Abstract
Background
Ayurvedic intervention (Brahmi Vati with Saraswatarista) is explored for their possible role in management of Generalized Anxiety Disorder (GAD), a common psychiatric disorder.
Objective
The objective of the study was to evaluate the efficacy of Brahmi Vati and Saraswatarista in GAD.
Methods
Study is a randomized controlled clinical trial. Patients (n = 50) of GAD (Diagnostic and Statistical Manual of Mental Disorders (DSM-5 criteria), 18–60 years of age, either sex participated in the study. Participants were randomly divided into two groups. Group A, received escitalopram 10 mg/day for first 10 days followed by 20 mg/day for next 50 days. Group B, received Ayurvedic intervention (Brahmi Vati 500 mg thrice a day (TID) and Saraswatarista 10 ml TID) for 60 days. Assessments were with clinical parameters like Hamilton Anxiety Rating Scale (HARS), GAD 7 scale (GAD 7), Beck Depression Inventory scale (BDI), Epworth sleepiness scale (ESS), Pittsburgh Sleep Quality Index (PSQI), WHO Quality of Life- BREF (WHOQOL-BREF), Clinical Global Improvement scale (CGI) and UKU-Side effect scale (UKU). These clinical assessments were measured on every 15th day during the intervention. Haemoglobin, liver function test (LFT), serum creatinine, serum urea were assessed before and after the study.
Results
Study results indicate that both the groups were comparable in HARS, GAD7, BDI, WHOQOL-Bref and CGI-Severity. Group B was better in PSQI (standard mean difference = 0.87, 95% CI: 0.28, 1.43), ESS (standard mean difference = 1.42, 95% CI: 0.78, 2.02), CGI [global improvement (standard mean difference = 0.82, 95% CI: 0.23,1.28) and efficacy index (standard mean difference = 0.97, 95% CI: 0.37,1.54)] and had better adverse events profile (standard mean difference = 0.79, 95% CI: 0.21, 1.36). Both the groups had a good safety profile assessed through liver and renal profiles.
Conclusion
Ayurveda interventions has additional advantages likes improvements in sleep profile, lesser adverse events and better global improvement in management of GAD.
CTRI Registration Number is CTRI/2020/09/027750.
Keywords: Generalized anxiety disorder, Brahmi vati, Saraswatarista, Escitalopram, Ayurveda, Randomized controlled trial
Highlights
-
•
Randomized controlled parallel group design provides strength to the study.
-
•
Generalized Anxiety disorder diagnosed as per DSM-5.
-
•
Comprehensive assessment of anxiety, depression, sleep, quality of life and disease.
-
•
Ayurveda intervention were comparable to escitalopram.
-
•
Additional advantages were in sleep, global improvement and lesser adverse events.
1. Introduction
Anxiety disorders are the most common mental illnesses worldwide and are associated with high comorbidity and mortality [1]. Generalized Anxiety Disorder (GAD) is one of the prevalent, severe, and incapacitating illnesses that is frequently misdiagnosed and undertreated. The core feature of GAD is worry which is excessive, chronic, pervasive, and is associated with physical, psychological symptoms that interferes with daily life [2]. It is marked by chronic course, high comorbidity, with low rate of remission and recovery [3].
Epidemiological study [4] on general population across 26 countries showed that the lifetime prevalence of GAD in 12-month, 30-day were 3.7%, 1.8% and 0.8% across the globe respectively. GAD affects women twice as frequently as it does men. Risk factors include low socioeconomic status, widowed, separated, divorced, middle age, comorbid psychiatric disorders [5], history of substance abuse [6], trauma [7] and family history of GAD [8]. GAD symptomatology varies in severity. GAD is associated with sleep disturbance in 60–70% of the patients [9]. A systematic review [10]assessed humanistic and economic outcomes among GAD patients and reported gross derangement in quality of life affecting psychosocial functioning, role function, work productivity and more disability days.
GAD is diagnosed correctly in one third of the cases [11], 60 % of those diagnosed are not treated [12]. GAD is managed through various pharmacological agents. First line psychopharmacological agents are selective serotonin reuptake inhibitor (SSRI) and selective norepinephrine reuptake inhibitor (SNRI) and second line are buspirone, benzodiazapines, pregabalin and second generation antipsychotics [13]. A systematic review showed the efficacy of escitalopram over placebo with a good acceptability [14]. Few of the demerits with the current pharmacological interventions include, a four-week wait for symptom alleviation, lack of response, incomplete remission, lingering symptoms, side effects, dependence, tolerance, and relapse risk [15]. Various factors including adverse effects are responsible for treatment seeking in complementary and alternative systems and anxiety forms the strongest factor [16].
Ayurveda is one of the most ancient systems of medicine and has documented various treatment modalities for the management of psychiatric disorders. ‘Chittodwega’ [17] mentioned in ayurvedic literature resembles manifestations of GAD. It is primarily caused by derangement of manasika doshas i.e., Rajas and Tamas. Brahmi Vati [18] and Saraswatarista [19] are together used by ayurvedic experts for the management of mental illnesses like GAD. A study [20] has showed that Brahmi Vati was effective in GAD. Escitalopram is one among SSRIs approved by United States Food and Drug Administration authority for the management of GAD [16]. Brahmi vati is a herbo mineral compound formulation and has various ingredients like Brahmi (Bacoppa monnieri (L.) Pennell), Shankhapushpi (Convolvulus pluricaulis Choisy), Vacha (Acorus calamus), Swarnamakshika (Chalcopyrite), Rasa sindoora (Red sulphide of mercury) and Jatamamsi (Nardostachys jatamamsi. DC) etc. [Table no 4 (supplementary data)]. Saraswatarista is an herbo-mineral hydro alcoholic formulation with 22 ingredients. Ingredients are Brahmi, shatavari (Asparagus racemosus Willd), vidarika (Pueraria tuberosa(Willd.)DC), Amrita (Tinospora cordifolia Willd.), Vacha (Acorus calamus Linn.), Ashwagandha (Withania somnifera (L.)Dunal) etc. [Table no 5 (supplementary data)]. Brahmi Vati and Saraswatarista on co administration has shown favourable outcome in our clinical settings. Hence this study was planned to compare the efficacy of Brahmi Vati along with Saraswatarista in management of GAD.
2. Materials and methods
2.1. Research design
The study was a randomised controlled parallel group design. Randomization was done using a block design with 25 blocks of 2. Online software (Random Number Generator software) was used for random sequence generation. The sequences were created by the principal investigator and sealed. Allocations concealment was through sealed opaque envelopes and were investigators blind. An impartial assistant from the research unit who was not involved in patient allocation unsealed the envelopes one by one after each patient gave their consent for the study and baseline assessment. The patients allocation in control and intervention groups were in the 1:1 ratio. Randomization, distribution, and administration of study-related materials were carried out by an independent research team. Adherence charts and counting of unused medications were noted to score the adherence. Assessment through clinical assessment tools was conducted on every 15th day. Laboratory parameters were evaluated at pre and post study.
Sample size calculation was based on a previous study [21]. And calculation was through the primary outcome of this study (Standard deviations were 3.23 and 4.28 in two groups, estimated effect size was 0.79). Total sample was 50, 25 in each arm with 5% alpha error, 80% power.
2.2. Patients
Patients attending the out patient department of KLE's Shri B M K Ayurveda Hospital Karnataka, India were recruited in the study. Reporting was as per CONSORT statement guidelines [22].Patients (n = 50) diagnosed as Generalized Anxiety Disorder as per Diagnostic and Statistical Manual of Mental Disorders (DSM-5) criteria [23] were recruited from outpatient department of the Institute.
2.2.1. Inclusion criteria
Patients of 18–60 years age of either sex. Patients with predominant anxiety and worry for more than 6 months and meeting the other DSM-5 diagnostic criteria for GAD were included in the study.
2.2.2. Exclusion criteria
Patients with lakshanas of Unmad (co-morbid psychiatric disorders); on psychotropic drugs four weeks prior to study; substance abuse like alcohol etc.; significant depression (BDI >17); other medical complications like hypertension, diabetes mellitus; pregnant and lactating women were excluded from the study.
2.2.3. Screening methods
Study brochures, pamplets, information were displayed at the prominent places of the hospital, institute, and in the camps conducted by the institute. Information brochures were circulated in various social media platforms connecting physicians, rural health workers, patients and care givers visiting psychiatric unit of the hospital. VBG and BRT was responsible for these activities and personally communicated to all the information seekers. Patients meeting the diagnostic criteria of GAD (DSM-5) were screened to enrol 50 patients in the study. Patients were recruited in the study were subjected to thorough examination and systematic data recording was done. Laboratory investigations including haemoglobin, liver function test, serum creatinine and serum urea were carried out in the clinical laboratory of the institute at baseline in all the patients.
2.2.4. Intervention
All the patients were randomly divided into two interventional groups: Group A and Group B. Group A received Tab escitalopram 10 mg/day for first 10 days, followed by 20 mg/day for next 50 days along with water after food. Group B received Brahmi Vati 500 mg thrice a day (TID) and Saraswatarista 10 ml TID after food with water for 60 days. Dosage of the drugs were as per the classical text books of Ayurveda [21,22].
Ingredients of Brahmi vati and Saraswatarista were purchased from reliable suppliers. All raw ingredients were authenticated at the Ministry of AYUSH-approved Ayurveda, Siddha, and Unani (ASU) drug testing facility, Central Research Facility (CRF), Karnataka Lingayat Education society (KLE) Shri B M Kankanwadi (BMK) Ayurveda Mahavidyalaya Belagavi. Each raw material and completed product underwent a qualitative analysis in accordance with API (Ayurvedic Pharmacopeia of India) standards. Saraswatarista was prepared in the GMP certified KLE Ayurveda pharmacy, Belagavi, Karnataka. Standard operating procedures were used in preparation of saraswatarista. Escitalopram was procured from Intas Pharmaceuticals Ltd. Ahmedabad Gujarat India (Batch No. N2003251) complying the standard procedures. Duration of intervention was 60 days with follow-up on every 15th day during treatment.
Patients were explained the nature and design of the study and informed consent was obtained. The study was approved by the Institutional Ethics Committee (Protocol Id- BMK/19/PG/KC/3). CTRI Registration Number is CTRI/2020/09/027750. Data collection was from February 2021 to June 2022. Patients were urged to follow the treatment plan throughout the study and notify the researchers on development of any untoward events. A drug compliance chart was used to evaluate drug compliance and was given to the patient at each appointment. It was instructed to fill it on daily basis, periodically send it to the investigator (VBG) and submit the compliance chart during the follow up visit. Unused drugs during the follow up visits were collected and cross checked with the adherence chart.
2.3. Criteria for assessment
2.3.1. Primary outcome measure
Hamilton Anxiety Rating Scale (HARS) [24]. Is a 14 item clinician administered clinical scale to asses the severity of anxiety. Scores of less than 17 indicates mild anxiety, moderate from 18 to 24 and severe anxiety is above 25.
2.3.2. Secondary outcome measure
The secondary outcomes measures were GAD 7 scale [25] (Is a self reported seven item measure that evaluates main manifestations of GAD. Low anxiety ranges from 0 to 4, mild anxiety from 5 to 9, moderate anxiety from 10 to 14, and severe anxiety above 15). Beck's Depression Inventory scale (BDI) [26] (is a self-reported, 21-item rating scale used to assess depressive symptoms. Total score of 0–13 is minimal depression, 14–19 is mild, 20–28 is moderate and 29–63 is severe depression), Epworth sleepiness scale (ESS) [27] (Is a 8 item scale measuring the day time sleepiness. Scores 0–5 is lower normal, 6–10 higher normal, 11–12 mild excessive, 13–15 moderate excessive, 16–24 is severe excessive daytime sleepiness), Pittsburgh Sleep Quality Index (PSQI) [28] (Is a 19 item self rated questionnaire assessing sleep quality and sleep disturbance. Scores above 5 suggest of sleep disturbance), World health organisation (WHO) Quality of Life- BREF (WHOQOL-BREF) [29] (Is a 26 item self reported questionnaire assessing quality of life in domains like physical health, psychological health, social relationships, and environment), Clinical Global Improvement scale (CGI) [30] (Is a 3 item observer rated clinical tool assessing severity of symptoms, treatment response and efficacy of treatments), UKU-side effect scale [31] (Is a 48 items global assessment of side effects for psychotrophic drugs assessing in domains like psychic, autonomic, neurological and others). Haemoglobin, serum creatinine, serum urea, liver function tests (total bilirubin, direct bilirubin, aspartate amino transferase, alanine amino transferase, alkaline phosphatase, total protein and albumin, albumin/globulin ratio) were the blood parameters. All the clinical assessment scales were evaluated at baseline, 15th, 30th, 45th, and 60th day. Blood assessments were done at base line and 60th day of interventions.
2.4. Statistical methods
SPSS Version 25.0 (IBM Corporation, Chicago, Illinois, United States) was used for the statistical analysis. By the χ2 test, the homogeneity between groups was assessed. A two-way repeated measure analysis of variance (rmANOVA) with a Bonferroni post-hoc test was used to compare the groups at different time intervals. Two time points within the same group were compared using the paired t-test. The independent sample t-test was used to compare the groups at a given time point. By comparing pre- and post-treatment changes, the treatment outcome was evaluated. The treatment effect was measured by the outcome from baseline to the 60th day of the intervention and the effect size was calculated using the partial Eta Square method. Effect size measurements were interpreted as follows: 0–0.2 minimal, 0.2–0.5 small, 0.5–0.8 medium, and above 0.8 high effect size [32]. Reporting values is done as mean ± standard deviation. Statistical significance was set at p < 0.05.
3. Results
3.1. Characterization of medicines
3.1.1. Brahmi vati
Qualitative analysis of each raw ingredients of the herbal drugs were macroscopic assessment (part, color, taste, odour). Physico chemical assessments were foreign matter, ash values, water and alcohol extractive values and loss on drying. Rasa sindhur (reddish brown colour, taste less, odour less, test for mercury-present, test for sulphur-present), swarna makshika bhasma (dark brown colour, taste less, loss on drying- 0.1%, acid soluble ash- 20.85%, qualitative test for iron-present). Finished product details are furnished. Macroscopic description is, tablet form, reddish brown colour and odourless. Physicochemical standards are loss on drying at 110 Centigrade was 3.05%, ash value was 23.18%, acid insoluble ash was 14.02%, water soluble extractive was 29.12%, alcohol soluble extractive was 25.44%, disintegration time was 20 min. Microbial test report (total bacterial count- 07 cfu/ml, total fungal count- 01 cfu/ml), bacteria like E.coli, S.aureas, P.aeruginosa, S.abony were absent. Impression of the sample was of standard quality.
3.1.2. Saraswatarista
Qualitative analysis of each raw herbal drugs were macroscopic assessment (part, color, taste, odour). Physico chemical assessments were foreign matter, ash values, water and alcohol extractive values, loss on drying, organoleptic characters were -liquid form, dark brown colour, fragrant odour and bitter taste. Physico chemical standards are specific gravity 1.10 g, total solids were 25.98% W/V, reducing sugars were 12.5%, alcohol content was 10% V/V and pH value was 3.57. Microbial test report (total bacterial count- 09 cfu/ml, total fungal count- 03 cfu/ml), bacteria like E.coli, S.aureas, P.aeruginosa, S.abony were absent. Impression of the sample was of standard quality.
3.2. Patient profile
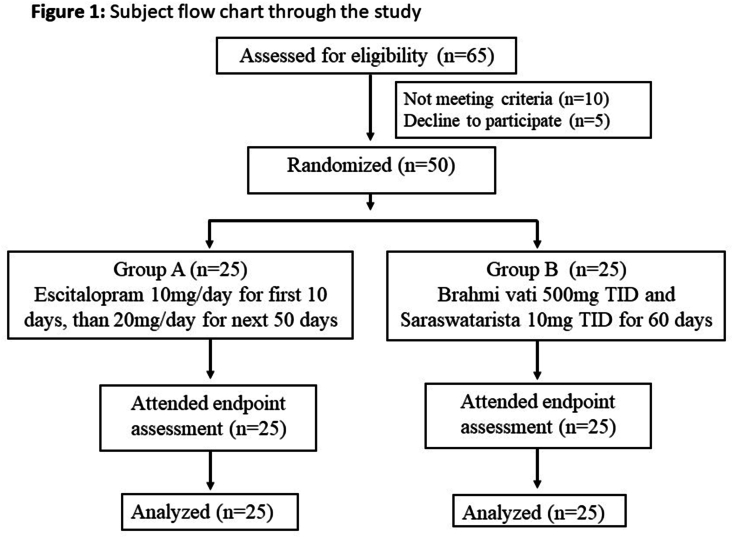
Sixty five patients meeting the diagnostic criteria (DSM-5) of GAD were screened. Not meeting the criteria were 10 due to significant comorbidity like depression, concomitant disease like diabetes mellitus. Five patients meeting the criteria declined to participate in the study. Reasons were apprehensions related to clinical trial, unable to comply with the protocol like frequency of visits, regularly filling the drug compliance forms etc. A total of 50 patients participated in the study. No patient dropped out of the study. The base line features like mean age (p = 0.37), gender (p = 0.22), educational status (p = 0.94), marital status (p = 0.30), diet status (p = 0.55), Shareerika prakurti (p = 0.60), manasika prakurti (p = 0.66), weight (p = 0.63), BMI (p = 0.59), duration of illness (p = 0.66), systolic blood pressure (p = 0.61), diastolic blood pressure (p = 0.48) were comparable between the groups except socio-economic status (p = 0.006). Most of the base line clinical features like HARS (p = 0.93), GAD7 (p = 0.79), BDI (p = 0.50), PSQI (p = 0.11), WHOQOL-Bref (p = 0.16), CGI severity (p = 0.33) were comparable between the groups except ESS (p = 0.01) (Table 1) (Fig. 1). Blood parameters including haemoglobin, liver function test, serum creatinine and serum urea were comparable between the groups at baseline.
Table. 1.
Patient profile at baseline.
| Clinical profile |
Group A (n = 25) |
Group B (n = 25) |
p |
|
|---|---|---|---|---|
| Age (Yrs) | 33.52 ± 11.22 | 30.96 ± 8.69 | 0.37 | |
| Gender | Male | 14 | 6 | 0.22 |
| Female | 11 | 19 | ||
| Socioeconomic Status | Higher class | 6 | 14 | 0.006 |
| Middle class | 15 | 8 | ||
| Lower class | 4 | 3 | ||
| Diet | Vegetarian | 8 | 10 | 0.55 |
| Non vegetarian | 17 | 15 | ||
| Education status | Primary | 5 | 5 | 0.94 |
| High school | 8 | 7 | ||
| Graduate | 12 | 13 | ||
| Marrital Status | Married | 11 | 10 | 0.30 |
| Unmarried | 12 | 15 | ||
| Widow | 2 | 0 | ||
| Shareerika Prakurti |
Vata | 2 | 0 | 0.60 |
| Kapha Vata | 3 | 3 | ||
| Pitta Kapha | 3 | 3 | ||
| Vata Kapha | 2 | 1 | ||
| Vata Pitta | 15 | 18 | ||
| Manasika Prakurti | Rajasika | 21 | 23 | 0.66 |
| Tamasika | 4 | 2 | ||
| Base line characteristics | HARS | 23.56 ± 6.12 | 23.40 ± 6.84 | 0.93 |
| GAD7 | 14.96 ± 2.03 | 15.12 ± 2.22 | 0.79 | |
| BDI | 12.84 ± 3.14 | 13.40 ± 2.71 | 0.50 | |
| ESS | 5.44 ± 3.01 | 7.28 ± 1.84 | 0.01 | |
| PSQI | 10.44 ± 3.33 | 8.92 ± 3.44 | 0.11 | |
| WHOQOL-Bref | 91.28 ± 6.93 | 88.52 ± 6.81 | 0.16 | |
| CGI-S | 3.48 ± 0.59 | 3.64 ± 0.57 | 0.33 | |
| Weight (Kgs) | 57.92 ± 9.68 | 59.17 ± 9.04 | 0.63 | |
| BMI | 22.68 ± 3.06 | 23.2 ± 3.72 | 0.59 | |
| Systolic Blood pressure (mm of Hg) | 122.8 ± 10.21 | 121.6 ± 6.24 | 0.61 | |
| Diastolic Blood pressure (mm of Hg) | 79.60 ± 4.54 | 78.8 ± 3.77 | 0.48 | |
| Duration of illness (Yrs, mean ± SD) | 2.44 ± 2.05 | 2.2 ± 1.7 | 0.66 | |
| Study completed | 25 | 25 | ||
Data are expressed in Mean and/or standard deviations (S.D.). HARS-Hamilton Anxiety Rating scale, GAD7- Generalized Anxiety disorder 7 scale, BDI- Beck's Depression Inventory scale, ESS-Epworth sleepiness scale, PSQI- Pittsburgh Sleep Quality Index, WHOQOL-BREF- WHO quality of life -BREF, CGI- Clinical Global Impression scales, BMI-Body Mass Index. UKU- UKU Side effect scale.
Fig. 1.
Subject flow chart through the study.
3.3. Primary outcome
HARS Assessments showed that outcomes were comparable between both the groups (p = 0.08). Both the groups showed significant improvement in HARS at all the five time points (p < 0.001). Effect size was minimal (standard mean difference = 0.06, CI: 0.50, 0.61). [Table 2 (supplementary data),].
Table. 2.
Effects on outcome variables.
| Sr No | Parameter | Interven tion | Baseline | 15th day | 30th day | 45th day | 60th day | Outcome difference (0–60day) | P value | Effect size |
|---|---|---|---|---|---|---|---|---|---|---|
| Primary Outcomes | ||||||||||
| 1. | HARS | A | 23.56 ± 6.12 | 21.16 ± 5.12 | 15.84 ± 4.26 | 12.16 ± 3.97 | 10.08 ± 4.25 | 13.68 ± 3.76 | 0.84 | 0.06 |
| B | 23.40 ± 6.84 | 20.84 ± 5.98 | 16.36 ± 5.43 | 12.08 ± 5.16 | 9.72 ± 4.24 | 13.48 ± 3.30 | ||||
| Secondary outcomes | ||||||||||
| 2. | GAD-7 | A | 14.96 ± 2.03 | 12.20 ± 1.96 | 8.92 ± 1.71 | 7.12 ± 1.88 | 5.84 ± 1.65 | 8.68 ± 1.62 | 0.30 | 0.33 |
| B | 15.12 ± 2.22 | 12.76 ± 2.26 | 9.80 ± 2.12 | 7.68 ± 2.08 | 6.44 ± 1.61 | 9.12 ± 1.36 | ||||
| 3. | BDI | A | 12.84 ± 3.14 | 11.64 ± 2.86 | 10.00 ± 2.45 | 6.88 ± 1.90 | 5.16 ± 2.43 | 7.8 ± 2.48 | 0.87 | 0.05 |
| B | 13.40 ± 2.71 | 12.60 ± 2.60 | 10.88 ± 2.30 | 7.32 ± 2.21 | 5.60 ± 1.63 | 7.68 ± 2.77 | ||||
| 4. | ESS | A | 5.44 ± 3.01 | 8.56 ± 3.07 | 9.08 ± 2.52 | 8.84 ± 2.32 | 8.40 ± 2.38 | −2.96 ± 2.30 | <0.001 | 1.42 |
| B | 7.28 ± 1.84 | 7.56 ± 2.14 | 7.20 ± 2.08 | 7.04 ± 1.95 | 6.92 ± 1.89 | 0.36 ± 1.18 | ||||
| 5. | PSQI | A | 10.44 ± 3.33 | 10.40 ± 3.14 | 8.88 ± 3.15 | 8.04 ± 2.86 | 8.04 ± 3.02 | 2.4 ± 1.87 | 0.003 | 0.87 |
| B | 8.92 ± 3.44 | 8.64 ± 3.23 | 6.20 ± 2.53 | 5.20 ± 2.24 | 4.64 ± 1.98 | 4.28 ± 2.42 | ||||
| 6. | WHO-QOL-BREF | A | 91.28 ± 6.93 | 92.32 ± 7.00 | 96.32 ± 7.10 | 99.52 ± 7.30 | 101.08 ± 7.04 | −8.36 ± 2.94 | 0.11 | 0.45 |
| B | 88.52 ± 6.81 | 89.60 ± 6.49 | 92.08 ± 6.36 | 95.04 ± 6.25 | 96.88 ± 5.61 | −9.80 ± 3.39 | ||||
| 7. | CGI-S | A | 3.48 ± 0.59 | 3.12 ± 0.44 | 2.60 ± 0.58 | 2.24 ± 0.44 | 2.16 ± 0.37 | 1.32 ± 0.47 | 1 | 0 |
| B | 3.64 ± 0.57 | 3.32 ± 0.56 | 2.80 ± 0.50 | 2.64 ± 0.57 | 2.32 ± 0.48 | 1.32 ± 0.47 | ||||
| 8. | CGI-GI | A | – | 3.08 ± 0.28 | 2.84 ± 0.37 | 2.36 ± 0.49 | 1.92 ± 0.49 | 0.8 ± 0.5 | 0.006 | 0.82 |
| B | – | 3.20 ± 0.41 | 3.00 ± 0.00 | 2.84 ± 0.37 | 2.40 ± 0.50 | 1.16 ± 0.37 | ||||
| 9. | CGI-E | A | – | 9.16 ± 0.80 | 8.04 ± 1.74 | 5.64 ± 1.50 | 5.16 ± 0.80 | 2.72 ± 1.86 | 0.001 | 0.97 |
| B | – | 9.96 ± 1.67 | 9.68 ± 1.03 | 8.36 ± 2.38 | 7.24 ± 2.35 | 4 ± 0 | ||||
| 10. | UKU | A | – | 0.28 ± 0.45 | 0.52 ± 0.50 | 0.48 ± 0.50 | 0.48 ± 0.50 | −0.48 ± 0.50 | 0.005 | 0.79 |
| B | – | 0 | 0.04 ± 0.20 | 0.12 ± 0.33 | 0.12 ± 0.33 | −0.12 ± 0.33 | ||||
| Clinical assessment | ||||||||||
| 11. | Weight (Kgs) | A | 57.92 ± 9.68 | 57.83 ± 9.7 | 57.91 ± 9.61 | 58.06 ± 9.82 | 58.11 ± 9.84 | −0.19 ± 5.96 | 1 | 0 |
| B | 59.17 ± 9.04 | 59.27 ± 8.99 | 59.42 ± 8.91 | 59.37 ± 8.97 | 59.37 ± 9.01 | −0.19 ± 0.71 | ||||
| 12. | BMI | A | 22.68 ± 3.06 | 22.64 ± 3.07 | 22.03 ± 5.02 | 22.08 ± 5.05 | 22.1 ± 5.07 | 0.58 ± 3.38 | 0.35 | 0.22 |
| B | 23.2 ± 3.72 | 23.26 ± 3.70 | 23.29 ± 3.67 | 23.62 ± 3.69 | 23.25 ± 3.71 | −0.05 ± 0.29 | ||||
| 13. | SBP (mm of Hg) | A | 122.8 ± 10.21 | 121.6 ± 10.27 | 121.6 ± 9.86 | 121.6 ± 8.98 | 122 ± 8.66 | 0.8 ± 4.93 | 0.74 | 0.09 |
| B | 121.6 ± 6.24 | 120 ± 7.63 | 120.8 ± 7.59 | 118.8 ± 7.25 | 121.2 ± 5.25 | 0.4 ± 3.51 | ||||
| 14. | DBP (mm of Hg) | A | 79.60 ± 4.54 | 78.4 ± 4.72 | 79.2 ± 4.93 | 78 ± 4.02 | 78 ± 5.77 | 1.6 ± 6.88 | 0.25 | 0.20 |
| B | 78.8 ± 3.77 | 77.6 ± 4.35 | 76.80 ± 4.76 | 78 ± 5.77 | 79.2 ± 4.93 | −0.4 ± 5.38 | ||||
Data are expressed in Mean and/or standard deviations (S.D.). HARS-Hamilton Anxiety Rating scale, GAD7- Generalized Anxiety disorder 7 scale, BDI- Beck's Depression Inventory scale, ESS-Epworth sleepiness scale, PSQI- Pittsburgh Sleep Quality Index, WHOQOL-BREF- WHO quality of life -BREF, CGI- S- Clinical Global Impression - Severity, CGI- GI- Clinical Global Impression – Global Improvement, CGI- EI- Clinical Global Impression – Efficacy Index, BMI-Body Mass Index. UKU- UKU Side effect scale, SBP- Systolic Blood Pressure, DBP- Diastolic Blood Pressure.
3.4. Secondary outcomes
3.4.1. Between group comparison
Between group comparison of outcomes in PSQI (p = 0.003), CGI-G (p = 0.006), CGI-E (p = 0.001) and UKU (p = 0.005) showed significant difference and outcomes of group B were better than group A. In parameters of GAD7 (p = 0.33), BDI (p = 0.05), WHOQOL-Bref (p = 0.11) and CGI-Severity no significant difference in outcomes were observed between groups. ESS (p < 0.001) showed significant difference between groups, however ESS scores increased in group A and trend of decrease were noted in group B.
3.4.2. Within group comparison
Within group assessment showed that in both the groups significant improvements were noted in most of the time points in GAD7 (p < 0.001), BDI (p < 0.001), PSQI (p < 0.001), WHOQOL-Bref (p < 0.001), CGI-S (p < 0.001). CGI-G scores showed significant improvements on 45th day [group A (p = 0.007), group B (p < 0.001)] and 60th day (p < 0.001) in both the groups. CGI-E scores showed significant improvements on all time points of group B and in group A, it was only at 45th day (p = 0.008) and 60th day (p < 0.001). ESS and UKU scores showed significant increase in all time points of group A and no such changes were observed in group B. (Table 2, Supplementary data).
3.4.3. Effect size
Effect size showed large effect in PSQI (standard mean difference = 0.87, 95% CI: 0.28, 1.43), ESS (standard mean difference = 1.42, 95% CI: 0.78, 2.02), CGI- Global improvement (standard mean difference = 0.82, 95% CI: 0.23,1.28) and CGI-Efficacy index (standard mean difference = 0.97, 95% CI: 0.37,1.54)]. And was of medium effect in UKU side effect scale (standard mean difference = 0.79, 95% CI: 0.21, 1.36). Effect size was small in GAD7 (standard mean difference = 0.33, 95% CI: −0.23, 0.88), WHOQOL-Bref (standard mean difference = 0.45, 95% CI: −0.11, 1.01), BMI (standard mean difference = 0.22, 95% CI: −0.34, 0.77), DBP (standard mean difference = 0.20, 95% CI: −0.36, 0.77) and minimal in HARS (standard mean difference = 0.06, 95% CI: −0.50, 0.61),BDI (standard mean difference = 0.05, 95% CI: −0.51, 0.60), SBP (standard mean difference = 0.09, 95% CI: −0.46, 0.65), CGI-S (standard mean difference = 0, 95% CI: −0.55, 0.55) and weight (standard mean difference = 0, 95% CI: −0.55, 0.55).
3.4.4. Adverse effects
Mild adverse effects noted in 13 patients of group A. UKU side effect scale assessment showed autonomic side effects 2 (giddiness), psychic side effects 2 (sleepiness, lethargy, fatigue). Group B showed adverse effects in 3 patients and they were psychic side effects 2 (sleepiness) and other forms was 1 (reduced appetite). Chi square test comparing number of adverse events between group showed significant higher adverse events in group A than group B (p = 0.006). UKU global assessment showed physician and patient assessment scores as 2 in psychic side effects. In other form of side effects, physician assessment was 1 and patient assessment was 2. Adverse events were mild to moderate category and occurred during the different time points of the interventions, not affected the functioning of the patients and needed no additional intervention.
Comparison of groups in terms of different time points showed that group B produced significant effects from 30th day of intervention, as CGI-G showed changes at 30th day (p = 0.04), 45th day (p < 0.001) and 60th day (p = 0.001). Due to adverse events related to sleep and day time drowsiness, significant differences were noted from 15th day onwards. PSQI showed significant difference at 15th day (p = 0.05), 30th day (p < 0.001), 45th day (p < 0.001) and 60th day (p < 0.001). ESS showed changes at 30th day (p = 0.01), 45th day (p = 0.007) and 60th day (p = 0.02). CGI-E showed changes in all time points, 15th day (p = 0.03), 30th day (p = 0.001), 45th day (p < 0.001) and 60th day (p < 0.001).
Laboratory parameters like haemoglobin, total bilirubin, direct bilirubin, AST, ALT, Total protein, albumin, AG ratio, alkaline phosphatase, serum creatinine, serum urea were within normal limit in both the groups, pre and post intervention (Table 3, supplementary data).
Table. 3.
Effects on safety variables.
| Sl. No | Outcome variable | Intervention | Baseline | 60th day | P |
|---|---|---|---|---|---|
| 1 | Hemoglobin (g/dl) | A | 11.76 ± 2.04 | 12.02 ± 1.7 | 0.08 |
| B | 11.91 ± 1.42 | 11.8 ± 1.46 | |||
| 2 | ESR (mm in 60 mints) | A | 18.1 ± 9.5 | 15.5 ± 7.9 | 1 |
| B | 17.5 ± 7.5 | 17.3 ± 7.1 | |||
| 3 | Total Bilirubin (mg/dL) | A | 0.4 ± 0.05 | 0.4 ± 0.06 | 0.82 |
| B | 0.4 ± 0.03 | 0.4 ± 0.05 | |||
| 4 | Direct Bilirubin (mg/dL) | A | 0.1 ± 0 | 0.1 ± 0 | 1 |
| B | 0.1 ± 0 | 0.1 ± 0.1 | |||
| 5 | AST (IU/mL) | A | 22.9 ± 5.08 | 24.3 ± 8.6 | 0.03 |
| B | 23.3 ± 4.32 | 21.5 ± 5.8 | |||
| 6 | ALT (IU/mL) | A | 24.4 ± 6.9 | 23 ± 5.5 | 0.33 |
| B | 25.9 ± 4.1 | 22.6 ± 6.4 | |||
| 7 | Albumin (mg/dL) | A | 4.05 ± 0.1 | 3.9 ± 0.1 | 0.01 |
| B | 3.9 ± 0.2 | 4.0 ± 0.1 | |||
| 8 | A/G ratio | A | 1.2 ± 0.1 | 1.2 ± 0.1 | 0.24 |
| B | 1.1 ± 0.1 | 1.1 ± 0.1 | |||
| 9 | Total Protein (mg/dL) | A | 7 ± 0.2 | 7.1 ± 0.2 | 0.75 |
| B | 7.0 ± 0.2 | 7.0 ± 0.2 | |||
| 10 | Alkaline Phosphatase (IU/mL) | A | 119.3 ± 17.2 | 121.9 ± 13.7 | 0.22 |
| B | 121.6 ± 16.2 | 119.7 ± 9.6 | |||
| 11 | Serum Creatinine (mg/dL) | A | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.40 |
| B | 0.8 ± 0.1 | 0.8 ± 0.8 | |||
| 12 | Serum Urea (mg/dL) | A | 22.5 ± 4.0 | 22.3 ± 3.6 | 0.33 |
| B | 22.6 ± 3.8 | 22.5 ± 3.4 |
ESR- Erythrocyte sedimentation rate, AST-Aspartate aminotransferase, ALT-Alanine transaminase, A/G-Albumin globulin ratio.
4. Discussion
The study demonstrated that the effect of Ayurveda interventions (Brahmi vati and Saraswatarista) were comparable to escitalopram. Both the interventions produced comparable outcomes in anxiety, depression, quality of life and disease severity (HARS, GAD7, BDI, WHOQOL-Bref and CGI-Severity). Ayurveda interventions showed significantly better outcome improvements compared to escitalopram in night sleep, global improvement and efficacy index (PSQI, CGI-G and CGI-E). Escitalopram showed significant worsening of adverse events profile and day time sleepiness (UKU and ESS) compared to ayurveda group. However Ayurveda groups showed trends of decrease in day time sleepiness and lesser adverse events profile. Mild adverse events were more frequent in escitalopram group (13 patients) compared to ayurveda group (3 patients). Both the interventions showed good safety profile as liver function tests, serum creatinine, serum urea and haemoglobin levels were within the normative ranges at both pre and post assessments.
Review of patient profile in the study showed that more patients were middle age (32.24 yrs),female gender (60%), mean duration of illness was 2.32 yrs, weight (58.54 kgs), BMI (22.94), moderate (HARS-23.4) to severe anxiety (GAD7-15), mild to moderate depression (13.12), severity was mildly ill (CGI-S- 3.56), normal day time sleepiness (ESS- 6.36) and disturbed night sleep (PSQI -9.68).
Effect of escitalopram and ayurveda interventions were comparable in primary outcome (HARS). Both the groups had comparable improvements in few of the secondary outcomes like GAD7, BDI, WHOQOL-Bref and CGI-Severity. Within group comparison showed that both groups produced significant and similar improvements in HARS, GAD7, BDI, WHOQOL-Bref and CGI-Severity. Ayurveda interventions produced better outcomes in PSQI, CGI-G and CGI-E. Escitalopram showed significant higher adverse events profile (UKU) compared to ayurveda group. Adverse event profile in escitalopram showed significant worsening at different time points. Day time sleepiness scores (ESS) were significantly high in escitalopram group and it worsened at different time points, though they were in the normative ranges. Systolic and diastolic blood pressures were within the normative ranges in both the groups during the entire study and showed no significant changes. Weight and BMI showed no significant changes with both the interventions. Liver function tests, serum creatinine, serum urea and haemoglobin levels were within normative ranges in both the interventional groups. Ayurveda group showed better global improvements (CGI-GI) and efficacy (CGI-E), this could be due to better adverse effect profiles in ayurveda group. Effect size was large in ESS, PSQI, CGI-GI, CGI-E and medium in UKU side effect scale favouring Ayurveda group. Anxiety levels decreased from moderate-severe (HARS, GAD7) to mild category. Depression (BDI) reduced from mild to moderate category to minimal depression in both the groups. Day time sleepiness scores were within the normative ranges in both the groups. Night sleep disturbance reduced from disturbed levels to normal ranges in ayurveda group and remained disturbed in escitalopram group. Clinical global impressions-severity scores reduced from mild to border line ill category in both the groups. Clinical global impressions-global improvements progressed from minimally improved (15th day) to much improved scores (60th day) in both the groups. Clinical global impressions-efficacy index progressed from minimal (15th day) to moderate therapeutic effect (60th day) in both the groups.
Similar to our study, a flexible dose of escitalopram (10–20 mg/day) for 8 weeks showed to be safe, effective and well tolerated in patients of GAD [33]. A study [34] with a pooled data from double blind placebo controlled trials with 850 patients showed that escitalopram (10 mg/day in first 4 week and 20 mg/day in next 4 weeks) was effective and well tolerated in GAD and observed side effects were nausea, ejaculation disorder, fatigue, insomnia, decreased libido, and anorgasmia. Most common adverse effect was fatigue or somnolence in our study and similar finding is also reported [35].
A clinical study showed that Brahmi vati was effective in patients of GAD and it decreased anxiety and depression, improved disturbed sleep and quality of life and was comparable to Manasamitra vataka (a compound herbo mineral ayurveda formulation available in tablet form) [23]. These findings are similar to our current study. Another study [36] showed that Brahmi vati reduced anxiety and improved sleep profiles in patients of essential hypertension. Saraswatarista has various ingredients with anxiolytic, nootropic, antioxidant properties and produced decreased immobility in forced swim test and absence of general stimulation in open field test, suggestive of antidepressant activity [37] in animal model. Brahmi has stress-relieving and antioxidant properties. It also lowers lipid peroxidation in the rat prefrontal cortex, hippocampus and striatum and helps in the repair of abnormalities in neurotransmission and has antidepressant properties [38]. Shankhpushpi has anxiolytic, antidepressant, antioxidant, hypolipidemic, tranquillizing, antistress, immunomodulatory and analgesic activity [39]. Increase in brain monoamine and GABA neurotransmitter, anxiolytic effects were noted with Jatamamsi (Nardostachys jatamansi) [40]. Guduchi (Tinospora cordifolia) counteracts the depressive-like behaviour in mice and decreased the brain's monoamine oxidase activity, which led to higher concentrations of brain monoamines [41]. Other studies have also demonstrated the effect of Ayurveda interventions in GAD. A study [26]showed that effect of Manasamitra vataka was comparable to clonazepam in GAD with comorbid social phobia and it reduced anxiety, depression and improved quality of life. It also showed that add on effect of shirodhara (ayurveda therapy of oil dripping on forehead) with Brahmi taila (Ayurveda medicated oil) was helpful in reduction of daytime sleepiness.
4.1. Strengths of the study
Strengths of the study are randomized controlled parallel group design, 60 days of intervention and gold standard control. Gross clinical assessments like anxiety, depression, day time sleepiness, night sleep profile, quality of life, adverse effect profile, assessment of global improvement and efficacy index were helpful in better global assessments. Safety profile of the drugs assessed through liver function test, serum creatinine, serum urea and haemoglobin levels is also note worthy component.
4.2. Limitations of the study
Study has limitations. Though sample size was adequate to demonstrate the statistical significance, larger population will help in viewing the better picture of the outcomes. Hence warrants multi centric study. Socio-economic status was not comparable between the groups. Middle class were more in escitalopram and lower class in Ayurveda group. This could be limitation as socioeconomic status can effect the disease and participants through factors like nutrition and environment. Assessment through the subjective parameters is the limitations of the study and assessments through cortisol, electrophysiological parameters would have given more strength to the study. Study samples were of middle age and further studies are needed to extrapolate to other populations. Detailed assessment of Brahmi Vati and Saraswatarista through other qualitative phytochemical analysis like phytoestrogen assessment, reverse phase- HPLC analysis, steroid estimation, HPTLC assessment etc would have been beneficial. Drug to drug interaction between Brahmi Vati and Saraswatarista needs to be studied. Ayurveda group showed improvement in sleep profile of GAD patients. A study in GAD patients with sleep disturbance can through a more light on its utility. Additionally, it is restricted to the regional and cultural populations of the study area. Long term follow up study will be beneficial.
5. Conclusion
Current study showed that ayurveda drugs (Brahmi vati along with saraswatarista) were comparable to escitalopram in management of GAD by decreasing anxiety, depression, improving quality of life. Ayurveda drugs showed advantages over escitalopram in improving night sleep, decreasing day time drowsiness, better adverse effect profile, better global improvement and efficacy index. Ayurveda drugs showed a better comprehensive improvements compared to escitalopram. Long term studies are needed to have a better picture of efficacy and adverse events profiles. Further studies are needed to understand the pharmacokinetic and pharmacodynamic properties of the ayurveda drugs.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Declaration of generative AI in scientific writing
Nothing to disclose.
Author contributions
VBG - Methodology, Writing - Original draft preparation,Visualization, Data collection, Writing - Reviewing and Editing.
BRT - Conceptualization, Methodology, Writing - Original draft preparation, Writing -Reviewing and Editing, Statistical analysis.
SH - Methodology, Writing - Original draft preparation, Visualization, Data collection, Writing - Reviewing and Editing.
RT - Visualization, Data collection, Writing - Reviewing and Editing.
Acknowledgements
We would like to thank the principal, staff and PG scholars of KAHER's BMK Ayurveda Mahavidyalaya college and hospital for their support.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jaim.2024.101033.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Stein D.J., Scott K.M., de Jonge P., Kessler R.C. Epidemiology of anxiety disorders: from surveys to nosology and back. Dialogues Clin Neurosci. 2017;19(2):127–136. doi: 10.31887/DCNS.2017.19.2/dstein. PMID: 28867937; PMCID: PMC5573557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeMartini J., Patel G., Fancher T.L. Generalized anxiety disorder. Ann Intern Med. 2019;170(7):ITC49–ITC64. doi: 10.7326/AITC201904020. PMID: 30934083. [DOI] [PubMed] [Google Scholar]
- 3.Kelly K.M., Mezuk B. Predictors of remission from generalized anxiety disorder and major depressive disorder. J Affect Disord. 2017;208:467–474. doi: 10.1016/j.jad.2016.10.042. PMID: 27863710; PMCID: PMC5515235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruscio A.M., Hallion L.S., Lim C.C.W., Aguilar-Gaxiola S., Al-Hamzawi A., Alonso J., et al. Cross-sectional comparison of the Epidemiology of DSM-5 generalized anxiety disorder across the globe. JAMA Psychiatr. 2017;74(5):465–475. doi: 10.1001/jamapsychiatry.2017.0056. PMID: 28297020; PMCID: PMC5594751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grant B.F., Hasin D.S., Stinson F.S., Dawson D.A., June Ruan W., Goldstein R.B., et al. Prevalence, correlates, co-morbidity, and comparative disability of DSM-IV generalized anxiety disorder in the USA: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Psychol Med. 2005;35(12):1747–1759. doi: 10.1017/S0033291705006069. PMID: 16202187. [DOI] [PubMed] [Google Scholar]
- 6.Bolton J.M., Sareen J. Lifetime mood, anxiety, and drug use disorders are common in the United States population. Evid Base Ment Health. 2006;9(4):113. doi: 10.1136/ebmh.9.4.113. PMID: 17065310. [DOI] [PubMed] [Google Scholar]
- 7.Brawman-Mintzer O., Monnier J., Wolitzky K.B., Falsetti S.A. Patients with generalized anxiety disorder and a history of trauma: somatic symptom endorsement. J Psychiatr Pract. 2005;11(3):212–215. doi: 10.1097/00131746-200505000-00010. PMID: 15920396. [DOI] [PubMed] [Google Scholar]
- 8.McLaughlin K.A., Behar E., Borkovec T.D. Family history of psychological problems in generalized anxiety disorder. J Clin Psychol. 2008;64(7):905–918. doi: 10.1002/jclp.20497. PMID: 18509873; PMCID: PMC4081441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Papadimitriou G.N., Linkowski P. Sleep disturbance in anxiety disorders. Int Rev Psychiatr. 2005;17(4):229–236. doi: 10.1080/09540260500104524. PMID: 16194794. [DOI] [PubMed] [Google Scholar]
- 10.Revicki D.A., Travers K., Wyrwich K.W., Svedsäter H., Locklear J., Mattera M.S., et al. Humanistic and economic burden of generalized anxiety disorder in North America and Europe. J Affect Disord. 2012;140(2):103–112. doi: 10.1016/j.jad.2011.11.014. PMID: 22154706. [DOI] [PubMed] [Google Scholar]
- 11.Wittchen H.U., Kessler R.C., Beesdo K., Krause P., Höfler M., Hoyer J. Generalized anxiety and depression in primary care: prevalence, recognition, and management. J Clin Psychiatry. 2002;63(Suppl 8):24–34. PMID: 12044105. [PubMed] [Google Scholar]
- 12.Wang P.S., Lane M., Olfson M., Pincus H.A., Wells K.B., Kessler R.C. Twelve-month use of mental health services in the United States: results from the National Comorbidity Survey Replication. Arch Gen Psychiatr. 2005;62(6):629–640. doi: 10.1001/archpsyc.62.6.629. PMID: 15939840. [DOI] [PubMed] [Google Scholar]
- 13.Strawn J.R., Geracioti L., Rajdev N., Clemenza K., Levine A. Pharmacotherapy for generalized anxiety disorder in adult and pediatric patients: an evidence-based treatment review. Expet Opin Pharmacother. 2018;19(10):1057–1070. doi: 10.1080/14656566.2018.1491966. PMID: 30056792; PMCID: PMC6340395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Slee A., Nazareth I., Bondaronek P., Liu Y., Cheng Z., Freemantle N. Pharmacological treatments for generalised anxiety disorder: a systematic review and network meta-analysis. Lancet. 2019;393(10173):768–777. doi: 10.1016/S0140-6736(18)31793-8. Erratum in: Lancet. 2019 Apr 27;393(10182):1698. PMID: 30712879. [DOI] [PubMed] [Google Scholar]
- 15.Katzman M.A. Current considerations in the treatment of generalized anxiety disorder. CNS Drugs. 2009;23(2):103–120. doi: 10.2165/00023210-200923020-00002. PMID: 19173371. [DOI] [PubMed] [Google Scholar]
- 16.Astin J.A. Why patients use alternative medicine: results of a national study. JAMA. 1998 May 20;279(19):1548–1553. doi: 10.1001/jama.279.19.1548. PMID: 9605899. [DOI] [PubMed] [Google Scholar]
- 17.Trikamji Y., editor. Charaka samhita of charaka, vimana sthana; Roganika vimanam. second ed. Chaukhabha Sanskrit series; Varanasi: 2017. p. 254. [chapter 6], verse 5. [Google Scholar]
- 18.Pathak R., Sara Sangraha Ayurveda, Prakarana Vati. Chaukhamba Publication; 2009. Vol1,Varanasi; pp. 455–456. [Google Scholar]
- 19.Mishra B., Dr.Kanjiv Lochan(English translation) Chaukhamba Publications; Varanasi: 2008. Bhaisajya ratnavali of govind dasji bhisagratna; p. 1115. [Google Scholar]
- 20.Khot S.G., Tubaki B.R., Gonugade V.B. Efficacy of Brahmi vati in generalised anxiety disorder - randomized double blind comparative clinical trial. J Ayurveda Integr Med. 2022;13(2) doi: 10.1016/j.jaim.2022.100552. PMID: 35325682; PMCID: PMC8943402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tubaki B.R., Chandrashekar C.R., Sudhakar D., Prabha T.N., Lavekar G.S., Kutty B.M. Clinical efficacy of Manasamitra Vataka (an Ayurveda medication) on generalized anxiety disorder with comorbid generalized social phobia: a randomized controlled study. J Alternative Compl Med. 2012;18(6):612–621. doi: 10.1089/acm.2010.0778. PMID: 22784349. [DOI] [PubMed] [Google Scholar]
- 22.Schulz K.F., Altman D.G., Moher D., CONSORT Group CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 2010 Mar 24;8:18. doi: 10.1186/1741-7015-8-18. PMID: 20334633; PMCID: PMC2860339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.American Psychiatric Association, DSM-5 Task Force . fifth ed. American Psychiatric Publishing, Inc.; 2013. Diagnostic and statistical manual of mental disorders: DSM-5™. 2013. [DOI] [Google Scholar]
- 24.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32(1):50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. PMID: 13638508. [DOI] [PubMed] [Google Scholar]
- 25.Spitzer R.L., Kroenke K., Williams J.B., Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006 May 22;166(10):1092–1097. doi: 10.1001/archinte.166.10.1092. PMID: 16717171. [DOI] [PubMed] [Google Scholar]
- 26.Beck A.T., Steer R.A., Carbin M.G. Psychometric properties of the Beck depression inventory: twenty-five years of evaluation. Clin Psychol Rev. 1988;(1):77–100. [Google Scholar]
- 27.Johns M.W. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 28.Buysse D.J., Reynolds CF 3rd, Monk T.H., Berman S.R., Kupfer D.J. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatr Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. PMID: 2748771. [DOI] [PubMed] [Google Scholar]
- 29.The World Health Organization Quality of Life Assessment (WHOQOL) Development and general psychometric properties. Soc Sci Med. 1998;46(12):1569–1585. doi: 10.1016/s0277-9536(98)00009-4. PMID: 9672396. [DOI] [PubMed] [Google Scholar]
- 30.Guy W., editor. ECDEU assessment manual for psychopharmacology. US Department of Health, Education, and Welfare Public Health Service Alcohol, Drug Abuse, and Mental Health Administration; Rockville, MD: 1976. [Google Scholar]
- 31.Lingjaerde O., Ahlfors U.G., Bech P., Dencker S.J., Elgen K. The UKU side effect rating scale. A new comprehensive rating scale for psychotropic drugs and a cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatr Scand Suppl. 1987;334:1–100. doi: 10.1111/j.1600-0447.1987.tb10566.x. PMID: 2887090. [DOI] [PubMed] [Google Scholar]
- 32.Cohen J. 2 ed. L. Erlbaum Associates; 1988. Statistical power analysis for the behavioral sciences. [Google Scholar]
- 33.Davidson J.R., Bose A., Korotzer A., Zheng H. Escitalopram in the treatment of generalized anxiety disorder: double-blind, placebo controlled, flexible-dose study. Depress Anxiety. 2004;19(4):234–240. doi: 10.1002/da.10146. PMID: 15274172. [DOI] [PubMed] [Google Scholar]
- 34.Goodman W.K., Bose A., Wang Q. Treatment of generalized anxiety disorder with escitalopram: pooled results from double-blind, placebo-controlled trials. J Affect Disord. 2005;87(2–3):161–167. doi: 10.1016/j.jad.2004.11.011. PMID: 15982747. [DOI] [PubMed] [Google Scholar]
- 35.Lenze E.J., Rollman B.L., Shear M.K., Dew M.A., Pollock B.G., Ciliberti C., Costantino M., Snyder S., Shi P., Spitznagel E., Andreescu C., Butters M.A., Reynolds CF 3rd. Escitalopram for older adults with generalized anxiety disorder: a randomized controlled trial. JAMA. 2009;301(3):295–303. doi: 10.1001/jama.2008.977. PMID: 19155456; PMCID: PMC2840403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mishra D., Tubaki B.R. Effect of Brahmi vati and Sarpagandha Ghana vati in management of essential hypertension - a randomized, double blind, clinical study. J Ayurveda Integr Med. 2019;10(4):269–276. doi: 10.1016/j.jaim.2017.04.001. PMID: 29242090; PMCID: PMC6938844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parekar R.R., Jadhav K.S., Marathe P.A., Rege N.N. Effect of Saraswatarishta in animal models of behavior despair. J Ayurveda Integr Med. 2014;5(3):141–147. doi: 10.4103/0975-9476.140469. PMID: 25336844; PMCID: PMC4204283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sairam K., Dorababu M., Goel R.K., Bhattacharya S.K. Antidepressant activity of standardized extract of Bacopa monniera in experimental models of depression in rats. Phytomedicine. 2002;9(3):207–211. doi: 10.1078/0944-7113-00116. PMID: 12046860. [DOI] [PubMed] [Google Scholar]
- 39.Agarwa P., Sharma B., Fatima A., Jain S.K. An update on Ayurvedic herb Convolvulus pluricaulis Choisy. Asian Pac J Trop Biomed. 2014;4(3):245–252. doi: 10.1016/S2221-1691(14)60240-9. PMID: 25182446; PMCID: PMC3868798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Razack S., Kandikattu H.K., Venuprasad M.P., Amruta N., Khanum F., Chuttani K., Mishra A.K. Anxiolytic actions of Nardostachys jatamansi via GABA benzodiazepine channel complex mechanism and its biodistribution studies. Metab Brain Dis. 2018;33(5):1533–1549. doi: 10.1007/s11011-018-0261-z. PMID: 29934858. [DOI] [PubMed] [Google Scholar]
- 41.Dhingra D., Goyal P.K. Evidences for the involvement of monoaminergic and GABAergic systems in antidepressant-like activity of tinospora cordifolia in mice. Indian J Pharmaceut Sci. 2008;70(6):761–767. doi: 10.4103/0250-474X.49118. PMID: 21369437; PMCID: PMC3040870. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.