Abstract
A possible immunomodulatory role of granulocyte colony-stimulating factor (G-CSF) was investigated in an experimental pneumococcal meningitis model in rabbits. Animals were pretreated with G-CSF (10 μg/kg subcutaneously twice a day) starting 48 h before in vivo and ex vivo experiments, causing a five- to six-fold increase in the peripheral leukocyte level. Meningitis was induced by intracisternal inoculation of ∼4 × 105 CFU of Streptococcus pneumoniae type 3. Neutrophil pleocytosis and interleukin-8 (IL-8) levels were significantly attenuated in G-CSF-pretreated animals compared to untreated animals (P < 0.05). Furthermore, G-CSF pretreatment significantly delayed alterations in cerebrospinal fluid (CSF) tumor necrosis factor alpha and IL-1β levels, as well as protein and glucose levels (P < 0.05). No difference in CSF bacterial concentrations was found, whereas the blood bacterial concentration was significantly decreased in G-CSF-pretreated animals (P < 0.05). Ex vivo chemotaxis of neutrophils isolated from G-CSF-pretreated animals was significantly decreased compared to that of neutrophils from untreated animals (P < 0.05). In conclusion, G-CSF pretreatment attenuates meningeal inflammation and enhances systemic bacterial killing. Further preclinical studies are required to investigate whether this may affect the clinical course of meningitis and thus whether G-CSF treatment may have a beneficial role in pneumococcal meningitis.
The pathophysiology of pneumococcal meningitis has been studied intensively throughout the last decade in animal models (see reference 32 for a review). Pneumococci or pneumococcal cell wall fragments induce a local inflammatory response characterized by neutrophil influx into the brain or the cerebrospinal fluid (CSF) (42). Various types of anti-inflammatory treatments (e.g., antibodies to cytokines [33, 36], leukocyte-endothelial adhesion molecules [11, 35, 43], inhibitors of neutrophil activation products [22, 24], and inhibitors of neuro-excitatory amino acids [25]) reduce the development of increased intracranial pressure, brain edema, cerebral ischemia, or neural injury. It has been speculated that an influx of neutrophils is the primary cause of these changes.
Another approach to improve our understanding of the role of the neutrophils in the pathophysiology of bacterial meningitis is to increase the number of neutrophils in the peripheral blood over the course of the meningitis. Granulocyte colony-stimulating factor (G-CSF) is a glycoprotein which stimulates proliferation and differentiation of hematopoietic progenitor cells and increases the total number of neutrophils in the blood (31; for a review, see reference 4).
G-CSF treatment has previously been demonstrated to improve survival in nonneutropenic models of systemic infections (27). However, conflicting results on survival have been obtained in various animal studies on local infections (12, 16, 28, 44). The explanation for this remains to be defined, but it could be due to the influence of G-CSF treatment on local host defense mechanisms. By using the rabbit meningitis model, it is possible to study the kinetics of the pleocytosis, since sequential CSF tappings can be performed.
The purpose of this study was to investigate the influence of pretreatment with G-CSF on the kinetics of local inflammation in an experimental pneumococcal meningitis model in rabbits. Our initial working hypothesis was that by increasing the number of neutrophils in peripheral blood, the rate of influx of neutrophils in the CSF would increase. However, our data showed that G-CSF-induced elevation of the peripheral leukocyte (WBC) level was associated with a consistent decrease in CSF pleocytosis, likely because of impaired chemotaxis by the G-CSF-induced neutrophils and/or impaired production of local cytokines.
MATERIALS AND METHODS
Bacterial strain.
The bacterial strain used was a Streptococcus pneumoniae type 3 strain (68034). The frozen organisms were thawed and grown on 5% blood agar plates for 24 h, and the colonies were suspended in beef broth to an optical density of 0.35 at 540 nm and incubated for 1 h. The test organism was diluted in sterile beef broth to a final concentration of approximately 2 × 106 CFU/ml (1 × 106 to 6 × 106 CFU/ml; there was no significant difference between the G-CSF-treated group and the untreated control group), as confirmed by quantitative cultures, and 0.2 ml was used for intracisternal inoculation.
G-CSF treatment.
Animals were pretreated with G-CSF (Neupogen; kindly provided by Amgen, Hellerup, Denmark) (10 μg/kg subcutaneously [s.c.] twice a day) starting 48 h before in vivo and ex vivo experiments. After bacterial inoculation, no further G-CSF was given.
Ex vivo experiments.
All ex vivo studies were carried out with corresponding blood cells from G-CSF-treated and untreated animals in at least six independent experiments.
(i) Separation of rabbit neutrophils and monocytes.
Blood was drawn from rabbits sedated with fentanyl/fluanisone (Hypnorm; Janssen Pharmaceutica N.V., Beerse, Belgium) (0.1 ml/kg intravenously) from a central ear artery into 4% (wt/vol) citrated anticoagulated tubes, mixed 1/6 with 5% (wt/vol) dextran (Statens Serum Institut, Copenhagen, Denmark), and left to sediment. WBC-rich plasma was layered over Histopaque (1.082 g/ml; Sigma Chemical Co., St. Louis, Mo.) and centrifuged for 30 min at 600 × g. The mononuclear band was carefully removed, and then the plasma and the density medium above the neutrophil pellet were removed. Neutrophils and monocytes were washed in 0.2% (wt/vol) saline to remove erythrocytes and resuspended in minimal essential medium (MEM) (Statens Serum Institut) for neutrophil chemotaxis and monocyte cytokine production assays or in Krebs Ringer buffer (KRB) (Statens Serum Institut) for chemiluminescence assay. Means of 4 × 108 and 3 × 107 neutrophils and 1 × 107 and 1 × 108 monocytes were isolated from 50 ml of blood taken from G-CSF-treated and untreated rabbits, respectively.
(ii) Neutrophil chemotaxis.
Chemotaxis was measured in triplicate by using a modified Boyden chamber assay as previously described (19). Briefly, the upper compartment, containing 106 cells/ml in a final volume of 500 μl suspended in MEM with 1% (vol/vol) human serum albumin (Statens Serum Institut), was separated from the lower compartment by a cellulose filter (3-μm pore size; Millipore, Bedford, Mass.). The lower compartment contained the following chemoattractant: 10−8 M fMLP (Sigma Chemical Co.), a 1:200 dilution of zymosan-activated human or rabbit serum (both prepared at our laboratory), or MEM as a control in a final volume of approximately 500 μl. The chambers were incubated at 37°C in CO2 for 2.5 h. Cells migrating to the lower surface of the filter were stained with hematoxylin and counted automatically by a computer-assisted image analysis system (Image House, Copenhagen, Denmark) in five randomly chosen fields per filter.
(iii) Neutrophil chemiluminescence.
A previously described luminol-enhanced chemiluminescence assay was used (20). Briefly, 5 × 105 neutrophils suspended in KRB with 7 × 10−5 M luminol (Sigma Chemical Co.) were tested in duplicate with the following stimulus: 2 × 10−6 M fMLP, 2 mg of zymosan per ml, or KRB as a control in a final volume of 1 ml. Peak chemiluminescence was recorded with an LKB 1251 instrument (Bio-Tec Instruments, Winooski, Vt.).
(iv) Cytokine production from mononuclear cells.
Cytokine production from mononuclear cells was assayed as previously described (23). However, we used only freshly prepared mononuclear cells in this study. Briefly, 5 × 105 mononuclear cells were incubated in microtiter wells (Nunc, Roskilde, Denmark) at 37°C in ambient air with whole S. pneumoniae type 3 or lipopolysaccharide (LPS), each in a twofold dilution from 5 × 103 to 5 × 107 CFU or from 9.8 pg to 2.5 μg, respectively, in RPMI medium (Statens Serum Institut) in a final volume of 0.2 ml. After 24 h of incubation, the plates were stored at −80°C for subsequent cytokine analysis.
In vivo experiments. (i) Experimental meningitis model.
The Danish animal experiment inspectorate approved the experimental protocols. A modification of the model originally described by Dacey and Sande (5) was used. New Zealand White rabbits, approximately 2.5 kg in weight, were anesthetized with midazolam (Dormicum; F. Hoffmann-La Roche AG, Basel, Switzerland) (0.5 mg/kg s.c.) and Hypnorm (0.35 ml/kg intramuscularly), and a dental acrylic helmet containing a half turnbuckle was attached to the skull. The next day the rabbits were reanesthetized with ethyl carbamate (Urethane; Fluka Kemi AG, Buchs, Switzerland) (1.75 g/kg s.c.) and pentobarbital (Nykomed, Roskilde, Denmark) (10 mg/kg) and immobilized in a stereotaxic frame. A 25-gauge spinal needle was introduced into cisterna magna for the inoculation of 0.2 ml of bacterial suspension and repetitive CSF sampling every second hour. The rate of removal of CSF did not exceed the rate of CSF formation, which is approximately 0.4 ml/h (38). Blood samples were taken from a central ear artery. After testing for bacterial concentration and WBC count, CSF and blood samples were centrifuged and supernatants were immediately stored at −80°C for subsequent analysis. Animals were euthanized 16 h after bacterial inoculation. After 16 h from the time of bacterial inoculation, the infected rabbits were starting to be clinically unstable because of sepsis and the continued anesthesia. At that time, the inflammatory response in the CSF was pronounced. The objective of the study was to monitor the kinetics of this response. For these reasons, the rabbits were sacrificed at this time with 50 mg of pentobarbital (Nykomed) per kg.
(ii) Bacterial concentration.
Bacterial titers in blood and CSF were determined by plating undiluted sample and 10-fold serial dilutions on 5% blood agar plates (Statens Serum Institut). The lowest detectable bacterial concentration was 50 CFU/ml.
(iii) WBC, glucose, lactate, and protein levels.
WBC and differential counts were determined on an automatic cell counter (Swelab, Årsta, Sweden). The lowest detectable WBC level was 100 × 103 cells/ml. Lactate and glucose contents were measured with commercial kits (Sigma Chemical Co.). Protein content was determined as described by Lowry et al. (26).
(iv) TNF-α.
Tumor necrosis factor alpha (TNF-α) levels in CSF and blood were measured by bioassay as previously described (9, 13). Briefly, 5 × 104 cells (WEHI 164, subclone 13; kindly provided by T. Espevik) per well in RPMI 1640 with 0.25 μg of actinomycin D (A 9415; Sigma Chemical Co.) per ml were incubated with a standard (human TNF-α, T 6674; Sigma Chemical Co.) or 10-μl samples in triplicate on microtiter plates (167008; Nunc) for 18 to 24 h in ambient air at 37°C. MTT (M 2128; Sigma Chemical Co.) at 1 mg/ml was used to indicate cell kill; 20% (wt/vol) sodium dodecyl sulfate (L 4509; Sigma Chemical Co.) was dissolved in 50% (vol/vol) DMF (D 8654; Sigma Chemical Co.) and in water and used as the lysing buffer, and the optical density was measured on an enzyme-linked immunosorbent assay (ELISA) reader (Bio-Tec) at 570 nm. The coefficient of variation was less than 10%. The lowest detection level was 50 pg/ml.
(v) IL-1β.
Interleukin-1β (IL-1β) levels in CSF were measured in a two-step assay which was a modification of the assay of Gearing et al. (10). In the first step, 2 × 105 cells (per well) of the murine T-cell line EL-4, subclone NOB 1, which produces IL-2 upon exposure to IL-1, in RPMI 1640 medium were incubated with a standard (human IL-1β; Sigma Chemical Co.) or 10-μl CSF samples in triplicate on microtiter plates (167008; Nunc) for 18 to 24 h in ambient air at 37°C. In the second step, 100 μl of the supernatants was carefully collected for subsequent analysis of IL-2 content with a commercial mouse IL-2 ELISA kit (Genzyme Diagnostics, Cambridge, Mass.), essentially as described by the manufacturer. The coefficient of variation was less than 20%. The lowest detection level was 30 pg/ml.
(vi) IL-8.
IL-8 levels in CSF and blood were measured with a modification of a rabbit-specific ELISA as previously described (14, 21). Briefly, microtiter plates (Maxisorb 439454; Nunc) were coated overnight with WS-4 (a kind gift of K. Matsushima, Tokyo, Japan), a mouse monoclonal antibody to human and rabbit IL-8, at 0.5 μg/ml in 0.05 M carbonate buffer (pH 9.6) at 4°C. After the plates were washed with phosphate-buffered saline containing 0.05% (vol/vol) Tween 20 (buffer A), the unbound sites were blocked by adding 150 μl of 1% (vol/vol) bovine serum albumin in buffer A (buffer B) per well at 37°C for 1 h. After another wash, the standard (rabbit-IL-8; a kind gift of K. Matsushima) or samples diluted in buffer B were added in duplicate and incubated overnight at 4°C. Five washes were carried out between each of the subsequent steps. Goat anti-human IL-8 immunoglobulin G (R&D Systems Europe Ltd., Abingdon, United Kingdom) at 1 μg/ml in buffer A (buffer C) was added as the detection antibody and incubated at 37°C for 2 h. The specificity of goat anti-human IL-8 for rabbit IL-8 has previously been tested by Western blot analysis and found to be similar to that of guinea pig anti-rabbit IL-8 (unpublished observations). In the next step, alkaline phosphatase-conjugated rabbit anti-goat immunoglobulin G (R&D Systems Europe Ltd.) diluted 1:3,000 in buffer C was added and incubated for an additional 1 h at 37°C. o-Phenylenediamine (Sigma Chemical Co.) at a concentration of 0.8 mg/ml in 0.05 M citric acid/sodium phosphate buffer containing a 1/1,000 dilution of 30% (vol/vol) hydrogen peroxide was added and allowed to react for 30 min at room temperature. The enzyme reaction was stopped by the addition of 100 μl of 4.5 N H2SO4, and the optical density at 490 nm was measured with the ELISA reader. The lowest detection level was 20 pg/ml. The coefficient of variation was less than 10%.
Statistical analysis.
All results are provided as medians and ranges (minimum to maximum). Comparison between groups was performed by nonparametric tests (Mann-Whitney test for unpaired data and Wilcoxon signed rank test for paired data). P < 0.05 was considered significant.
RESULTS
Effect of G-CSF treatment on neutrophil pleocytosis.
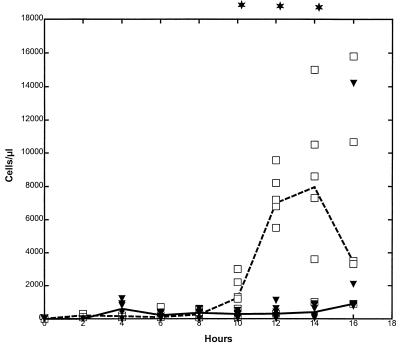
Intracisternal challenge with pneumococci resulted in neutrophil pleocytosis in untreated animals, with peak levels at 12 to 14 h after the bacteria were inoculated, whereas in G-CSF-treated animals, the CSF WBC response was significantly attenuated (P < 0.05) (Fig. 1). This was despite the fact that the blood WBC level (103 cells/μl) was increased approximately five- to sixfold in G-CSF-treated animals compared to untreated animals at the time of bacterial inoculation (28.7 [20.4 to 35.4] versus 5.4 [3.7 to 8.3], respectively; P = 0.0001) and throughout the study (data not shown).
FIG. 1.
Median CSF WBC levels in G-CSF-pretreated and untreated rabbits during experimental pneumococcal meningitis. G-CSF (10 μg/kg) was administered twice a day starting 48 h before intracisternal bacterial challenge (0 h). The CSF WBC level was significantly attenuated in the G-CSF-treated group (closed symbols [median, solid line]) compared to the untreated control group (open symbols [median, dashed line]) between 10 and 14 h (n = 6 for both groups). ∗, P < 0.05 (Mann-Whitney test).
CSF cytokine kinetics.
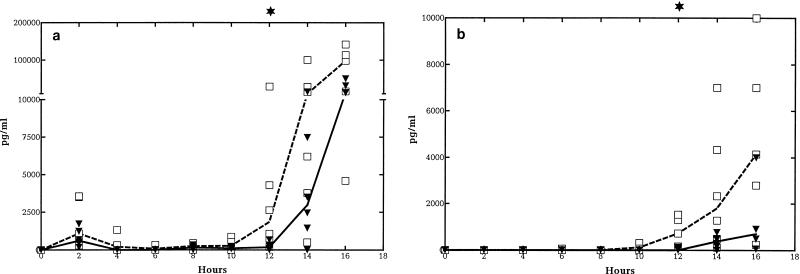
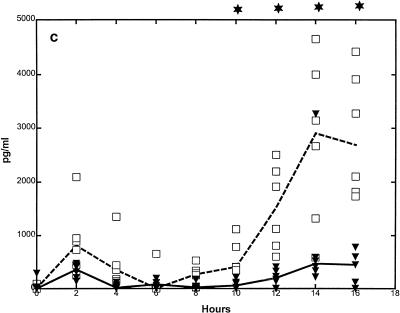
Median CSF TNF-α levels (picograms per milliliter) were lower in G-CSF-treated than in nontreated animals from the start of meningeal inflammation (from approximately 8 to 10 h) and were significantly reduced at 12 h (175 [0 to 666] versus 1,848 [195 to 29,030], respectively; P = 0.015) (Fig. 2a). Median CSF IL-1β levels (picograms per milliliter) were also lower from the start of meningeal inflammation and were significantly reduced at 12 h (713 [155 to 1,524] versus 0 [0 to 120], respectively; P = 0.004) (Fig. 2b). Similar but more consistent trends over longer periods were observed for IL-8 levels in CSF, with a significant difference from 8 h throughout the study period (P < 0.05) (Fig. 2c).
FIG. 2.
Median CSF cytokine kinetics in G-CSF-treated and untreated rabbits during experimental pneumococcal meningitis. Symbols are as described for Fig. 1. G-CSF (10 μg/kg) was administered twice a day starting 48 h before intracisternal bacterial challenge (0 h). CSF TNF-α (a) and IL-1β (b) levels were significantly attenuated at 12 h in the G-CSF-treated group. CSF IL-8 levels (c) were significantly attenuated in the G-CSF-treated group between 10 and 16 h. (n = 6 for both groups.)
The corresponding cytokine levels in plasma were low and not significantly different according to usage of G-CSF (for both groups, 0 to 1,000 pg/ml for TNF and <30 pg/ml for IL-8; P > 0.05), confirming that cytokines are locally produced in bacterial meningitis.
Cytokine production from rabbit monocytes ex vivo.
To address whether the attenuated levels of proinflammatory cytokines in the CSF were due to impaired ability of the monocytes to produce cytokines, monocytes were isolated and stimulated with live pneumococci or LPS. However, no difference in release of cytokines was detected regardless of whether the animals were pretreated with G-CSF or not (for both groups the range of measurements were 0 to 18,817 pg/ml for TNF-α and 279 to 5,000 pg/ml for IL-1β; P > 0.05).
Rabbit neutrophil chemotaxis ex vivo.
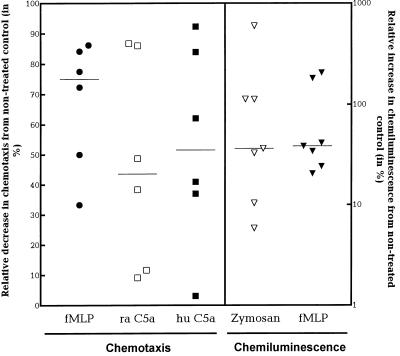
Another possible cause of the diminished pleocytosis is that the neutrophils in the peripheral blood had a reduced ability to migrate towards a chemotactic gradient. In fact, chemotaxis for various chemoattractants (fMLP and rabbit and human C5a) was reduced by more than 40% in neutrophils isolated from G-CSF-treated animals compared to neutrophils from untreated animals (Fig. 3) (P < 0.05).
FIG. 3.
Effect of G-CSF treatment on rabbit neutrophil function ex vivo. G-CSF (10 μg/kg) was administered twice a day, starting 48 h before isolation of neutrophils. Data are expressed as the relative decrease (chemotaxis) or increase (chemiluminescence) for G-CSF-treated rabbits compared to corresponding untreated rabbits in at least six independent experiments. Chemotaxis (number of migrated neutrophils) to fMLP and to rabbit (ra) and human (hu) C5a was significantly decreased in the G-CSF-treated animals compared to the untreated animals. Chemiluminescence (peak value in millivolts) with opsonized zymosan and fMLP was significantly increased in the G-CSF-treated animals compared to the untreated animals. (P < 0.05 [Wilcoxon signed rank test] for all experiments.)
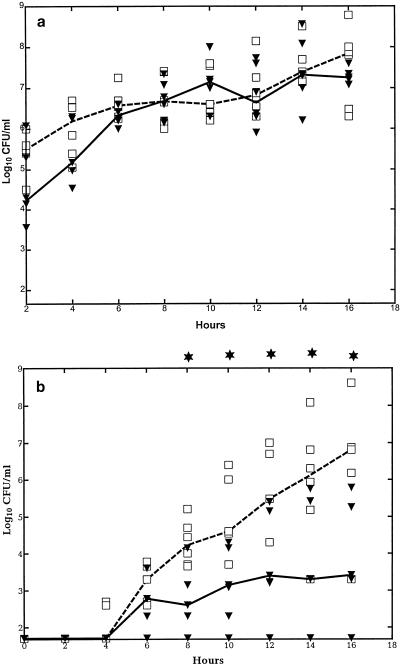
Bacterial growth. (i) Bacterial concentration in CSF.
During the 16-h study period, the bacterial concentration in the CSF increased exponentially by more than 2 log10 CFU/ml but without significant differences in concentration according to whether the rabbits were pretreated with G-CSF or not (Fig. 4a) (P > 0.05).
FIG. 4.
Median bacterial concentration in CSF (a) and in blood (b) in G-CSF-treated and untreated rabbits during experimental pneumococcal meningitis. Symbols are as described for Fig. 1. G-CSF (10 μg/kg) was administered twice a day starting 48 h before intracisternal bacterial challenge (0 h). The bacterial concentration in blood was significantly reduced in the G-CSF-treated animals compared to the untreated animals between 8 and 16 h. (n = 6 for both groups.)
(ii) Bacterial concentration in blood.
The rabbits started to become bacteremic 4 to 6 h after the bacteria were inoculated in the CSF. Thereafter, the growth curve was exponential for both groups (Fig. 4b). Although the times that the rabbits started to become bacteremic were similar regardless of pretreatment with G-CSF at all subsequent time points, G-CSF pretreatment was consistently associated with a decreased concentration of pneumococci in the blood (P < 0.05).
Luminol-enhanced chemoluminescence (percentage of the peak value in millivolts) was significantly enhanced in neutrophils isolated from G-CSF-pretreated rabbits compared to untreated rabbits when opsonized zymosan and fMLP were used as stimuli (36.3% [5.8 to 596.0%] and 38.5% [20.4 to 205.9%], respectively; Wilcoxon signed rank test, P < 0.05).
Indices of meningeal inflammation and blood-brain barrier damage.
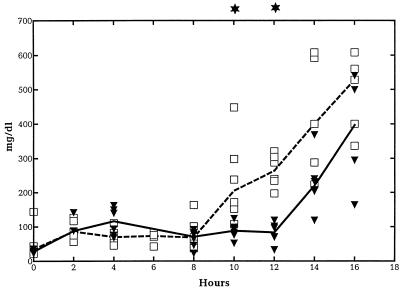
To assess the metabolic activity within the central nervous system, levels of glucose and lactate in CSF were measured. Levels of glucose in CSF (milligrams per deciliter) remained stable throughout the study period for rabbits pretreated with G-CSF, whereas they started to decrease from 12 h in the course of bacterial meningitis in untreated animals. The difference became statistically significant at 16 h after bacterial inoculation (99.7 [65 to 135] versus 43.0 [27 to 101]; P < 0.05). The lactate concentration in the CSF gradually increased from 10 h in both groups, without any consistent difference (data not shown). Finally, to obtain an impression of the damage to the blood-brain barrier, levels of protein in CSF were measured. The CSF protein concentration gradually increased late in the course of the meningitis, but this increase started significantly later in rabbits pretreated with G-CSF, with the difference being statistically significant at 10 and 12 h (Fig. 5) (P < 0.05).
FIG. 5.
Median levels of protein in CSF in G-CSF-treated and untreated rabbits during experimental pneumococcal meningitis. Symbols are as described for Fig. 1. G-CSF (10 μg/kg) was administered twice a day starting 48 h before intracisternal bacterial challenge (0 h). The levels of protein in CSF were significantly reduced in the G-CSF-treated animals compared to the untreated animals at 10 and 12 h and at 16 h, respectively. (n = 6 for both groups.)
DISCUSSION
In the present study, we found that pretreatment of rabbits with G-CSF attenuated the enhanced neutrophil pleocytosis in pneumococcal meningitis, despite a five- to sixfold increase in peripheral WBCs when the bacteria were inoculated. Two other studies have previously addressed the role of G-CSF in neutrophil recruitment to an inflamed site in vivo. A study by Price et al. found impaired neutrophil migration to skin chambers in normal humans with G-CSF-induced neutrocytosis (31), whereas Nelson et al. found an elevated number of neutrophils in brochioalveolar lavage of rats with Klebsiella pneumoniae pneumonia pretreated with G-CSF (28). Differences in the organs and bacteria studied, as well as differences in animal species used, may well explain these conflicting findings.
In the present study, we further investigated possible explanations for why G-CSF pretreatment results in an attenuated pleocytosis. Theoretically, these include (i) a reduction in CSF bacterial concentration, (ii) an inhibition of proinflammatory cytokine production within the central nervous system, (iii) a reduction in neutrophil chemotactic ability per se, and (iv) a modulation of adhesion molecules on neutrophils and vascular endothelium (and hence impaired rolling, adhesion, and diapedesis over the blood-brain barrier).
(i) In this study, no difference was found in the CSF bacterial concentration regardless of whether the rabbits were pretreated with G-CSF. Thus, diminished bacterial antigenic stimulation of host cells responsible for initiating and sustaining the inflammatory response within the central nervous system is an unlikely explanation for the observed attenuated pleocytosis in G-CSF-pretreated animals.
The fact that a lower number of neutrophils in the CSF does not affect the growth of bacteria in CSF in experimental models of meningitis is in accordance with several other studies in which the CSF WBC level was reduced either by nitrogen mustard-induced peripheral neutropenia (8, 41), blockage of integrins with anti-CD18 monoclonal antibodies (35, 43), or inhibition of selectins with fucoidin (unpublished observations). Moreover, an enhanced neutrophil pleocytosis in pneumococcal meningitis after treatment with fMLP intracisternally has previously been shown not to influence bacterial growth in the CSF (41). Previous studies on the effect of G-CSF on local bacterial or fungal killing have generated conflicting results (12, 16, 28, 44), so caution should be used when findings from one model of infections are extrapolated to other models.
(ii) We found consistently decreased CSF IL-8 levels in the G-CSF-pretreated animals compared to the untreated animals from 8 h after the bacterial inoculation until the end of the study. In contrast, only a slight delay in CSF elevation of TNF-α and IL-1β in the G-CSF-pretreated animals compared to the untreated animals was observed. A reduction in levels of TNF-α and IL-1β in CSF after intracisternal treatment with monoclonal antibodies to TNF-α and IL-1β has previously been shown to reduce neutrophil pleocytosis in experimental pneumococcal and Haemophilus influenzae-induced meningitis (33, 36). IL-8 has been demonstrated to be an important chemokine for neutrophil influx in vivo (1) and in vitro (37) but has not previously been addressed in models of bacterial meningitis. In further support of a role of IL-8 as the responsible chemokine in attracting neutrophils into the CSF, we have recently shown that treatment with a monoclonal antibody to IL-8 attenuates neutrophil pleocytosis in pneumococcal meningitis (unpublished data). Thus, decreased production of IL-8, rather than TNF-α and IL-1β, appears to be the most likely explanation for the attenuated pleocytosis, if chemokines really do play a key role as chemoattractants in bacterial meningitis.
The cellular source of IL-8 detected in the CSF has not at present been identified. Various local cells within the CNS are able to produce IL-8, including microglia (7), astrocytes (2), and endothelial cells (17). For the production of other cytokines (e.g., IL-1β) during experimental meningitis, it has been suggested that an influx of monocytes from the blood compartment during the infection plays a key role in this respect (45). Monocytes and macrophages can also produce large amounts of IL-8 (3). We are fairly certain that the IL-8 measured in the CSF is not produced by the incoming neutrophils, since a blockage of the entry of neutrophils by the selectin blocker fucoidin did not inhibit IL-8 release in the CSF during the course of bacterial meningitis (unpublished data). Furthermore, the attenuated IL-8 production is likely not due to a general inhibition of cytokine production from monocyte-derived cells by G-CSF, since we found that G-CSF pretreatment starting 48 h before stimulation of rabbit monocytes with pneumococci or LPS ex vivo does not influence TNF-α or IL-1β production. This is in accordance with two human studies, where G-CSF pretreatment for longer than 24 h did not influence TNF-α or IL-1β production after stimulation of whole blood with LPS (6, 15). Further investigations are necessary to determine the relative importance of local cells versus incoming monocytes as producers of CSF IL-8 during bacterial meningitis.
(iii) In the present study, neutrophils obtained from rabbits which were pretreated with G-CSF had reduced chemotaxis towards three different chemotactic agents compared with neutrophils from untreated rabbits. This observation is in accordance with two studies of human neutrophils (18, 39). Thus, the diminished neutrophil pleocytosis in bacterial meningitis associated with G-CSF pretreatment may well be caused by an impaired mobility of the neutrophils towards a chemotactic gradient. The exact mechanism behind this remains to be defined.
(iv) An impaired ability of neutrophils to roll and adhere to the endothelium and/or impaired diapedesis over the blood-brain barrier is a potential cause of the attenuated pleocytosis observed in our study. Adhesion molecules (e.g., selectins, integrins, and immunoglobulins) are important for neutrophil adherence to the vascular endothelium and subsequent transmigration. Various up- and down-regulation of adhesion molecules occurs during G-CSF treatment (40). A down-regulation of the selectin leukocyte adhesion molecule-1 on neutrophils (30) and/or an increase in levels of soluble L- and E-selectins in serum occurs during G-CSF treatment (29). Intravenous administration of fucoidin, which binds to and blocks selectins (11) as well as peptides that mimic selectins (34), results in a decreased neutrophil pleocytosis in experimental meningitis. Thus, an altered expression or competitive binding of selectins may explain the decreased pleocytosis seen in our model after G-CSF administration.
Thus, a complex range of causes may explain the attenuated pleocytosis observed after G-CSF pretreatment in this experimental meningitis model. We have shown that the local production of proinflammatory cytokines and the ability of the neutrophils to move against a chemotactic gradient are impaired in animals which were pretreated with G-CSF. However, as discussed above, several other potential mechanisms may also be important and should be further studied.
Beside its influence on neutrophil pleocytosis and CSF cytokine levels, G-CSF pretreatment caused an attenuated enhancement of CSF protein levels and an attenuated decrease in CSF glucose levels. This supports the opinion that a reduction in meningeal inflammation may prevent disruption of the blood-brain barrier during bacterial meningitis (32). The influence of G-CSF pretreatment on brain damage or outcome in experimental pneumococcal meningitis was not studied in the present study. Further studies with experimental models suitable for this purpose should be performed.
Another important finding in this study was that G-CSF pretreatment limited the secondary bacteremia in experimental meningitis. We found in the present study that G-CSF-treated rabbits, all with high numbers of neutrophils in the blood, had significantly reduced blood bacterial concentrations compared to untreated rabbits. Human studies have previously found that G-CSF treatment increases neutrophil phagocytic and fungicidal activity and oxidative burst ex vivo, whereas little is known about the effect on bacterial killing (40). Here we found a significantly increased chemiluminescence activity of rabbit neutrophils after G-CSF pretreatment. Based on this, we assume that the observed increased bacterial killing in the blood after G-CSF pretreatment may be due to an increase in the total number of peripheral neutrophils and an increased ability of individual cells to respond.
Others have claimed that prevention of blood-brain barrier disruption by treatment with monoclonal antibodies to integrins alone or in combination with dexamethasone accounts for a delay in the secondary bacteremia in pneumococcal and H. influenzae-induced meningitis (35, 43). However, an increased number of peripheral neutrophils could be responsible for this decrease in secondary bacteremia, since rabbits treated with monoclonal antibodies to integrins or dexamethasone had significantly increased WBC counts in the blood (35, 43). Moreover, rabbits with neutropenia induced by nitrogen mustard had increased blood bacterial concentrations compared to untreated rabbits in pneumococcal meningitis, despite the fact that no difference in meningeal inflammation was seen between groups (8).
In conclusion, G-CSF pretreatment reduces meningeal inflammation and blood bacterial concentration in experimental pneumococcal meningitis. Whether this attenuated inflammatory response affects the clinical course of the meningitis should be further investigated in animal models before G-CSF treatment is used in patients with bacterial meningitis.
ACKNOWLEDGMENTS
This work was supported in part from the Læge Sofus Carl Emil Friis’, Ebbe Celinder’s, Ydes’, and Købmand Svend Hansen’s foundation and the Danish Biotechnology program.
We thank Terrence O’Reilly for invaluable help with the rabbit model and useful scientific discussions and K. Matushima for providing rabbit IL-8 and WS-4. We also thank Bo Sønder Hansen, Jacob Vang, Anne Asanovski, and Hanne Tamstorf for skillful technical assistance.
REFERENCES
- 1.Akahoshi T, Endo H, Kondo H, Kashiwazaki S, Kasahara T, Mukaida N, Harada A, Matsushima K. Essential involvement of interleukin-8 in neutrophil recruitment in rabbits with acute experimental arthritis induced by lipopolysaccharide and interleukin-1. Lymphokine Cytokine Res. 1994;13:113–116. [PubMed] [Google Scholar]
- 2.Aloisi F, Carè A, Borsellino G, Gallo P, Rosa S, Bassani A, Cabibbo A, Testa U, Levi G, Peschle C. Production of hemolymphopoietic cytokines (IL-6, IL-8, colony-stimulating factors) by normal human astrocytes in response to IL-1 beta and tumor necrosis factor-alpha. J Immunol. 1992;149:2358–2366. [PubMed] [Google Scholar]
- 3.Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines—CXC and CC chemokines. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- 4.Clark S C, Kamen R. The human hematopoietic colony-stimulating factors. Science. 1987;236:1229. doi: 10.1126/science.3296190. [DOI] [PubMed] [Google Scholar]
- 5.Dacey R G, Sande M A. Effect of probenecid on cerebrospinal fluid concentrations of penicillin and cephalosporin derivatives. Antimicrob Agents Chemother. 1974;6:437–441. doi: 10.1128/aac.6.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dalhoff K, Hansen F, Dromann D, Schaaf B, Aries S P, Braun J. Inhibition of neutrophil apoptosis and modulation of the inflammatory response by granulocyte colony-stimulating factor in healthy and ethanol-treated human volunteers. J Infect Dis. 1998;178:891–895. doi: 10.1086/515350. [DOI] [PubMed] [Google Scholar]
- 7.Ehrlich L C, Hu S X, Sheng W S, Sutton R L, Rockswold G L, Peterson P K, Chao C C. Cytokine regulation of human microglial cell IL-8 production. J Immunol. 1998;160:1944–1948. [PubMed] [Google Scholar]
- 8.Ernst J D, Decazes J M, Sande M A. Experimental pneumococcal meningitis: role of leukocytes in pathogenesis. Infect Immun. 1983;41:275–279. doi: 10.1128/iai.41.1.275-279.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Espevik T, Nissen-Meyer J. A highly sensitive cell line, WEHI 164 clone 13, for measuring cytotoxic/tumor necrosis factor from human monocytes. J Immunol Methods. 1986;95:99–105. doi: 10.1016/0022-1759(86)90322-4. [DOI] [PubMed] [Google Scholar]
- 10.Gearing A J H, Bird C R, Bristow A, Poole S, Thorpe R. A simple sensitive bioassay for interleukin-1 which is unresponsive to 103 U/ml of interleukin-2. J Immunol Methods. 1987;99:7–11. doi: 10.1016/0022-1759(87)90025-1. [DOI] [PubMed] [Google Scholar]
- 11.Granert C, Raud J, Xie X, Lindquist L, Lindbom L. Inhibition of leukocyte rolling with polysaccharide fucoidin prevents pleocytosis in experimental meningitis in the rabbit. J Clin Investig. 1994;93:929–936. doi: 10.1172/JCI117098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graybill J R, Bocanegra R, Lambros C, Luther M F. Granulocyte colony stimulating factor therapy of experimental cryptococcal meningitis. J Med Vet Mycol. 1997;35:243–247. doi: 10.1080/02681219780001221. [DOI] [PubMed] [Google Scholar]
- 13.Hansen M B, Nielsen S E, Berg K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J Immunol Methods. 1989;119:203–210. doi: 10.1016/0022-1759(89)90397-9. [DOI] [PubMed] [Google Scholar]
- 14.Harada A, Sekido N, Kuno K, Akiyama M, Kasahara T, Nakanishi I, Mukaida N, Matsushima K. Expression of recombinant rabbit IL-8 in Escherichia coli and establishment of the essential involvement of IL-8 in recruiting neutrophils into lipopolysaccharide-induced inflammatory site of rabbit skin. Int Immunol. 1993;5:681–690. doi: 10.1093/intimm/5.6.681. [DOI] [PubMed] [Google Scholar]
- 15.Hartung T, Döcke W D, Gantner F, Krieger G, Sauer A, Stevens P, Volk H D, Wendel A. Effect of granulocyte colony-stimulating factor treatment on ex vivo blood cytokine response in human volunteers. Blood. 1995;85:2482–2489. [PubMed] [Google Scholar]
- 16.Held T K, Mielke M E A, Chedid M, Unger M, Trautmann M, Huhn D, Cross A S. Granulocyte colony-stimulating factor worsens the outcome of experimental Klebsiella pneumoniae pneumonia through direct interaction with the bacteria. Blood. 1998;91:2525–2535. [PubMed] [Google Scholar]
- 17.Hofman F M, Chen P, Jeyaseelan R, Incardona F, Fisher M, Zidovetzki R. Endothelin-1 induces production of the neutrophil chemotactic factor interleukin-8 by human brain-derived endothelial cells. Blood. 1998;92:3064–3072. [PubMed] [Google Scholar]
- 18.Höglund M, Håkansson L, Venge P. Effects of in vivo administration of G-CSF on neutrophil functions in healthy volunteers. Eur J Haematol. 1997;58:195–202. doi: 10.1111/j.1600-0609.1997.tb00947.x. [DOI] [PubMed] [Google Scholar]
- 19.Jensen P, Kharazmi A. Computer-assisted image analysis assay of human neutrophil chemotaxis in vitro. J Immunol Methods. 1991;144:43–48. doi: 10.1016/0022-1759(91)90228-8. [DOI] [PubMed] [Google Scholar]
- 20.Kharazmi A, Høiby N, Döring G, Valerius N H. Pseudomonas aeruginosa exoproteases inhibit human neutrophil chemiluminescence. Infect Immun. 1984;44:587–591. doi: 10.1128/iai.44.3.587-591.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ko Y, Mukaida N, Panyutich A, Voitenok N N, Matsushima K, Kawai T, Kasahara T. A sensitive enzyme-linked immunosorbent assay for human interleukin-8. J Immunol Methods. 1992;149:227–235. doi: 10.1016/0022-1759(92)90254-q. [DOI] [PubMed] [Google Scholar]
- 22.Koedel U, Bernatowicz A, Paul R, Frei K, Fontana A, Pfister H W. Experimental pneumococcal meningitis: cerebrovascular alterations, brain edema, and meningeal inflammation are linked to the production of nitric oxide. Ann Neurol. 1995;37:313–323. doi: 10.1002/ana.410370307. [DOI] [PubMed] [Google Scholar]
- 23.Kurtzhals J A, Kemp M, Poulsen L K, Hansen M B, Kharazmi A, Theander T G. Interleukin-4 and interferon-gamma production by Leishmania stimulated peripheral blood mononuclear cells from nonexposed individuals. Scand J Immunol. 1995;41:343–349. doi: 10.1111/j.1365-3083.1995.tb03577.x. [DOI] [PubMed] [Google Scholar]
- 24.Leib S L, Kim Y S, Chow L L, Sheldon R A, Tauber M G. Reactive oxygen intermediates contribute to necrotic and apoptotic neuronal injury in an infant rat model of bacterial meningitis due to group B streptococci. J Clin Investig. 1996;98:2632–2639. doi: 10.1172/JCI119084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leib S L, Kim Y S M, Ferriero D M, Täuber M G. Neuroprotective effect of excitatory amino acid antagonist kynurenic acid in experimental bacterial meningitis. J Infect Dis. 1996;173:166–171. doi: 10.1093/infdis/173.1.166. [DOI] [PubMed] [Google Scholar]
- 26.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 27.Mooney D P, Gamelli R L, O’Reilly M, Hebert J C. Recombinant human granulocyte colony-stimulating factor and Pseudomonas burn wound sepsis. Arch Surg. 1988;123:1353–1357. doi: 10.1001/archsurg.1988.01400350067010. [DOI] [PubMed] [Google Scholar]
- 28.Nelson S, Summer W, Bagby G, Nakamura C, Stewart L, Lipscomb G, Andresen J. Granulocyte colony-stimulating factor enhances pulmonary host defenses in normal and ethanol-treated rats. J Infect Dis. 1991;164:901–906. doi: 10.1093/infdis/164.5.901. [DOI] [PubMed] [Google Scholar]
- 29.Ohsaka A, Saionji K, Igari J. Granulocyte colony-stimulating factor administration increases serum concentrations of soluble selectins. Br J Haematol. 1998;100:66–69. doi: 10.1046/j.1365-2141.1998.00510.x. [DOI] [PubMed] [Google Scholar]
- 30.Ohsaka A, Saionji K, Sato N, Mori T, Ishimoto K, Inamatsu T. Granulocyte colony-stimulating factor down-regulates the surface expression of the human leucocyte adhesion molecule-1 on human neutrophils in vitro and in vivo. Br J Haematol. 1993;84:574–580. doi: 10.1111/j.1365-2141.1993.tb03130.x. [DOI] [PubMed] [Google Scholar]
- 31.Price T H, Chatta G S, Dale D C. Effect of recombinant granulocyte colony-stimulating factor on neutrophil kinetics in normal young and elderly humans. Blood. 1996;88:335–340. [PubMed] [Google Scholar]
- 32.Quagliarello V, Scheld W M. Bacterial meningitis: pathogenesis, pathophysiology, and progress. N Engl J Med. 1992;327:864–872. doi: 10.1056/NEJM199209173271208. [DOI] [PubMed] [Google Scholar]
- 33.Ramilo O, Saez Llorens X, Mertsola J, Jafari H, Olsen K D, Hansen E J, Yoshinaga M, Ohkawara S, Nariuchi H, McCracken G H., Jr Tumor necrosis factor alpha/cachectin and interleukin 1 beta initiate meningeal inflammation. J Exp Med. 1990;172:497–507. doi: 10.1084/jem.172.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rozdzinski E, Jones T, Burnette W N, Burroughs M, Tuomanen E. Antiinflammatory effects in experimental meningitis of prokaryotic peptides that mimic selectins. J Infect Dis. 1993;168:1422–1428. doi: 10.1093/infdis/168.6.1422. [DOI] [PubMed] [Google Scholar]
- 35.Saez-Llorens X, Jafari H S, Severien C, Parras F, Olsen K D, Hansen E J, Singer I I, McCracken G H. Enhanced attenuation of meningeal inflammation and brain edema by concomitant administration of anti-CD18 monoclonal antibodies and dexamethasone in experimental Haemophilus meningitis. J Clin Investig. 1991;88:2003–2011. doi: 10.1172/JCI115527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saukkonen K, Sande S, Cioffe C, Wolpe S, Sherry B, Cerami A, Tuomanen E. The role of cytokines in the generation of inflammation and tissue damage in experimental gram-positive meningitis. J Exp Med. 1990;171:439–448. doi: 10.1084/jem.171.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith W B, Gamble J R, Clark Lewis I, Vadas M A. Interleukin-8 induces neutrophil transendothelial migration. Immunology. 1991;72:65–72. [PMC free article] [PubMed] [Google Scholar]
- 38.Spector R, Lorenzo A V. Inhibition of penicillin transport from the cerebrospinal fluid after intracisternal inoculation of bacteria. J Clin Investig. 1974;54:316–325. doi: 10.1172/JCI107767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spiekermann K, Emmendoerffer A, Elsner J, Raeder E, Lohmann Matthes M L, Prahst A, Link H, Freund M, Welte K, Roesler J. Altered surface marker expression and function of G-CSF-induced neutrophils from test subjects and patients under chemotherapy. Br J Haematol. 1994;87:31–38. doi: 10.1111/j.1365-2141.1994.tb04866.x. [DOI] [PubMed] [Google Scholar]
- 40.Spiekermann K, Roesler J, Emmendoerffer A, Elsner J, Welte K. Functional features of neutrophils induced by G-CSF and GM-CSF treatment: differential effects and clinical implications. Leukemia. 1997;11:466–478. doi: 10.1038/sj.leu.2400607. [DOI] [PubMed] [Google Scholar]
- 41.Tauber M G, Borschberg U, Sande M A. Influence of granulocytes on brain edema, intracranial pressure, and cerebrospinal fluid concentrations of lactate and protein in experimental meningitis. J Infect Dis. 1988;157:456–464. doi: 10.1093/infdis/157.3.456. [DOI] [PubMed] [Google Scholar]
- 42.Tuomanen E, Liu H, Hengstler B, Zak O, Tomasz A. The induction of meningeal inflammation by components of the pneumococcal cell wall. J Infect Dis. 1985;151:859–868. doi: 10.1093/infdis/151.5.859. [DOI] [PubMed] [Google Scholar]
- 43.Tuomanen E I, Saukkonen K, Sande S, Cioffe C, Wright S D. Reduction of inflammation, tissue damage, and mortality in bacterial meningitis in rabbits treated with monoclonal antibodies against adhesion-promoting receptors of leukocytes. J Exp Med. 1989;170:959–969. doi: 10.1084/jem.170.3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yasuda H, Ajiki Y, Shimozato T, Kasahara M, Kawada H, Iwata M, Shimizu K. Therapeutic efficacy of granulocyte colony-stimulating factor alone and in combination with antibiotics against Pseudomonas aeruginosa infections in mice. Infect Immun. 1990;58:2502–2509. doi: 10.1128/iai.58.8.2502-2509.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zysk G, Brück W, Huitinga I, Fischer F R, Flachsbarth F, van Rooijen N, Nau R. Elimination of blood-derived macrophages inhibits the release of interleukin-1 and the entry of leukocytes into the cerebrospinal fluid in experimental pneumococcal meningitis. J Neuroimmunol. 1997;73:77–80. doi: 10.1016/s0165-5728(96)00173-7. [DOI] [PubMed] [Google Scholar]