Abstract
Background & Aims
Quantifying alcohol intake is crucial for subclassifying participants with steatotic liver disease (SLD) and interpreting clinical trials of alcohol-related liver disease (ALD) and metabolic and alcohol-related liver disease (MetALD). However, the accuracy of self-reported alcohol intake is considered imprecise. We compared the diagnostic and prognostic utility of self-reported alcohol intake with blood-based biomarkers of alcohol intake: phosphatidylethanol (PEth) and carbohydrate-deficient transferrin (CDT).
Methods
We studied 192 participants from two randomized controlled trials on MetALD and ALD, all with current or former excessive alcohol intake (≥24/36 [♀/♂] grams daily for at least 1 year) and biopsy-proven liver disease. We assessed self-reported alcohol intake, PEth, and CDT at four time points. We collected follow-up data on hepatic decompensation and death manually through electronic medical records.
Results
Most participants were male (n = 161, 84%) with a mean age of 59 (SD 9) years and 73 participants reported 1-week abstinence before inclusion; the remaining reported a median alcohol intake of 43 g/day. Median PEth was 0.5 μmol/L (IQR: 0.0–1.3) and %CDT = 1.9 (IQR: 1.6–2.3). Of 32 patients reporting at least 6 months of abstinence; 27 (84%) was confirmed by PEth <0.05 μmol/L. Self-reported alcohol intake correlated well with PEth (r = 0.617) and moderately with CDT (r = 0.316). Self-reported alcohol intake, PEth, and CDT all predicted hepatic decompensation and death. However, PEth showed the highest prediction, surpassing self-reported alcohol intake (Harrel’s C, PEth = 0.80 vs. self-reported = 0.68, p = 0.026).
Conclusions
Self-reported abstinence can be considered reliable in clinical trials. However, PEth is superior in predicting hepatic decompensation and death in patients with MetALD and ALD.
Impact and implications
An accurate quantification of alcohol intake is crucial in the clinical phenotyping of patients with steatotic liver disease and when designing clinical trials. This study found self-reported abstinence to be reliable but phosphatidylethanol was a more accurate prognostic biomarker of hepatic decompensation and death in a clinical trial setting. Findings may inform the design of future trials in patients with steatotic liver disease.
Keywords: alcohol use disorder, alcohol-related liver disease, cirrhosis, steatotic liver disease, biomarker
Graphical abstract
Highlights
-
•
Self-reported abstinence for 6 months is reliable and confirmed by low PEth levels in most cases.
-
•
Self-reported alcohol intake shows a good correlation with PEth and a moderate correlation with CDT.
-
•
PEth, CDT, and self-reported intake each independently predict hepatic decompensation and death.
-
•
However, PEth is more effective than self-reported intake in predicting hepatic decompensation and death.
-
•
Self-reported alcohol abstinence is reliable in a clinical trial setting and can be used for stratification purposes.
Introduction
Alcohol is a leading cause of liver cirrhosis and liver-related mortality worldwide.1 The steatotic liver disease (SLD) nomenclature provides a framework to subclassify patients within the disease spectrum according to alcohol intake.2 However, reliable methods to quantify alcohol intake are needed for correct subclassification.3,4 With the growing focus on early detection of chronic liver disease, there is an increasing interest in developing treatments for patients diagnosed with SLD and excessive alcohol intake (alcohol-related liver diseases [ALDs] and metabolic and alcohol-related liver disease [MetALD]).5,6 However, only a few clinical trials testing treatments for patients with SLD and a history of excessive alcohol intake have been performed. Consequently, there are limited data available on how to ideally design randomized clinical trials (RCTs) and quantify alcohol intake in this group of patients. The diagnoses of MetALD and ALD are based on the patient’s self-reported historic intake of alcohol.2,7 Furthermore, because alcohol is the leading cause of liver damage, accurate quantification of intake is crucial for trials in patients with MetALD and ALD.8 However, reporting of alcohol intake is subject to recall bias and stigma in healthcare settings.9,10 Besides the self-reported alcohol intake, phosphatidylethanol (PEth) and carbohydrate-deficient transferrin (CDT) are already in use as markers of alcohol intake.11 PEth is an abnormal phospholipid formed in the presence of ethanol, and integrated in the erythrocyte membrane when alcohol is present in the blood.12 PEth is recommended to confirm alcohol abstinence in patients on the liver transplant waiting-list, as it is independent of disease severity, where it can detect alcohol consumption during the past 2–4 weeks.13 CDT is a measure of the carbohydrate-deficient isoform of transferrin, which increases with alcohol intake by inhibition of the glycosylation of transferrin.14 CDT can detect alcohol consumption during the past 2–3 weeks.15 Although both PEth and CDT are capable of detecting alcohol consumption, their accuracy in predicting clinical outcomes compared with self-reported intake is unknown.16,17 Our primary aim was to investigate the reliability and prognostic performance of self-reported alcohol intake and abstinence, compared with PEth and CDT, in clinical trial participants with an alcohol intake corresponding to metabolic-dysfunction associated steatotic liver disease (MASLD), MetALD, and ALD. The secondary aim was to explore the reliability of self-reported alcohol intake over time in this setting.
Participants and methods
Study design
This study consisted of participants from two RCTs performed at Odense University Hospital in Denmark. The first trial (GALA-RIF trial) was an 18-month treatment trial, testing rifaximin-α as an anti-fibrotic treatment in patients with ALD.18 The second trial (GALA-POSTBIO trial) was a 6-month treatment with ReFerm® (Nordic Rebalance, Hillerød, Denmark), a postbiotic product made by fermenting oat gruel composition with Lactobacillus plantarum DSM 9843, to attenuate liver hepatic stellate cell activity in patients with ALD and advanced liver fibrosis.19 All participants were between 18 and 75 years of age, had biopsy-verified liver disease, and a current or previous history of an excessive high-risk alcohol intake (≥1 year with ≥24 g/day for women and ≥36 g/day for men), as defined by the Danish Health Authority at the time of the trials. A history of decompensation was an exclusion criterion in the GALA-RIF trial. Child-Pugh C and model for end-stage liver disease-sodium (MELD-Na) score ≥15 were exclusion criteria in GALA-POSTBIO. None of the participants were listed for liver transplantation at inclusion or during the trials. All participants signed an informed consent before any investigations in line with the Declaration of Helsinki.20 Both trials were approved by the local Ethical Committee of Southern Denmark (S-20140078 and S-17200163) and registered in clinical trial registries before the enrollment of participants (EudraCT: 2014–001856-51 and ClinicalTrials.gov: NCT03863730).
Investigations
Self-reported alcohol intake
All information on participants' alcohol intake was collected by trained personnel in a private examination room in both trials. We assessed the alcohol history by a short, protocolized interview with questions on current and previous alcohol consumption (Table S1). Participants were asked about their short- (past week) and long-term (6 months) alcohol abstinence status. For participants reporting an ongoing alcohol intake, the current daily alcohol intake was reported in Danish units (1 unit = 12 g of alcohol) and converted to grams of alcohol per day.
Blood sampling
We collected blood for biomarkers of alcohol intake at four visits in both trials. In GALA-RIF trial, the first visit was a screening biopsy visit. The second visit was at trial inclusion. The third and fourth visit was 1 and 18 months after trial inclusion, respectively. In the GALA-POSTBIO trial, the four visits were: trial inclusion and start of treatment, after 4 weeks of treatment, after 24 weeks of treatment, and finally 6–8 weeks after end of treatment. All blood samples were performed in a fasting state and stored immediately at -80 °C.
Measurement of alcohol biomarkers
PEth was measured from whole blood aliquots. All analyses were done by the Department of Biochemistry and Immunology at University Hospital of Southern Denmark, Vejle using liquid chromatography-tandem mass spectrometry, according to routine protocol. CDT was measured in plasma from participants in the GALA-RIF trial. We used the Siemens® N Latex CDT immunoassay platform as per the instructions of the manufacturer, at the Department of Biochemistry in the Hospital of South West Jutland. We used PEth level of <0.05 μmol/L and CDT level of <1.7 %CDT for detecting abstinence or an intake corresponding to MASLD.21,22
Histology
Liver biopsies were performed in relation to trial enrollment for all participants. Liver fibrosis was staged according to the Kleiner fibrosis score and steatosis, lobular inflammation, and ballooning were scored according to the non-alcoholic fatty liver disease activity score (NAS).23 All biopsies were evaluated by two expert pathologists blinded to the clinical data and treatment group of the participants.
Outcome data
We collected outcome data regarding hepatic decompensating events and death from the participants’ electronic medical records. The follow-up period for participants in the GALA-RIF trial was from the trial inclusion period (March 2015–May 2020) until death or end of follow-up in January 2023. The follow-up period for participants in the GALA-POSTBIO trial was from the start of the trial inclusion period (March 2019–January 2021) until death or end of follow-up in April 2023. We used the BAVENO VII criteria to qualify decompensating events (overt ascites, variceal bleeding or overt hepatic encephalopathy).24
Statistics
Parametric data are presented using means and standard deviations, whereas the non-parametric data are presented as medians with 25th and 75th percentiles. We performed statistical tests using the Student t test for continuous parametric data, the Wilcoxon rank-sum and Kruskal-Wallis test for comparison of continuous non-parametric data, and Χ2 test for categorical data. We assessed the correlation over time for self-reported alcohol intake and the biomarkers of alcohol consumption with Spearman’s rho. Only participants with complete data for PEth and self-reported alcohol intake are included in the Sankey plot. For time-to-event (decompensation or death) analysis we used Cox regression by conducting univariable and multivariable analyses including self-reported alcohol intake, PEth and CDT together with age, sex, treatment group, liver fibrosis stage, and BMI. Parameters with a value of p <0.10 from the univariable analysis were included in the multivariable analysis, where we applied stepwise backward elimination. We used C-statistics to calculate Harrel’s C to compare the prognostic ability of self-reported alcohol intake, PEth, and CDT. Self-reported alcohol intake subclassified patients according to the steatotic liver disease nomenclature.2 The corresponding groups were for PEth: an intake corresponding to MASLD <0.21 μmol/L, MetALD 0.21–0.42 μmol/L, and ALD >0.42 μmol/L, and for CDT: an intake corresponding to MASLD <1.78 %CDT, MetALD 1.78-2.08 %CDT, and ALD >2.08 %CDT.17,22 We used Youden’s index to calculate the optimal cut-off in our cohort. We considered a p <0.05 as statistically significant and used STATA 18 (Stata Corp., College Station, TX, USA) for all analyses.
Results
Baseline characteristics
We included 192 participants of which data from 136 (71%) participants was derived from the GALA-RIF trial and 56 (29%) from the GALA-POSTBIO trial (Table S2). Most participants were men (84%) with a mean BMI of 29 (SD ±6), the mean age was 59 years (SD ±9). Of the 192 participants, 186 (97%) presented with at least one cardiometabolic risk factor. The distribution of liver fibrosis stage according to Kleiner fibrosis score (F0/1/2/3/4) was 7/37/63/23/6 with a higher proportion of advanced fibrosis (≥F3) in the group reporting abstinence (Table 1).
Table 1.
Baseline participant characteristics.
| Total |
Non-abstinence |
Abstinence >1 week |
|
|---|---|---|---|
| N = 192 | n = 119 | n = 73 | |
| Trial, GALA-RIF trial, n (%) | 136 (71) | 96 (81) | 40 (55) |
| Age at inclusion, years | 59 (±9) | 60 (±8) | 58 (±9) |
| Sex, male, n (%) | 161 (84) | 102 (86) | 59 (81) |
| Body mass index, kg/m2 | 29 (±6) | 30 (±6) | 29 (±6) |
| Smoking, n (%) | |||
| Never smoker | 51 (26) | 33 (28) | 18 (25) |
| Previous smoker | 67 (35) | 42 (34) | 25 (34) |
| Current smoker | 68 (35) | 44 (37) | 24 (33) |
| Missing | 6 (3) | 6 (8) | |
| Type 2 diabetes, n (%) | 34 (18) | 21 (18) | 13 (18) |
| Write presence of cardiometabolic risk factors∗, n (%) | 186 (97) | 115 (97) | 71 (97) |
| Alanine transaminase, U/L | 35 (24–56) | 44 (30–69.5) | 27 (20–34) |
| Gamma-glutamyl transferase, U/L | 115 (54.5–291.5) | 185.5 (78.5–358.5) | 63.5 (35.0–131.5) |
| Alkaline phosphatase, U/L | 72.5 (55.5–95.0) | 74.0 (64.0–93.0) | 71.0 (35.5–98.0) |
| Bilirubin, µmol/L | 11 (7–15) | 11 (7–16) | 10 (7–15) |
| Platelets, 109/L | 202 (165–244) | 208 (172–245) | 193 (149–240) |
| INR | 1 (1–1.1) | 1 (1–1.1) | 1 (1–1.1) |
| Albumin, g/L | 43 (41–46) | 43 (41–46) | 44 (41–46) |
| Kleiner fibrosis score, n (%) | |||
| F0 | 7 (3.6) | 5 (4.2) | 2 (2.7) |
| F1 | 37 (19.3) | 22 (18.5) | 15 (20.5) |
| F2 | 67 (34.9) | 47 (39.5) | 20 (27.4) |
| F3 | 38 (19.8) | 28 (23.5) | 10 (13.7) |
| F4 | 39 (20.3) | 15 (12.6) | 24 (32.9) |
| Missing | 4 (2.1) | 2 (1.7) | 2 (1.7) |
| Transient elastography (FibroScan), kPa | 11.4 (7.9–18.4) | 10.2 (8.1–17.3) | 13.2 (7.1–20.5) |
| CAP score, dB/m | 304 (±60.6) | 317 (±59) | 284 (±59) |
| Alcohol | |||
| Alcohol intake for non-abstinent, g/day | 43 (24–69) | ||
| Years of excessive alcohol intake, n (%): | |||
| 1–5 | 19 (9.9) | 9 (7.6) | 10 (13.7) |
| 6–10 | 28 (14.6) | 20 (16.8) | 8 (11.0) |
| 11–20 | 49 (25.5) | 28 (23.5) | 21 (28.8) |
| 21–30 | 40 (20.8) | 27 (22.7) | 13 (17.8) |
| >30 | 47 (24.5) | 32 (26.9) | 15 (20.5) |
| Missing | 9 (4.7) | 3 (2.5) | 6 (8.2) |
| Phosphatidylethanol, μmol/L | 0.5 (0.0–1.3) | 0.9 (0.4–1.8) | 0.0 (0.0–0.1) |
| Carbohydrate-deficient transferrin†, %CDT | 1.9 (1.6–2.3) | 2.1 (1.7–2.6) | 1.7 (1.6–1.9) |
Values are reported as mean ± standard deviation, counts (proportion), and median (IQR).
Cardiometabolic criteria as defined in relation to SLD (2).
Only available in GALA-RIF trial. CAP, controlled attenuation parameter; INR, international normalized ratio.
Alcohol intake at study inclusion
At baseline, 32 of 192 (20%) participants reported alcohol abstinence for ≥6 months and 73 of 192 (38%) participants self-reported alcohol abstinence for ≥1 week. The proportion of self-reported abstinence for ≥1 week was 64% in the GALA-POSTBIO trial (64%) and 12% in the GALA-RIF trial. The median self-reported alcohol intake for participants with an ongoing alcohol intake was 43 g/day (IQR 24–69). The median PEth level was 0.5 μmol/L (IQR 0.0–1.3), with participants reporting abstinence for ≥1 week having significantly lower PEth levels compared with participants reporting ongoing alcohol intake (0.0 [IQR 0.0–0.1) vs. 0.9 [IQR 0.4–1.8] μmol/L, p <0.001). The median CDT level was 1.9 (IQR 1.6–2.3) with participants reporting abstinence for ≥1 week having significantly lower CDT levels compared with participants reporting ongoing alcohol intake (1.7 [IQR 1.6–1.9] vs. 2.1 [IQR 1.7–2.6] %CDT, p <0.001).
Comparison between self-reported alcohol intake and biomarkers of alcohol consumption
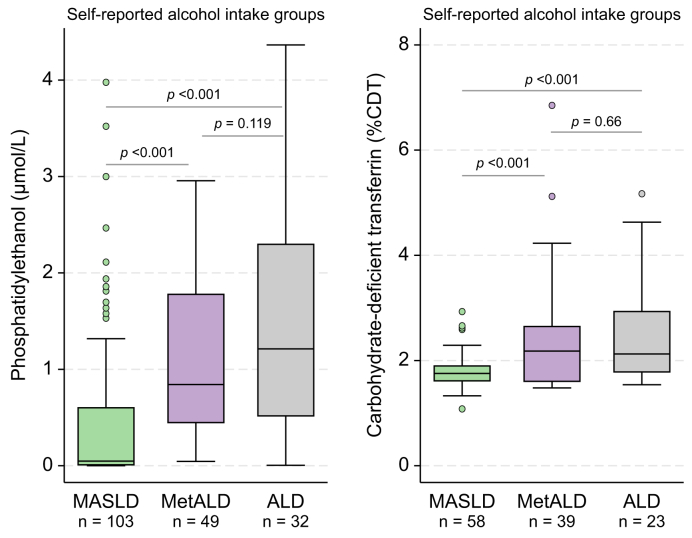
At inclusion, 44 of 73 (60%) of participants reporting short-term abstinence (≥1 week) had PEth levels <0.05 μmol/L. In participants reporting long-term alcohol abstinence (≥6 months) at inclusion, 27 of 32 (84%) had a PEth <0.05 μmol/L (Table S2). In participants with available CDT measurement and reporting short-term abstinence (≥1 week) 18 of 40 (45%) had CDT levels <1.7 %CDT indicating an intake corresponding to MASLD. Participants reporting long-term alcohol abstinence (≥6 months), eight of 16 (50%) had CDT levels <1.7 %CDT. Self-reported alcohol intake correlated significantly better with PEth (r = 0.617) compared with CDT at baseline (r = 0.316, p = 0.004) (Fig. S1). According to the self-reported alcohol intake, 103 (56%) had a daily alcohol intake corresponding to MASLD (<20/30 g/day), 49 (26%) had an intake corresponding to MetALD (20/30–50/60 g/day), whereas 32 (19%) had an intake corresponding to ALD (>50/60 g/day) at inclusion (Fig. S2). PEth and CDT separated the group with a self-reported intake corresponding to MASLD from the groups with an intake corresponding to MetALD or ALD (p <0.001, Fig. 1). There was no difference in PEth and CDT levels between the groups reporting intake corresponding to MetALD and ALD (Fig. 1).
Fig. 1.
Self-reported alcohol intake and blood-based biomarkers of alcohol intake.
Box plots of baseline PEth and CDT according to the defined self-reported alcohol intake groups: an intake corresponding to MASLD <20/30 g (♀/♂); MetALD, 20-50 g/30–60 g (♀/♂), and ALD, >50/60 g (♀/♂) with p values obtained using the Wilcoxon rank-sum test. Levels of alcohol intake are corresponding to the limits of MetALD and ALD. PEth was measured in 184 patients, while %CDT was measured in 120 patients. ALD, alcohol-related liver disease; MASLD, metabolic-dysfunction associated steatotic liver disease; MetALD, metabolic dysfunction and ALD.
Reliability of self-reported alcohol intake over time
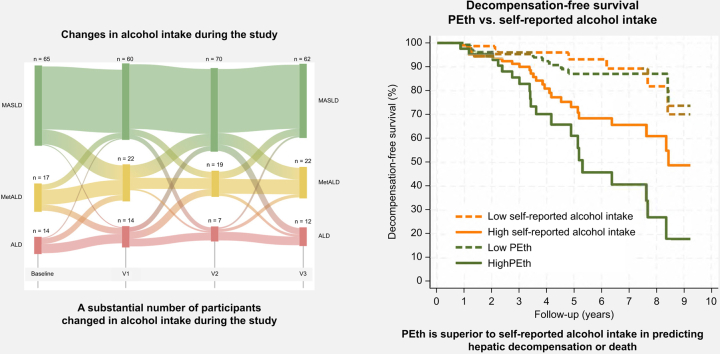
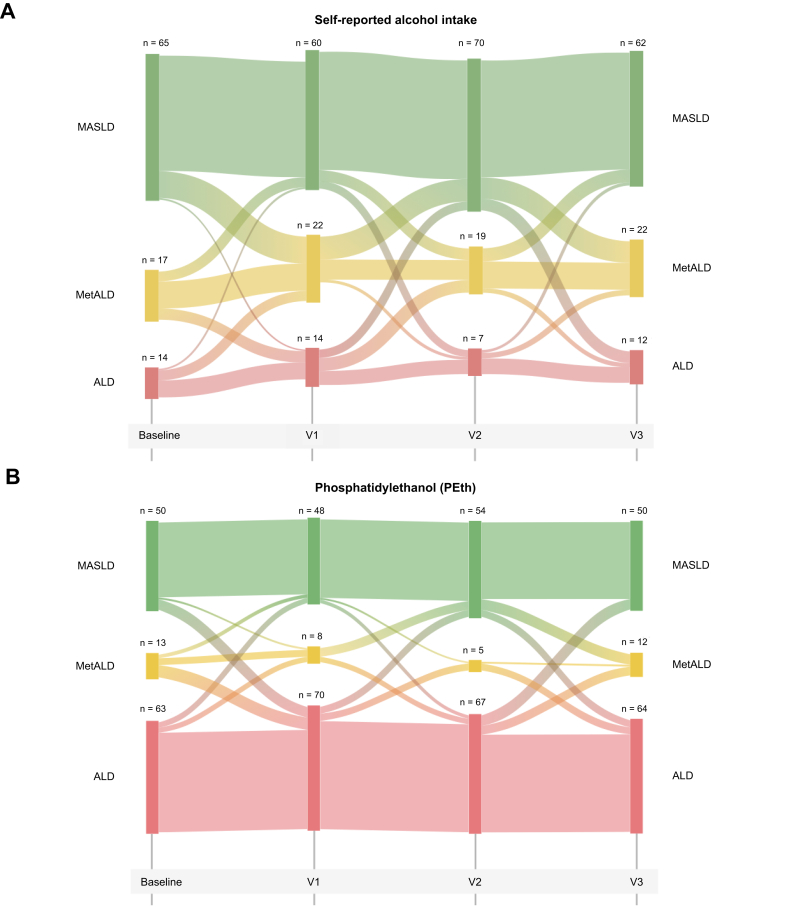
We assessed the association between self-reported alcohol intake and the biomarkers of alcohol consumption (PEth, CDT, and gamma-glutamyl transferase) at the four study visits. The correlation between self-reported alcohol intake and PEth and CDT levels remained relatively stable throughout the study (Fig. S3). Based on the repeated assessment of alcohol intake during the study periods, we found a substantial proportion of participants that changed their alcohol consumption during the study according to self-reported alcohol. These changes were not reflected in changes of PEth levels (Fig. 2).
Fig. 2.
Sankey plot over the flow of participants between self-reported alcohol intake and PEth groups over the study time.
Self-reported alcohol intake group is defined as an intake corresponding to MASLD <20/30 g (♀/♂); MetALD, 20–50 g/30–60 g (♀/♂), and ALD >50/60 g (♀/♂). Levels of PEth corresponding to the limits of MetALD and ALD. PEth groups defined as low <0.21 μmol/L, MetALD 0.21–0.42 μmol/L, and ALD >0.42 μmol/L. Only participants with complete data for PEth and self-reported alcohol intake are included in the Sankey plot. ALD, alcohol-related liver disease; MetALD, metabolic dysfunction and ALD; MASLD, metabolic-dysfunction associated steatotic liver disease; PEth, phosphatidylethanol.
We assessed if liver disease severity influenced PEth measurements. In a multivariable regression model, adjusting for self-reported alcohol intake, disease severity as measured by Kleiner fibrosis stage had no statistically significant influence on PEth levels (β = 0.06, 95% CI -0.07 to 0.18, p = 0.384).
Quantification of alcohol intake to predict hepatic decompensation and death
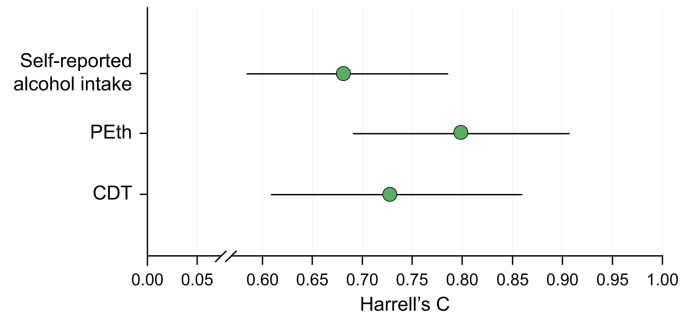
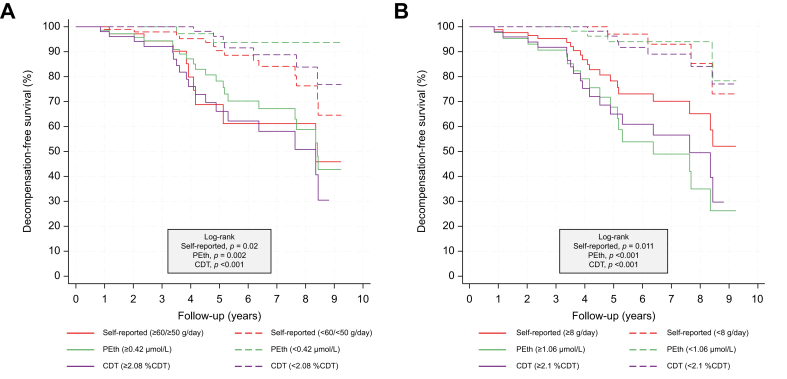
We followed the participants from inclusion in the GALA-RIF trial for a median of 3.7 years (IQR 2.7–6.2) and 2.5 years (IQR 2.2–3.2) from inclusion in the GALA-POSTBIO trial. A total of 35 events (hepatic decompensation or death) were recorded during the follow-up period, where 29 (82%) events were observed in the group of self-reported non-abstinence participants and six (18%) in participants self-reporting abstinence at baseline. All three methods to quantify alcohol intake (self-reported, PEth, and CDT) predicted hepatic decompensation and death (Table 2). In three separate multivariable analyses, we found that higher self-reported alcohol intake (HR = 1.84, 95% CI 1.25–2.71, p = 0.002), increasing levels of PEth (hazard ratio [HR] = 1.66, 95% CI 1.31–2.11, p <0.001) and CDT (HR = 1.27, 95% CI 1.12–1.4, p <0.001) independently predicted hepatic decompensation and death. BMI and fibrosis score were also predictors in all multivariable analyses (Table 2). Baseline levels of PEth had the best prognostic performance (Harrell’s C = 0.80, 95% CI 0.69–0.91) followed by CDT (Harrell’s C = 0.73, 95% CI 0.61–0.86) and self-reported alcohol intake (Harrell’s C = 0.68, 95% CI 0.58–0.79) (Fig. 3). PEth was significantly better than self-reported alcohol intake (p = 0.026) to predict hepatic decompensation and death, whereas no significant difference was found between PEth and CDT (p = 0.23) or CDT and self-reported alcohol intake (p = 0.49). To illustrate the prognostic performance of self-reported alcohol intake, PEth and CDT, we stratified participants with complete data into high and low groups. We used the cut-off corresponding to heavy intake of alcohol for each method (self-reported alcohol intake: ≥60 g/day, PEth: ≥0.42 μmol/L, CDT: ≥2.08 %CDT). For all three assessment methods, the prognosis was significantly worse in the group with high alcohol intake compared with the group with low alcohol intake (Fig. 4A). Finally, we calculated the optimized cut-off values using Youden’s index (self-reported alcohol intake = 8 g/day, PEth = 1.06 μmol/L, and CDT = 2.1 %CDT). Here all methods to quantify alcohol intake predicted time-to-decompensation and death accurately (self-reported alcohol in p = 0.011, PEth p <0.001, and CDT p <0.001, Fig. 4B), with corresponding AUROCs of 0.61 for self-reported alcohol intake (sensitivity = 83% and specificity = 39%), 0.78 for PEth (sensitivity = 78% and specificity = 75%) and 0.72 for CDT (sensitivity = 72% and specificity = 71%).
Table 2.
Predictors of hepatic decompensation or death.
| Variable | Univariable |
Multivariable Self-reported alcohol intake |
Multivariable PEth |
Multivariable CDT |
||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | |
| Age, years | 1.03 (0.99–1.08) | 0.128 | ||||||
| Female, yes | 1.81 (0.86–3.81) | 0.117 | ||||||
| BMI, kg/m2 | 0.88 (0.82–0.95) | 0.001 | 0.88 (0.82–0.95) | 0.001 | 0.92 (0.85–0.99) | 0.048 | 0.87 (0.76–0.92) | <0.001 |
| Fibrosis score, 1 stage | 1.8 (1.23–2.2) | 0.002 | 2.28 (1.48–3.52) | <0.001 | 1.96 (1.29–2.98) | 0.002 | 1.85 (1.12–3.08) | 0.017 |
| Self-reported alcohol intake, 100 g/day | 1.63 (1.11–2.41) | 0.014 | 1.84 (1.25–2.71) | 0.002 | ||||
| PEth, μmol/L | 1.77 (1.41–2.21) | <0.001 | 1.66 (1.31–2.11) | <0.001 | ||||
| CDT, %CDT∗ | 1.22 (1.1–1.36) | <0.001 | 1.27 (1.12–1.4) | <0.001 | ||||
Univariable and multivariable Cox regression of 192 participants with 35 events of hepatic decompensation or death. Variables from the univariable analysis with p <0.10 are carried forward into the multivariable analysis.
For CDT data was only available in the GALA-RIF trial (n = 136, 25 events). CDT, Carbohydrate-deficient transferrin; HR, hazard ratio; PEth, phosphatidyl-ethanol.
Fig. 3.
Harrel’s C statistic with 95% CIs for baseline self-reported alcohol intake, PEth, and CDT on prognostic performance to predict hepatic decompensation and death.
PEth had the highest (Harrell’s C = 0.80, 95% CI 0.69-0.91) followed by CDT (Harrell’s C = 0.73, 95% CI 0.61–0.86) and self-reported alcohol intake (Harrell’s C = 0.68, 95% CI 0.58–0.79). PEth was significantly better than self-reported alcohol intake (p = 0.026) to predict hepatic decompensation and death. No difference between PEth and CDT or CDT and self-reported. CDT, carbohydrate-deficient transferrin; PEth, phosphatidylethanol.
Fig. 4.
Kaplan–Meier plot of decompensation-free survival using baseline levels.
Cut-offs at (A) self-reported alcohol intake ≥60 g/day for men and ≥50 g/day for women, Peth ≥0.42 μmol/L and CDT ≥2.08 %CDT (B) by Youden’s index: self-reported alcohol intake ≥8 g/day, PEth ≥1.06 μmol/L and CDT ≥2.1 %CDT. p values are obtained by log-rank test between the high and low group for each assessment. CDT, carbohydrate-deficient transferrin; PEth, phosphatidylethanol.
Discussion
In this study, we investigated the reliability and prognostic performance of self-reported alcohol intake, compared with PEth and CDT assessed in clinical trials of participants with an intake corresponding to MASLD, MetALD, and ALD. We found that self-reported alcohol intake was highly correlated with levels of PEth and moderately with levels of CDT. Interestingly, the prognostic performance of PEth to predict hepatic decompensation and death was superior to self-reported alcohol intake, independent of disease severity. Participants who reported being alcohol abstinent for at least 6 months were highly correlated with low PEth indicating that self-reporting of alcohol abstinence is reliable in clinical trials of patients with an intake corresponding to MASLD, MetALD, and ALD.
There is increasing focus on the development of treatments for patients in the spectrum of steatotic liver disease where well-designed clinical trials are crucial for the interpretation of treatment efficacy.8 However, across the spectrum of steatotic liver disease, alcohol intake affect outcomes.25 Therefore, the ability to correctly assess alcohol consumption is of the highest importance.10 We showed that self-reported long-term alcohol abstinence was reliable for the majority of the participants in our two trials of patients with an intake corresponding to MASLD, MetALD, and ALD. Our findings suggest that a simple interview about alcohol intake history serve to determine eligibility for clinical trials in MetALD and ALD including status of alcohol abstinence. This will of course be highly dependent on culture, clinical setting, and the potential consequence of reporting excessive alcohol use.
The new nomenclature of steatotic liver disease subclassifies patients with cardiometabolic risk factors according to self-reported alcohol intake. In our study, PEth and CDT were shown to be good at distinguishing participants with a low self-reported intake of alcohol corresponding to MASLD (<20/30 g/day) from participants with an excessive alcohol intake. These findings suggest that both PEth and CDT may be useful to support the diagnosis of MASLD, which has shown to be difficult25,26 However, PEth and CDT could not significantly discriminate between participants reporting alcohol intake corresponding to MetALD (20–50/30–60 g/day) and ALD (>50/60 g/day). We believe that objective measurements are necessary to discriminate between MetALD and ALD, as it likely has implications for prognosis.4,27 Future research in larger datasets, should aim to optimize the cut-offs of PEth and CDT for differentiating between MetALD and ALD. In addition, an external validation of our optimized cut-offs by Youden’s index for self-reported alcohol intake, PEth and CDT would be of value.
Although PEth and self-reported alcohol intake had a high correlation, PEth had the highest accuracy (Harrel’s C = 0.80) in predicting hepatic decompensation and death. It is therefore important to consider how to implement this information in clinical practice. Measurements of PEth may be used to encourage patients with SLD to reduce their alcohol intake. However, PEth measurements may impact trust in the patient–doctor relationship. we believe that PEth should be measured as part of all clinical trials involving patients with SLD to support data on self-reported alcohol intake for the interpretation of treatment efficacy.
Our study has important strengths. First, the prognostic performance of the methods used to quantify alcohol intake is compared against the hard clinical outcome of decompensation or death. Second, our study population covers the whole spectrum of biopsy-proven asymptomatic and symptomatic patients with an alcohol intake corresponding to MASLD, MetALD, and ALD from no fibrosis (F0) to cirrhosis (F4). Third, data were derived from two RCTs with protocolized data collection.
However, our study also carries limitations. Our study only includes patients willing to participate in a study about patients with a history of excessive alcohol intake. Thus, the accuracy of self-reported intake may be more correct than in a clinical setting. In settings where excessive alcohol intake may decide whether a patient is eligible for a treatment trial, or if treatment options are dependent on alcohol intake, self-reported alcohol intake may be insufficient. The results are also limited by the lack of validated cut-off levels for both PEth and CDT in relation to stratifying levels of alcohol intake between MetALD and ALD. Finally, our study was a post hoc analysis, resulting in the assessment of self-reported alcohol intake not aligning with the detection periods for PEth and CDT and differences in the assessment of patients between the trials.
In conclusion, self-reported alcohol intake has high accuracy in assessing abstinence for clinical trials of patients with an alcohol intake corresponding to MASLD, MetALD, and ALD. However, PEth is superior to self-reported alcohol intake to predict hepatic decompensation and death and could be considered measured and reported in all future trials trial of patients with steatotic liver disease as it holds prognostic significance.
Abbreviations
ALD, alcohol-related liver disease; ALT, alanine transaminase; AST, aspartate aminotransferase; CAP, controlled attenuation parameter; CDT, carbohydrate-deficient transferrin; GGT, gamma-glutamyl transferase; HR, hazard ratio; INR, international normalized ratio; NAS, NAFLD Activity Score; MASLD, metabolic-associated steatotic liver disease; MetALD, metabolic dysfunction and ALD; PEth, phosphatidylethanol; RCTs, randomized clinical trials; SLD, steatotic liver disease.
Authors’ contributions
Conceived the study: EDH, NT, AK, MI. Acquired funding: AK. Project administration: MI, AK. Developed the methodology: EDH, NT, AK, MI. Data curation: EDH, NT, SJ, CDH, KT, JKH, KPL, PA, MT, MI. Clinical investigations: EDH, NT, SJ, MLB, CDH, SD, KT, JKH, GHJ, KPL, PA, MT, MI. Accessed and verified the data: EDH, NT, SJ, PA, MT, AK, MI. Formal analyses: EDT, NT, MI. Performed validation: MI, AK. Performed visualizations: EDH, NT. Supervision: MT, AK, MI. Wrote the original draft: EDH, NT, MI. Reviewing and editing subsequent drafts: all authors. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Data availability statement
The datasets analyzed during the current study are not publicly available because of data privacy but are available from the corresponding author on reasonable request and subject to approval from the relevant ethical and institutional committees. Any requests for data access will be evaluated on a case-by-case basis to ensure compliance with applicable data protection regulations and ethical standards.
Financial support
The European Union’s Program for Science and Innovation ‘GALAXY’ (fund number 668031) and The Novo Nordisk Foundation. Challenge grant ‘MicrobLiver’, NNF15OC0016692, Novo Nordisk Foundation. Siemens Healthcare provided the CDT test kits free of charge. They had no role in study design, data collection, decision to publish or preparation of the article.
Conflicts of interest
JKH has received a speaking fee from Norgine. KPL has received a speaking fee, support for travels from Siemens, and is a co-founder and board member for Evido. ET has received speaking fees from Echosens, NovoNordisk, Orphalan, and Dr Falk and participated in advisory boards for Boehringer, NovoNordisk, Pfizer, Orphalan, Univar, Alexion, and Siemens Healthineers. JT has received speaking and/or consulting fees from Versantis, Gore, Boehringer-Ingelheim, Falk, Grifols, Genfit, and CSL Behring. MT has received speaking fees from Siemens Healthcare, Norgine, Echosens, Tillotts pharma, Takeda and Madrigal; consulting fees from GE Healthcare, Boehringer Ingelheim and GSK, is vice chair on the board of Alcohol & Society (NGO) and co-founder and board member for Evido. AK has served as speaker for Novo Nordisk, Norgine, Siemens, and Nordic Bioscience and participated in advisory boards for Norgine, Siemens, Resalis Therapeutics, Boehringer Ingelheim, and Novo Nordisk. Research support: Norgine, Siemens, Nordic Bioscience, AstraZeneca, Echosens. Board member and co-founder Evido. EDH, NT, SJ, MLB, CDH, SD, PA, IV, KB, KT, GHJ, and TB declare no conflicts of interest.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgements
We thank project nurses and medical students at FLASH Liver Research Centre, Lise Ryborg, Louise Just and the Department of Biochemistry at the Hospital of South West Jutland.
Footnotes
Author names in bold designate shared co-first authorship.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2024.101200.
Supplementary data
The following are the Supplementary data to this article:
Multimedia component 1
References
- 1.Asrani S.K., Devarbhavi H., Eaton J., et al. Burden of liver diseases in the world. J Hepatol. 2019;70:151–171. doi: 10.1016/j.jhep.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 2.Rinella M.E., Lazarus J.V., Ratziu V., et al. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol. 2023;78:1966–1986. [Google Scholar]
- 3.Israelsen M., Torp N., Johansen S., et al. MetALD: new opportunities to understand the role of alcohol in steatotic liver disease. Lancet Gastroenterol Hepatol. 2023;8:866–868. doi: 10.1016/S2468-1253(23)00206-6. [DOI] [PubMed] [Google Scholar]
- 4.Israelsen M., Torp N., Johansen S., et al. Validation of the new nomenclature of steatotic liver disease in patients with a history of excessive alcohol intake: an analysis of data from a prospective cohort study. Lancet Gastroenterol Hepatol. 2024;9:218–228. doi: 10.1016/S2468-1253(23)00443-0. [DOI] [PubMed] [Google Scholar]
- 5.Ginès P., Graupera I., Lammert F., et al. Screening for liver fibrosis in the general population: a call for action. Lancet Gastroenterol Hepatol. 2016;1:256–260. doi: 10.1016/S2468-1253(16)30081-4. [DOI] [PubMed] [Google Scholar]
- 6.Karlsen T.H., Rutter H., Carrieri P., et al. The EASL-Lancet Commission on liver health in Europe: prevention, case-finding, and early diagnosis to reduce liver-related mortality. Lancet. 2024;403:P1522–P1524. doi: 10.1016/S0140-6736(24)00204-6. [DOI] [PubMed] [Google Scholar]
- 7.Singal A.K., Mathurin P. Diagnosis and treatment of alcohol-associated liver disease. JAMA. 2021;326:165. doi: 10.1001/jama.2021.7683. [DOI] [PubMed] [Google Scholar]
- 8.Mellinger J., Winder G.S., Fernandez A.C. Measuring the alcohol in alcohol-associated liver disease: choices and challenges for clinical research. Hepatology. 2021;73:1207–1212. doi: 10.1002/hep.31539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karlsen T.H., Sheron N., Zelber-Sagi S., et al. The EASL-Lancet Liver Commission: protecting the next generation of Europeans against liver disease complications and premature mortality. Lancet. 2022;399:61–116. doi: 10.1016/S0140-6736(21)01701-3. [DOI] [PubMed] [Google Scholar]
- 10.Åberg F., Byrne C.D., Pirola C.J., et al. Alcohol consumption and metabolic syndrome: clinical and epidemiological impact on liver disease. J Hepatol. 2023;78:191–206. doi: 10.1016/j.jhep.2022.08.030. [DOI] [PubMed] [Google Scholar]
- 11.Andresen-Streichert H., Müller A., Glahn A., et al. Alcohol biomarkers in clinical and forensic contexts. Dtsch Arztebl Int. 2018;115:309–315. doi: 10.3238/arztebl.2018.0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aradottir S., Asanovska G., Gjerss S., et al. PHosphatidylethanol (PEth) concentrations in blood are correlated to reported alcohol intake in alcohol-dependent patients. Alcohol Alcohol. 2006;41:431–437. doi: 10.1093/alcalc/agl027. [DOI] [PubMed] [Google Scholar]
- 13.Gundlach J.P., Braun F., Mötter F., et al. Phosphatidylethanol (PEth) for monitoring sobriety in liver transplant candidates: preliminary results of differences between alcohol-related and non-alcohol-related cirrhosis candidates. Ann Transpl. 2022;27 doi: 10.12659/AOT.936293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bortolotti F., Sorio D., Bertaso A., et al. Analytical and diagnostic aspects of carbohydrate deficient transferrin (CDT): a critical review over years 2007-2017. J Pharm Biomed Anal. 2018;147:2–12. doi: 10.1016/j.jpba.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 15.Allen J.P., Wurst F.M., Thon N., et al. Assessing the drinking status of liver transplant patients with alcoholic liver disease. Liver Transpl. 2013;19:369–376. doi: 10.1002/lt.23596. [DOI] [PubMed] [Google Scholar]
- 16.Helander A., Péter O., Zheng Y. Monitoring of the alcohol biomarkers PEth, CDT and EtG/EtS in an outpatient treatment setting. Alcohol Alcohol. 2012;47:552–557. doi: 10.1093/alcalc/ags065. [DOI] [PubMed] [Google Scholar]
- 17.Morinaga M., Kon K., Uchiyama A., et al. Carbohydrate-deficient transferrin is a sensitive marker of alcohol consumption in fatty liver disease. Hepatol Int. 2022;16:348–358. doi: 10.1007/s12072-022-10298-8. [DOI] [PubMed] [Google Scholar]
- 18.Israelsen M., Madsen B.S., Torp N., et al. Rifaximin-α for liver fibrosis in patients with alcohol-related liver disease (GALA-RIF): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Gastroenterol Hepatol. 2023;8:P523–P532. doi: 10.1016/S2468-1253(23)00010-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bednarska O., Biskou O., Israelsen H., et al. A postbiotic fermented oat gruel may have a beneficial effect on the colonic mucosal barrier in patients with irritable bowel syndrome. Front Nutr. 2022;9 doi: 10.3389/fnut.2022.1004084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Medical Association Declaration of Helsinki Ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 21.Arnts J., Vanlerberghe B.T.K., Roozen S., et al. Diagnostic accuracy of biomarkers of alcohol use in patients with liver disease: a systematic review. Alcohol Clin Exp Res. 2021;45:25–37. doi: 10.1111/acer.14512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walther L., de Bejczy A., Löf E., et al. Phosphatidylethanol is superior to carbohydrate-deficient transferrin and γ-glutamyltransferase as an alcohol marker and is a reliable estimate of alcohol consumption level. Alcohol Clin Exp Res. 2015;39:2200–2208. doi: 10.1111/acer.12883. [DOI] [PubMed] [Google Scholar]
- 23.Kleiner D.E., Brunt E.M., Van Natta M., et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 24.de Franchis R., Bosch J., Garcia-Tsao G., et al. Baveno VII - renewing consensus in portal hypertension. J Hepatol. 2022;76:959–974. doi: 10.1016/j.jhep.2021.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nasr P., Wester A., Ekstedt M., et al. Misclassified alcohol-related liver disease is common in presumed metabolic dysfunction-associated steatotic liver disease and highly increases risk for future cirrhosis. Clin Gastroenterol Hepatol. 2024;22:1048–1057.e2. doi: 10.1016/j.cgh.2024.01.006. [DOI] [PubMed] [Google Scholar]
- 26.Staufer K., Huber-Schönauer U., Strebinger G., et al. Ethyl glucuronide in hair detects a high rate of harmful alcohol consumption in presumed non-alcoholic fatty liver disease. J Hepatol. 2022;77:918–930. doi: 10.1016/j.jhep.2022.04.040. [DOI] [PubMed] [Google Scholar]
- 27.Trebicka J., Fernandez J., Papp M., et al. The PREDICT study uncovers three clinical courses of acutely decompensated cirrhosis that have distinct pathophysiology. J Hepatol. 2020;73:842–854. doi: 10.1016/j.jhep.2020.06.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multimedia component 1
Data Availability Statement
The datasets analyzed during the current study are not publicly available because of data privacy but are available from the corresponding author on reasonable request and subject to approval from the relevant ethical and institutional committees. Any requests for data access will be evaluated on a case-by-case basis to ensure compliance with applicable data protection regulations and ethical standards.