Abstract
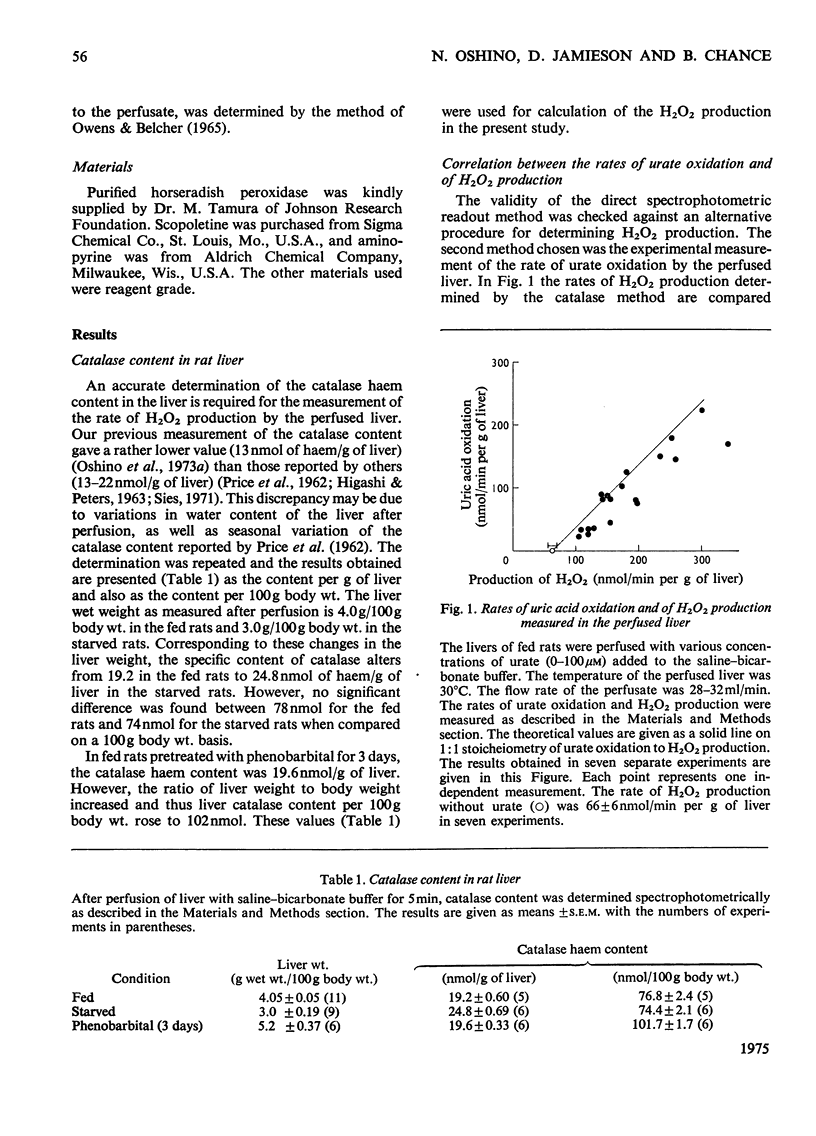
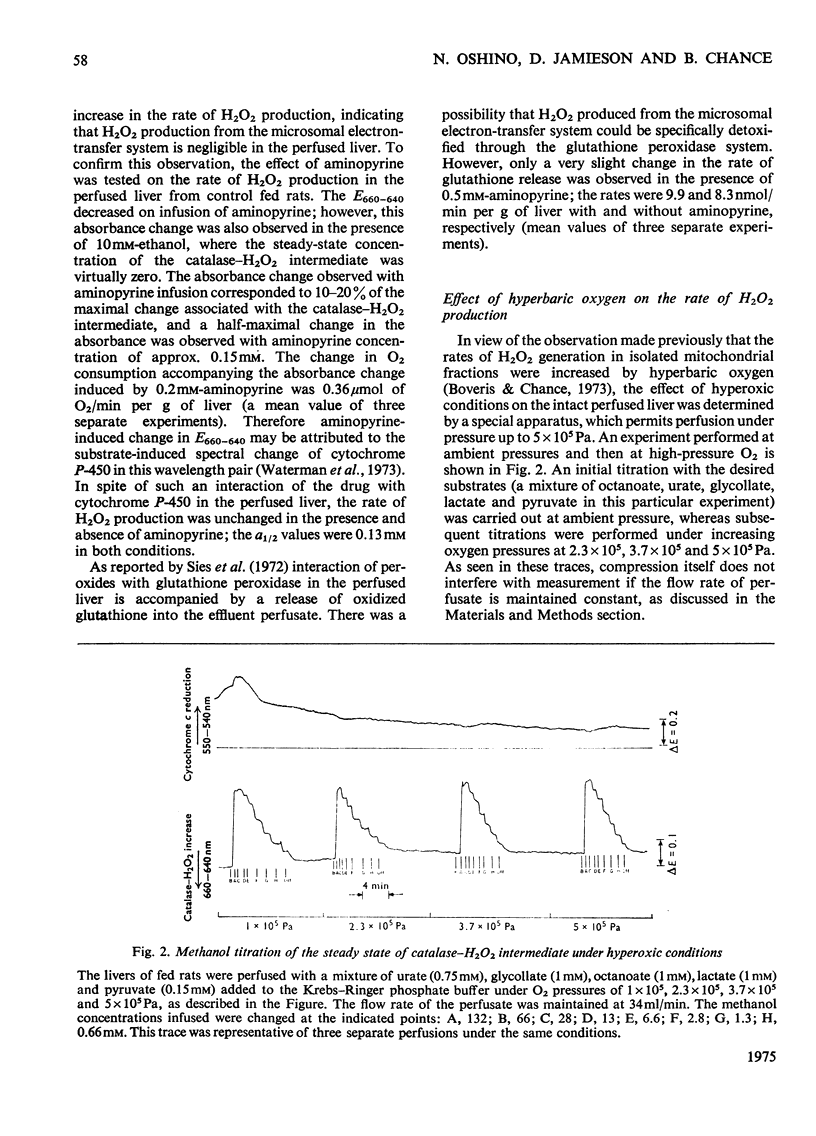
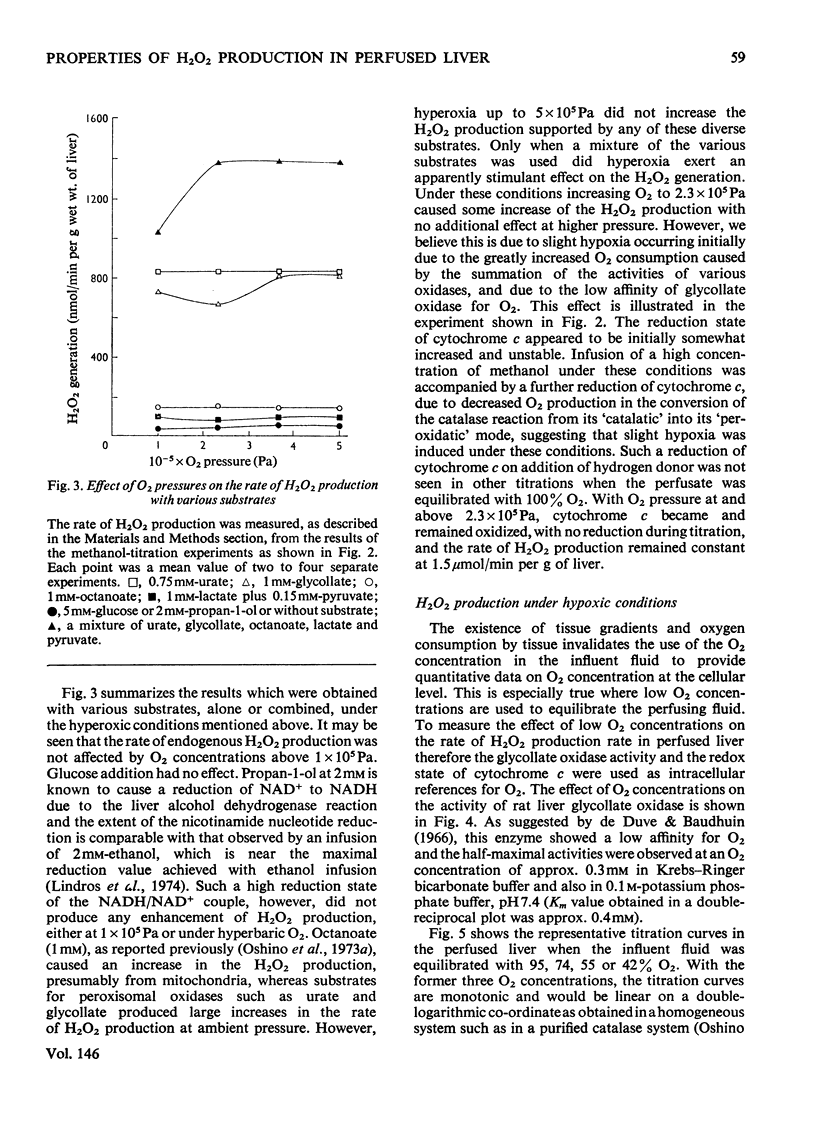
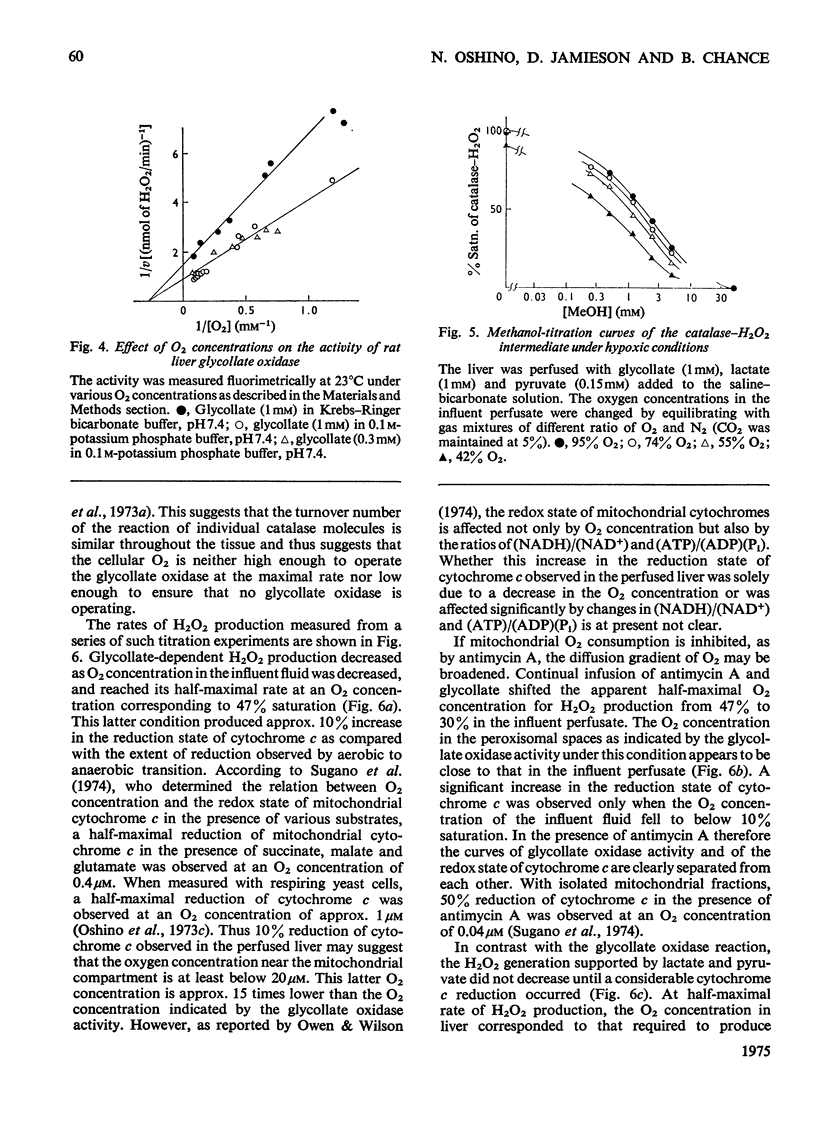
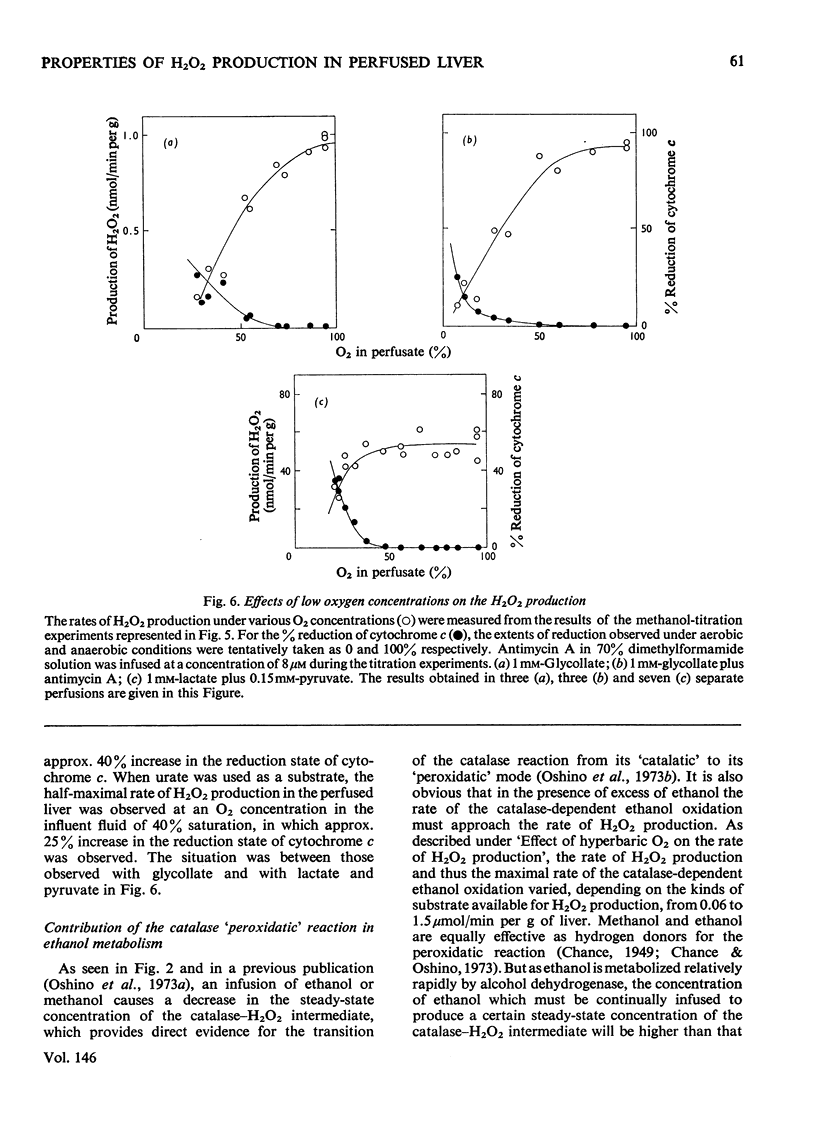
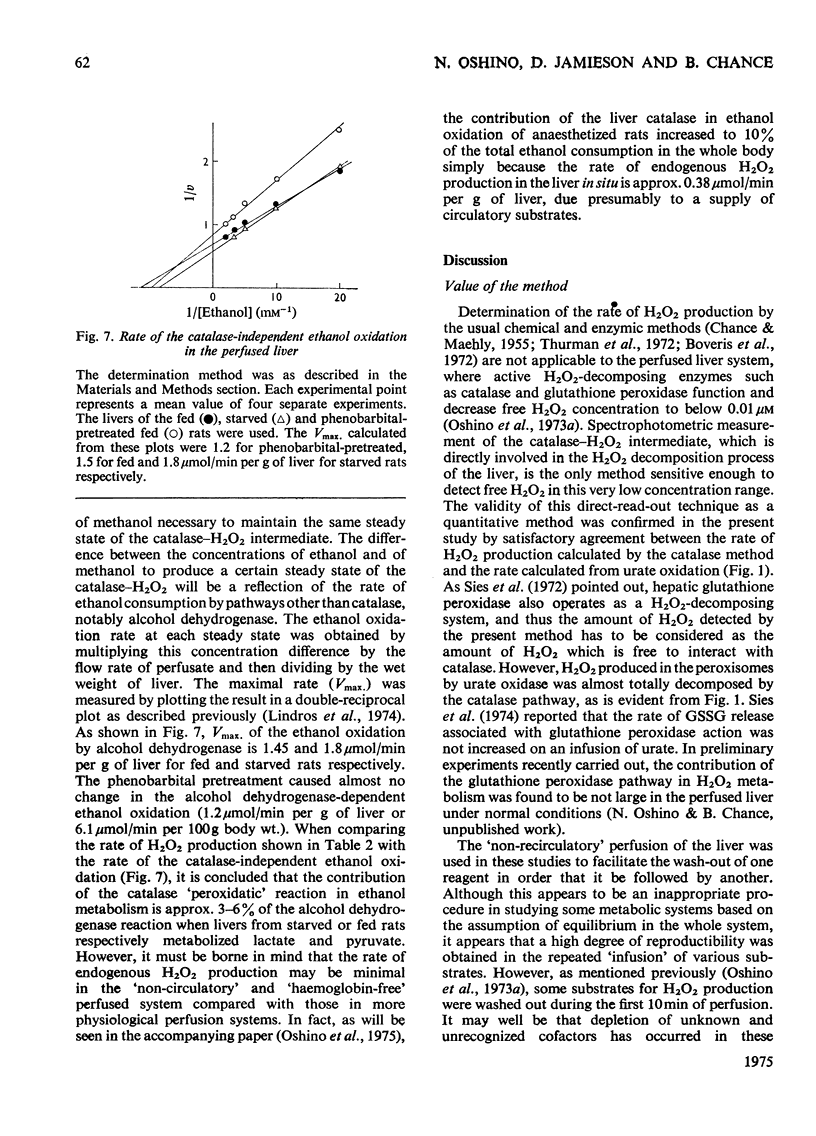
The properties of H2O2 production in the "haemoglobin-free", "non-circulatory" perfused liver of rats were examined. The H2O2 production with 1 mM-lactate and 0.15 mM-pyruvate was 82nmol/min per g of liver or 333nmol/min per 100g body wt. in the liver of fed rats at 30 degrees C. This rate decreased to almost half in the livers of starved and phenobarbital-pretreated rats. When H2O2 production was stimulated by urate infusion, almost all of the H2O2 produced by the uricase reaction was decomposed by the catalase reaction. During the demethylation reaction of aminopyrine, no change in H2O2 production was detected by the present method; thus microsomal H2O2 production observed in isolated subcellular fractions appeared not to contribute significantly to the H2O2 production in the whole organ. Whereas the rate of the glycolate-dependent H2O2 production was halved at an intracellular O2 concentration that caused a 10 percent increase in the reduction state of cytochrome c, the half-maximal rate of H2O2 production with lactate and pyruvate was observed at an O2 concentration that caused a 40 percent increase in the reduction state of cytochrome c in the liver. No further increase in the rates of H2O2 production was obtained by increasing O2 pressure up to 5 times 10(5) Pa. The rate of ethanol oxidation through the catalase "peroxidatic" reaction varied, depending on the substrate availability. The maximal capability of this pathway in ethanol oxidation reached approx. 1.5 mumol/min per g of liver, when a mixture of urate, glycollate and octanoate was infused to enhance H2O2 production.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beauchamp C., Fridovich I. A mechanism for the production of ethylene from methional. The generation of the hydroxyl radical by xanthine oxidase. J Biol Chem. 1970 Sep 25;245(18):4641–4646. [PubMed] [Google Scholar]
- Boveris A., Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J. 1973 Jul;134(3):707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveris A., Oshino N., Chance B. The cellular production of hydrogen peroxide. Biochem J. 1972 Jul;128(3):617–630. doi: 10.1042/bj1280617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANCE B. Spectrophotometry of intracellular respiratory pigments. Science. 1954 Nov 12;120(3124):767–775. doi: 10.1126/science.120.3124.767. [DOI] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- CLAYTON R. K. Role of H2O2 in the photooxidative killing of blue-green mutants of Rhodopseudomonas spheroides. J Bacteriol. 1961 Aug;82:314–315. doi: 10.1128/jb.82.2.314-315.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B., Oshino N. Analysis of the catalase--hydrogen peroxide intermediate in coupled oxidations. Biochem J. 1973 Mar;131(3):564–567. doi: 10.1042/bj1310564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B., Oshino N. Kinetics and mechanisms of catalase in peroxisomes of the mitochondrial fraction. Biochem J. 1971 Apr;122(2):225–233. doi: 10.1042/bj1220225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B. The State of Catalase in the Respiring Bacterial Cell. Science. 1952 Aug 22;116(3008):202–203. doi: 10.1126/science.116.3008.202. [DOI] [PubMed] [Google Scholar]
- De Duve C., Baudhuin P. Peroxisomes (microbodies and related particles). Physiol Rev. 1966 Apr;46(2):323–357. doi: 10.1152/physrev.1966.46.2.323. [DOI] [PubMed] [Google Scholar]
- Dixon M. The acceptor specificity of flavins and flavoproteins. 3. Flavoproteins. Biochim Biophys Acta. 1971 Mar 2;226(2):269–284. doi: 10.1016/0005-2728(71)90094-6. [DOI] [PubMed] [Google Scholar]
- ESTABROOK R. W., COOPER D. Y., ROSENTHAL O. THE LIGHT REVERSIBLE CARBON MONOXIDE INHIBITION OF THE STEROID C21-HYDROXYLASE SYSTEM OF THE ADRENAL CORTEX. Biochem Z. 1963;338:741–755. [PubMed] [Google Scholar]
- Fong K. L., McCay P. B., Poyer J. L., Keele B. B., Misra H. Evidence that peroxidation of lysosomal membranes is initiated by hydroxyl free radicals produced during flavin enzyme activity. J Biol Chem. 1973 Nov 25;248(22):7792–7797. [PubMed] [Google Scholar]
- HIGASHI T., PETERS T., Jr STUDIES ON RAT LIVER CATALASE. I. COMBINED IMMUNOCHEMICAL AND ENZYMATIC DETERMINATION OF CATALASE IN LIVER CELL FRACTIONS. J Biol Chem. 1963 Dec;238:3945–3951. [PubMed] [Google Scholar]
- Hildebrandt A. G., Speck M., Roots I. Possible control of hydrogen peroxide production and degradation in microsomes during mixed function oxidation reaction. Biochem Biophys Res Commun. 1973 Oct 1;54(3):968–975. doi: 10.1016/0006-291x(73)90789-4. [DOI] [PubMed] [Google Scholar]
- Keilin D., Hartree E. F. Properties of catalase. Catalysis of coupled oxidation of alcohols. Biochem J. 1945;39(4):293–301. [PMC free article] [PubMed] [Google Scholar]
- Lavelle F., Michelson A. M., Dimitrijevic L. Biological protection by superoxide dismutase. Biochem Biophys Res Commun. 1973 Nov 16;55(2):350–357. doi: 10.1016/0006-291x(73)91094-2. [DOI] [PubMed] [Google Scholar]
- Lieber C. S., DeCarli L. M. Hepatic microsomal ethanol-oxidizing system. In vitro characteristics and adaptive properties in vivo. J Biol Chem. 1970 May 25;245(10):2505–2512. [PubMed] [Google Scholar]
- Lindros K. O., Vihma R., Forsander O. A. Utilization and metabolic effects of acetaldehyde and ethanol in the perfused rat liver. Biochem J. 1972 Feb;126(4):945–952. doi: 10.1042/bj1260945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loschen G., Azzi A., Richter C., Flohé L. Superoxide radicals as precursors of mitochondrial hydrogen peroxide. FEBS Lett. 1974 May 15;42(1):68–72. doi: 10.1016/0014-5793(74)80281-4. [DOI] [PubMed] [Google Scholar]
- Loschen G., Flohé L., Chance B. Respiratory chain linked H(2)O(2) production in pigeon heart mitochondria. FEBS Lett. 1971 Nov 1;18(2):261–264. doi: 10.1016/0014-5793(71)80459-3. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. The utility of superoxide dismutase in studying free radical reactions. II. The mechanism of the mediation of cytochrome c reduction by a variety of electron carriers. J Biol Chem. 1970 Mar 25;245(6):1374–1377. [PubMed] [Google Scholar]
- Misra H. P., Fridovich I. The univalent reduction of oxygen by reduced flavins and quinones. J Biol Chem. 1972 Jan 10;247(1):188–192. [PubMed] [Google Scholar]
- Nakamura S., Kimura T. Studies on aggregated multienzyme systems. Stimulation of oxygen uptake of ferredoxin-nicotinamide adenine dinucleotide phosphate reductase-ferredoxin complex by cytochrome. J Biol Chem. 1972 Oct 25;247(20):6462–6468. [PubMed] [Google Scholar]
- Nakano M., Ushijima Y., Saga M., Tsutsumi Y., Asami H. Aliphatic L-alpha-hydroxyacid oxidase from rat livers: purification and properties. Biochim Biophys Acta. 1968 Aug 27;167(1):9–22. doi: 10.1016/0005-2744(68)90273-8. [DOI] [PubMed] [Google Scholar]
- OWENS C. W., BELCHER R. V. A COLORIMETRIC MICRO-METHOD FOR THE DETERMINATION OF GLUTATHIONE. Biochem J. 1965 Mar;94:705–711. doi: 10.1042/bj0940705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orrenius S., Ericsson J. L., Ernster L. Phenobarbital-induced synthesis of the microsomal drug-metabolizing enzyme system and its relationship to the proliferation of endoplasmic membranes. A morphological and biochemical study. J Cell Biol. 1965 Jun;25(3):627–639. doi: 10.1083/jcb.25.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshino N., Chance B., Sies H., Bücher T. The role of H 2 O 2 generation in perfused rat liver and the reaction of catalase compound I and hydrogen donors. Arch Biochem Biophys. 1973 Jan;154(1):117–131. doi: 10.1016/0003-9861(73)90040-4. [DOI] [PubMed] [Google Scholar]
- Oshino N., Jamieson D., Sugano T., Chance B. Optical measurement of the catalase-hydrogen peroxide intermediate (Compound I) in the liver of anaesthetized rats and its implication to hydrogen peroxide production in situ. Biochem J. 1975 Jan;146(1):67–77. doi: 10.1042/bj1460067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshino N., Oshino R., Chance B. The characteristics of the "peroxidatic" reaction of catalase in ethanol oxidation. Biochem J. 1973 Mar;131(3):555–563. doi: 10.1042/bj1310555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshino R., Oshino N., Chance B. Studies on yeast hemoglobin. The properties of yeast hemoglobin and its physiological function in the cell. Eur J Biochem. 1973 May;35(1):23–33. doi: 10.1111/j.1432-1033.1973.tb02805.x. [DOI] [PubMed] [Google Scholar]
- Owen C. S., Wilson D. F. Control of respiration by the mitochondrial phosphorylation state. Arch Biochem Biophys. 1974 Apr 2;161(2):581–591. doi: 10.1016/0003-9861(74)90341-5. [DOI] [PubMed] [Google Scholar]
- PRICE V. E., STERLING W. R., TARANTOLA V. A., HARTLEY R. W., Jr, RECHCIGL M., Jr The kinetics of catalase synthesis and destruction in vivo. J Biol Chem. 1962 Nov;237:3468–3475. [PubMed] [Google Scholar]
- REMMER H., MERKER H. J. [Enzyme induction and increase of endoplasmic reticulum in liver cells during phenobarbital (Luminal) therapy]. Klin Wochenschr. 1963 Mar 15;41:276–282. doi: 10.1007/BF01483392. [DOI] [PubMed] [Google Scholar]
- Sies H., Brauser B. Interaction of mixed function oxidase with its substrates and associated redox transitions of cytochrome P-450 and pyridine nucleotides in perfused rat liver. Eur J Biochem. 1970 Sep;15(3):531–540. doi: 10.1111/j.1432-1033.1970.tb01037.x. [DOI] [PubMed] [Google Scholar]
- Sies H., Bücher T., Oshino N., Chance B. Heme occupancy of catalase in hemoglobin-free perfused rat liver and of isolated rat liver catalase. Arch Biochem Biophys. 1973 Jan;154(1):106–116. doi: 10.1016/0003-9861(73)90039-8. [DOI] [PubMed] [Google Scholar]
- Sies H., Chance B. The steady state level of catalase compound I in isolated hemoglobin-free perfused rat liver. FEBS Lett. 1970 Dec;11(3):172–176. doi: 10.1016/0014-5793(70)80521-x. [DOI] [PubMed] [Google Scholar]
- Sies H., Gerstenecker C., Menzel H., Flohé L. Oxidation in the NADP system and release of GSSG from hemoglobin-free perfused rat liver during peroxidatic oxidation of glutathione by hydroperoxides. FEBS Lett. 1972 Oct 15;27(1):171–175. doi: 10.1016/0014-5793(72)80434-4. [DOI] [PubMed] [Google Scholar]
- Sugano T., Oshino N., Chance B. Mitochondrial functions under hypoxic conditions. The steady states of cytochrome c reduction and of energy metabolism. Biochim Biophys Acta. 1974 Jun 28;347(3):340–358. doi: 10.1016/0005-2728(74)90074-7. [DOI] [PubMed] [Google Scholar]
- TENENBAUM E. Appearance of crystalline needles in monkey kidney cell cultures infected with poliomyelitis virus and incubated at 22-30 degrees C. Nature. 1959 Nov 21;184(Suppl 21):1657–1658. doi: 10.1038/1841657a0. [DOI] [PubMed] [Google Scholar]
- Theorell H., Chance B., Yonetani T., Oshino N. The combustion of alcohol and its inhibition by 4-methyl-pyrazole in perfused rat livers. Arch Biochem Biophys. 1972 Aug;151(2):434–444. doi: 10.1016/0003-9861(72)90519-x. [DOI] [PubMed] [Google Scholar]
- Thurman R. G., Ley H. G., Scholz R. Hepatic microsomal ethanol oxidation. Hydrogen peroxide formation and the role of catalase. Eur J Biochem. 1972 Feb;25(3):420–430. doi: 10.1111/j.1432-1033.1972.tb01711.x. [DOI] [PubMed] [Google Scholar]
- Thurman R. G., Scholz R. Mixed function oxidation in perfused rat liver. The effect of aminopyrine on oxygen uptake. Eur J Biochem. 1969 Oct;10(3):459–467. doi: 10.1111/j.1432-1033.1969.tb00711.x. [DOI] [PubMed] [Google Scholar]
- Waterman M. R., Ullrich V., Estabrook R. W. Effect of substrate on the spin state of cytochrome P-450 in hepatic microsomes. Arch Biochem Biophys. 1973 Apr;155(2):355–360. doi: 10.1016/0003-9861(73)90124-0. [DOI] [PubMed] [Google Scholar]


