Abstract
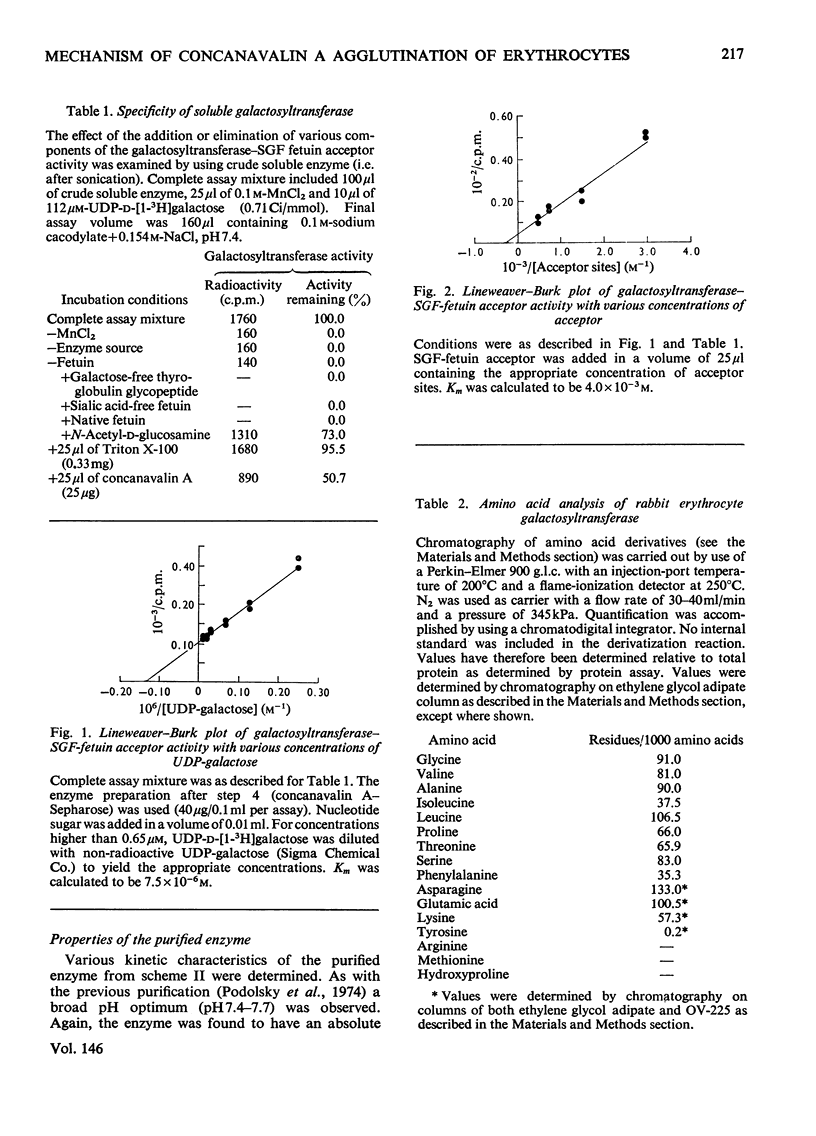
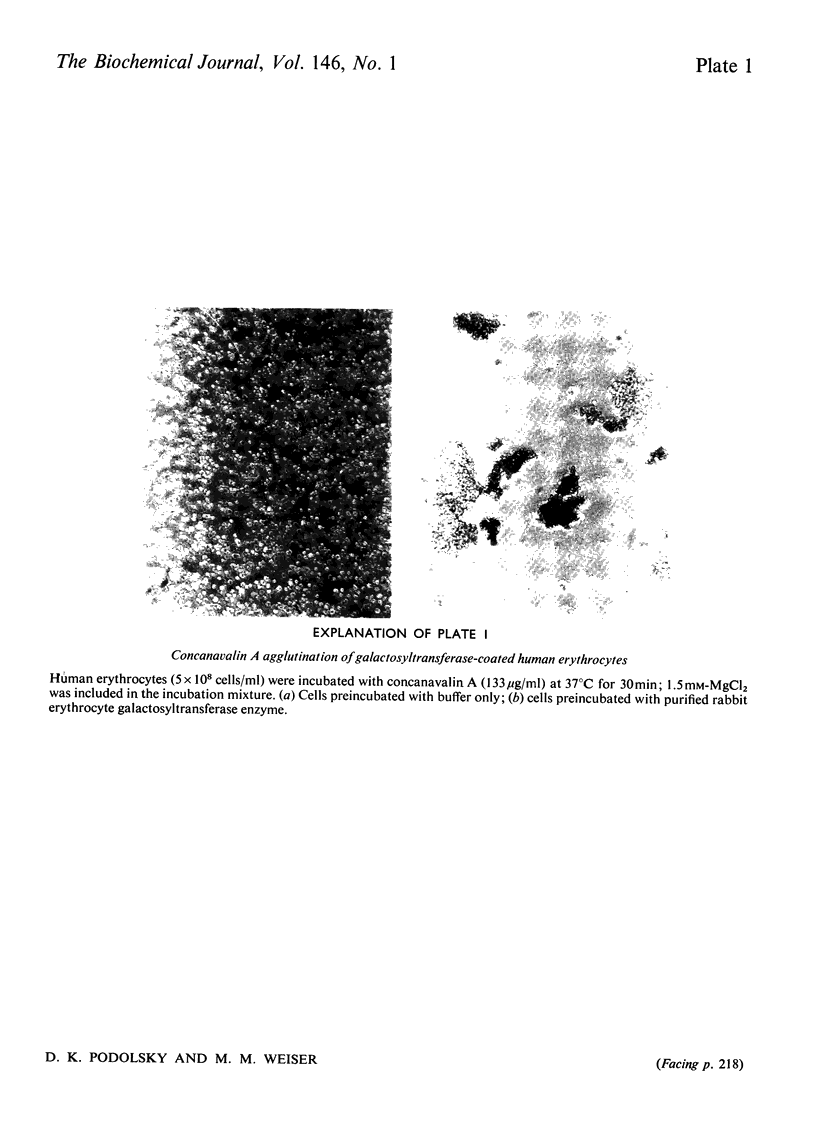
It has been previously observed that rabbit erythrocyte cell surface galactosyltransferase appears to play a role in concanavalin A agglutination of these erythrocytes (Podolsky et al., 1974). Further, a correlation between the occurrence or level of cell surface galactosyltransferase and concanavalin A agglutinability of other cell types has also been observed. The mechanism by which rabbit erythrocyte galactosyltransferase participates in concanavalin A agglutination has now been further defined. The enzyme was solubilized and purified. Characterization of the enzyme properties has shown them to be similar to those reported for other purified galactosyltransferases. Amino acid and carbohydrate analysis showed a high asparagine content and the presence of D-mannose. Specific alpha-mannosidase treatment of the enzyme showed that some of these D-mannose residues were terminal sugars. The purified enzyme also conferred concanavalin A agglutinability to non-agglutinable human erythrocytes. However, the ability to confer concanavalin A agglutinability was unrelated to the enzyme activity per se (as measured with fetuin acceptor) but appeared to be entirely dependent on the presence of terminal alpha-linked D-mannosyl residues in the enzyme structure. These findings suggest that the presence of terminal alpha-mannosidyl residues on cell surface glycoproteins such as galactosyltransferase may be the determining factor in agglutination of cells by concanavalin A.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan D., Auger J., Crumpton M. J. Glycoprotein receptors for concanavalin A isolated from pig lymphocyte plasma membrane by affinity chromatography in sodium deoxycholate. Nat New Biol. 1972 Mar 8;236(62):23–25. doi: 10.1038/newbio236023a0. [DOI] [PubMed] [Google Scholar]
- Caccam J. F., Eylar E. H. Glycoprotein biosynthesis: purification and characterization of a glycoprotein: galactosyl transferase from Ehrlich ascites tumor cell membranes. Arch Biochem Biophys. 1970 Apr;137(2):315–324. doi: 10.1016/0003-9861(70)90445-5. [DOI] [PubMed] [Google Scholar]
- Cline M. J., Livingston D. C. Binding of 3 H-concanavalin A by normal and transformed cells. Nat New Biol. 1971 Aug 4;232(31):155–156. doi: 10.1038/newbio232155a0. [DOI] [PubMed] [Google Scholar]
- GOLDSTEIN I. J., HOLLERMAN C. E., SMITH E. E. PROTEIN-CARBOHYDRATE INTERACTION. II. INHIBITION STUDIES ON THE INTERACTION OF CONCANAVALIN A WITH POLYSACCHARIDES. Biochemistry. 1965 May;4:876–883. doi: 10.1021/bi00881a013. [DOI] [PubMed] [Google Scholar]
- Janson V. K., Sakamoto C. K., Burger M. M. Isolation and characterization of agglutinin receptor sites. 3. Studies on the interaction with other lectins. Biochim Biophys Acta. 1973 Jan 2;291(1):136–143. doi: 10.1016/0005-2736(73)90068-0. [DOI] [PubMed] [Google Scholar]
- Kim Y. S., Perdomo J., Nordberg J. Glycoprortein biosynthesis in small intestinal mucosa. I. A study of glycosyltransferases in microsomal subfractions. J Biol Chem. 1971 Sep 10;246(17):5466–5476. [PubMed] [Google Scholar]
- Kim Y. S., Perdomo J., Whitehead J. S. Glycosyltransferases in human blood.I. Galactosyltransferase in human serum and erythrocyte membranes. J Clin Invest. 1972 Aug;51(8):2024–2032. doi: 10.1172/JCI107008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nicolson G. L. Topography of membrane concanavalin A sites modified by proteolysis. Nat New Biol. 1972 Oct 18;239(94):193–197. doi: 10.1038/newbio239193a0. [DOI] [PubMed] [Google Scholar]
- Noonan K. D., Burger M. M. Binding of ( 3 H)concanavalin A to normal and transformed cells. J Biol Chem. 1973 Jun 25;248(12):4286–4292. [PubMed] [Google Scholar]
- Noonan K. D., Burger M. M. The relationship of concanavalin A binding to lectin-initiated cell agglutination. J Cell Biol. 1973 Oct;59(1):134–142. doi: 10.1083/jcb.59.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan K. D., Renger H. C., Basilico C., Burger M. M. Surface changes in temperature-sensitive Simian virus 40-transformed cells. Proc Natl Acad Sci U S A. 1973 Feb;70(2):347–349. doi: 10.1073/pnas.70.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozanne B., Sambrook J. Binding of radioactively labelled concanavalin A and wheat germ agglutinin to normal and virus-transformed cells. Nat New Biol. 1971 Aug 4;232(31):156–160. doi: 10.1038/newbio232156a0. [DOI] [PubMed] [Google Scholar]
- Podolsky D. K., Weiser M. M., La Mont J. T., Isselbacher K. J. Galactosyltransferase and concanavalin A agglutination of cells. Proc Natl Acad Sci U S A. 1974 Mar;71(3):904–908. doi: 10.1073/pnas.71.3.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolsky D. K., Weiser M. M. Specific selection of mitotically active intestinal cells by concanavalin A-derivatized fibers. J Cell Biol. 1973 Aug;58(2):497–500. doi: 10.1083/jcb.58.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon N., Lis H. Lectins: cell-agglutinating and sugar-specific proteins. Science. 1972 Sep 15;177(4053):949–959. doi: 10.1126/science.177.4053.949. [DOI] [PubMed] [Google Scholar]
- So L. L., Goldstein I. J. Protein-carbohydrate interaction. 13. The interaction of concanavalin A with alpha-mannans from a variety of microorganisms. J Biol Chem. 1968 Apr 25;243(8):2003–2007. [PubMed] [Google Scholar]
- Spiro M. J., Spiro R. G. Glycoprotein biosynthesis: studies on thyroglobulin. Thyroid sialyltransferase. J Biol Chem. 1968 Dec 25;243(24):6520–6528. [PubMed] [Google Scholar]
- Steck T. L., Weinstein R. S., Straus J. H., Wallach D. F. Inside-out red cell membrane vesicles: preparation and purification. Science. 1970 Apr 10;168(3928):255–257. doi: 10.1126/science.168.3928.255. [DOI] [PubMed] [Google Scholar]
- Sumner J. B., Howell S. F. Identification of Hemagglutinin of Jack Bean with Concanavalin A. J Bacteriol. 1936 Aug;32(2):227–237. doi: 10.1128/jb.32.2.227-237.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- Tarentino A., Plummer T. H., Jr, Maley F. Studies on the oligosaccharide sequence of ribonuclease B. J Biol Chem. 1970 Aug 25;245(16):4150–4157. [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Weiser M. M. Intestinal epithelial cell surface membrane glycoprotein synthesis. I. An indicator of cellular differentiation. J Biol Chem. 1973 Apr 10;248(7):2536–2541. [PubMed] [Google Scholar]
- Wray V. P., Walborg E. F., Jr Isolation of tumor cell surface binding sites for concanavalin A and wheat germ agglutinin. Cancer Res. 1971 Dec;31(12):2072–2079. [PubMed] [Google Scholar]
- Zumwalt R. W., Roach D., Gehrke C. W. Gas-liquid chromatography of amino acids in biological substances. J Chromatogr. 1970 Dec;53(2):171–194. doi: 10.1016/s0021-9673(01)98457-2. [DOI] [PubMed] [Google Scholar]



