1991;3:579–585. doi: 10.1093/intimm/3.6.579. [DOI] [PubMed] [Google Scholar]
- 27.Rousset F, Garcia E, Defrance T, Peronne C, Vezzio N, Hsu D H, Kastelein R, Moore K W, Banchereau J. Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc Natl Acad Sci USA. 1992;89:1890–1893. doi: 10.1073/pnas.89.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schofield L, Villaquiran J, Ferreira A, Schellekens H, Nussenzweig R, Nussenzweig V. Gamma interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature. 1987;330:664–666. doi: 10.1038/330664a0. [DOI] [PubMed] [Google Scholar]
- 29.te Velde A, Malefijt R, Huijbens R J, de Vries J, Figdor C G. IL-10 stimulates monocyte Fc gamma R surface expression and cytotoxic activity. Distinct regulation of antibody-dependent cellular cytotoxicity by IFN-gamma, IL-4, and IL-10. J Immunol. 1992;149:4048–4052. [PubMed] [Google Scholar]
- 30.van der Poll T, Jansen J, Levi M, ten Cate H, ten Cate J, van Deventer S. Regulation of interleukin 10 release by tumor necrosis factor in humans and chimpanzees. J Exp Med. 1994;180:1985–1988. doi: 10.1084/jem.180.5.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang R, Charoenvit Y, Corradin G, De La Vega P, Franke E D, Hoffman S L. Protection against malaria by Plasmodium yoelii sporozoite surface protein 2 linear peptide induction of CD4+ T cell- and IFN-gamma-dependent elimination of infected hepatocytes. J Immunol. 1996;157:4061–4067. [PubMed] [Google Scholar]
- 32.Weiss W R, Mellouk S, Houghten R A, Sedegah M, Kumar S, Good M F, Berzofsky J A, Miller L H, Hoffman S L. Cytotoxic T cells recognize a peptide from the circumsporozoite protein on malaria-infected hepatocytes. J Exp Med. 1990;171:763–773. doi: 10.1084/jem.171.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wizel B, Houghten R, Church P, Tine J A, Lanar D E, Gordon D M, Ballou W R, Sette A, Hoffman S L. HLA-A2-restricted cytotoxic T lymphocyte responses to multiple Plasmodium falciparum sporozoite surface protein 2 epitopes in sporozoite-immunized volunteers. J Immunol. 1995;155:766–775. [PubMed] [Google Scholar]
- 34.Wizel B, Houghten R A, Parker K C, Coligan J E, Church P, Gordon D M, Ballou W R, Hoffman S L. Irradiated sporozoite vaccine induces HLA-B8-restricted cytotoxic T lymphocyte responses against two overlapping epitopes of the Plasmodium falciparum sporozoite surface protein 2. J Exp Med. 1995;182:1435–1445. doi: 10.1084/jem.182.5.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang C, Shi Y P, Udhayakumar V, Alpers M P, Povoa M M, Hawley W A, Collins W E, Lal A A. Sequence variations in the non-repetitive regions of the liver stage-specific antigen-1 (LSA-1) of Plasmodium falciparum from field isolates. Mol Biochem Parasitol. 1995;71:291–294. doi: 10.1016/0166-6851(95)00069-d. [DOI] [PubMed] [Google Scholar]
- 36.Zhu J, Hollingdale M R. Structure of Plasmodium falciparum liver stage antigen-1. Mol Biochem Parasitol. 1991;48:223–226. doi: 10.1016/0166-6851(91)90117-o. [DOI] [PubMed] [Google Scholar]