Abstract
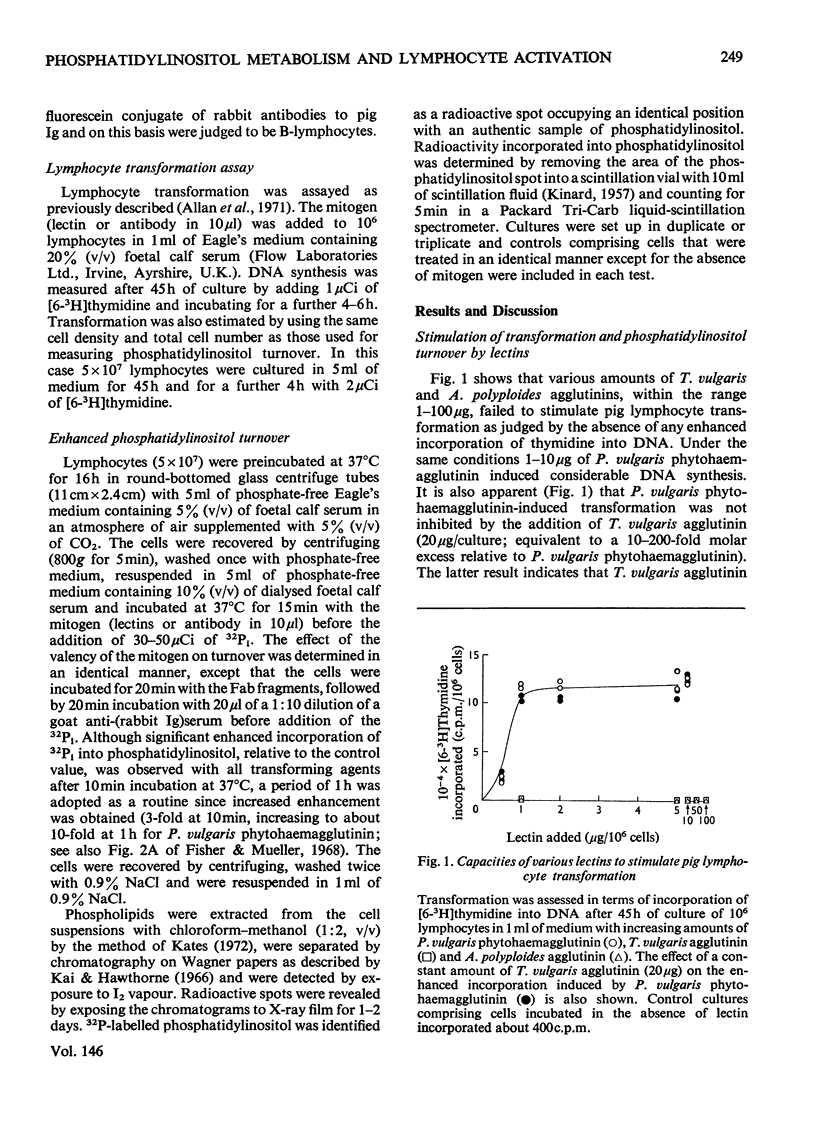
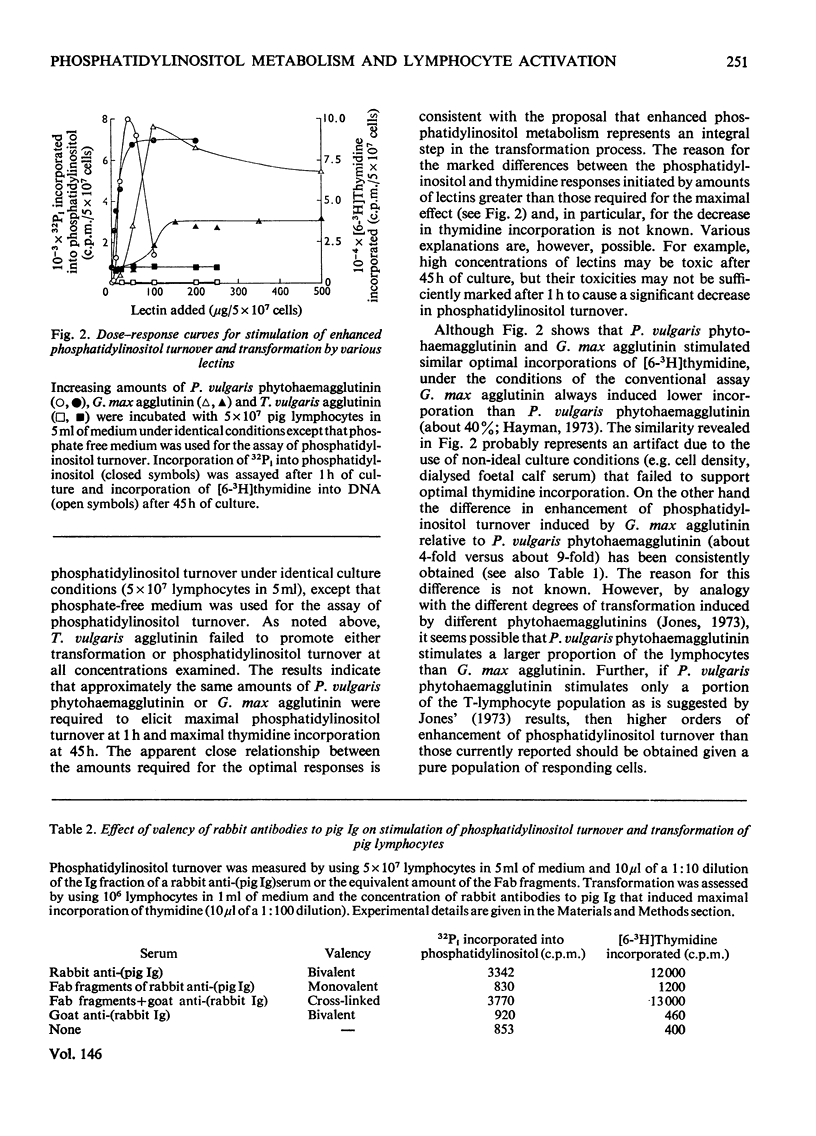
1. Various lectins [phaseolus vulgaris phytohaemagglutinin, Glycine max (soy-bean) agglutinin, Triticum vulgaris (wheat-germ) agglutinin and Axinella polyploides agglutinin] and antibodies to pig Ig (immunoglobulin) that are found by pig lymphocytes were assessed in terms of their capacities to stimulate lymphocyte transformation and to enhance phosphatidylinositol turnover. Transformation was measured after 45h of culture by incorporation of [6-(3)H]thymidine into DNA, whereas phosphatidylinositol metabolism was assessed after 1h of cultuis and G. max agglutinins and rabbit antibodies to pig Ig) increased phosphatidylinositol turnover, but non-transforming agents (T. vulgaris and A. polyploides agglutinins and Fab fragments of rabbit antibodies to pig Ig) failed to induce any significant enhancement. Subsequent cross-linkage of the bound, non-transforming Fab fragments with a goat antiserum to rabbit Ig stimulated transformation and phosphatidylinositol turnover. 3. Each transforming agent gave characteristic optimal dose responses that were similar for both phosphatidylinositol turnover and transformation. 4. The results indicate that activation of T- and B-lymphocytes is accompanied by enhanced phosphatidylinositol turnover and that in the case of B-cells this enhancement depends on the cross-linkage of surface receptors. They are consistent with the proposal that turnover represents an essential early step in the transformation process.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan D., Auger J., Crumpton M. J. Glycoprotein receptors for concanavalin A isolated from pig lymphocyte plasma membrane by affinity chromatography in sodium deoxycholate. Nat New Biol. 1972 Mar 8;236(62):23–25. doi: 10.1038/newbio236023a0. [DOI] [PubMed] [Google Scholar]
- Allan D., Auger J., Crumpton M. J. Interaction of phytohemagglutinin with plasma membranes of pig lymphocytes and thymus cells. Exp Cell Res. 1971 Jun;66(2):362–368. doi: 10.1016/0014-4827(71)90689-6. [DOI] [PubMed] [Google Scholar]
- Allen A. K., Neuberger A., Sharon N. The purification, composition and specificity of wheat-germ agglutinin. Biochem J. 1973 Jan;131(1):155–162. doi: 10.1042/bj1310155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elson C. J., Singh J., Taylor R. B. The effect of capping by anti-immunoglobulin antibody on the expression of cell surface immunoglobulin and on lymphocyte activation. Scand J Immunol. 1973;2(2):143–149. doi: 10.1111/j.1365-3083.1973.tb02025.x. [DOI] [PubMed] [Google Scholar]
- Fanger M. W., Hart D. A., Wells J. V., Nisonoff A. Requirement for cross-linkage in the stimulation of transformation of rabbit peripheral lymphocytes by antiglobulin reagents. J Immunol. 1970 Dec;105(6):1484–1492. [PubMed] [Google Scholar]
- Fisher D. B., Mueller G. C. An early alteration in the phospholipid metabolism of lymphocytes by phytohemagglutinin. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1396–1402. doi: 10.1073/pnas.60.4.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freinkel N., Dawson R. M. Role of inositol cyclic phosphate in stimulated tissues. Nature. 1973 Jun 29;243(5409):535–537. doi: 10.1038/243535a0. [DOI] [PubMed] [Google Scholar]
- Gordon Julius A., Blumberg Shmaryahu, Lis Halina, Sharon Nathan. Purification of soybean agglutinin by affinity chromatography On sepharose-N-epsilon-aminocaproyl-beta-D-galactopyranosylamine. FEBS Lett. 1972 Aug 1;24(2):193–196. doi: 10.1016/0014-5793(72)80765-8. [DOI] [PubMed] [Google Scholar]
- Greaves M., Janossy G. Elicitation of selective T and B lymphocyte responses by cell surface binding ligands. Transplant Rev. 1972;11:87–130. doi: 10.1111/j.1600-065x.1972.tb00047.x. [DOI] [PubMed] [Google Scholar]
- Hokin L. E. Dynamic aspects of phospholipids during protein secretion. Int Rev Cytol. 1968;23:187–208. doi: 10.1016/s0074-7696(08)60272-7. [DOI] [PubMed] [Google Scholar]
- Jones G. The number of reactive cells in mouse lymphocyte cultures stimulated by phytohemagglutinin, concanavalin A or histocompatibility antigen. J Immunol. 1973 Sep;111(3):914–920. [PubMed] [Google Scholar]
- KARNOVSKY M. L., WALLACH D. F. The metabolic basis of phagocytosis. III. Incorporation of inorganic phosphate into various classes of phosphatides during phagocytosis. J Biol Chem. 1961 Jul;236:1895–1901. [PubMed] [Google Scholar]
- Kai M., Hawthorne J. N. Incorporation of injected [32P] phosphate into the phosphoinositides of subcellular fractions from young rat brain. Biochem J. 1966 Jan;98(1):62–67. doi: 10.1042/bj0980062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapetina E. G., Michell R. H. Phosphatidylinositol metabolism in cells receiving extracellular stimulation. FEBS Lett. 1973 Apr 1;31(1):1–10. doi: 10.1016/0014-5793(73)80061-4. [DOI] [PubMed] [Google Scholar]
- Loor F. Lymphocyte membrane particle redistribution induced by a mitogenic-capping dose of the phytohemagglutinin of Phaseolus vulgaris. Eur J Immunol. 1973 Feb;3(2):112–116. doi: 10.1002/eji.1830030212. [DOI] [PubMed] [Google Scholar]
- Masuzawa Y., Osawa T., Inoue K., Nojima S. Effects of various mitogens on the phospholipid metabolism of human peripheral lymphocytes. Biochim Biophys Acta. 1973 Dec 20;326(3):339–344. [PubMed] [Google Scholar]
- Michell R. H., Lapetina E. G. Production of cyclic inositol phosphate in stimulated tissues. Nat New Biol. 1972 Dec 27;240(104):258–260. doi: 10.1038/newbio240258a0. [DOI] [PubMed] [Google Scholar]
- PORTER R. R. The hydrolysis of rabbit y-globulin and antibodies with crystalline papain. Biochem J. 1959 Sep;73:119–126. doi: 10.1042/bj0730119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak C. A. Relief from contact inhibition. Early increase in phospholipid turnover. J Cell Biol. 1972 Apr;53(1):231–234. doi: 10.1083/jcb.53.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resch K., Ferber E., Odenthal J., Fischer H. Early changes in the phospholipid metabolism of lymphocytes following stimulation with phytohemagglutinin and with lysolecithin. Eur J Immunol. 1971 Jun;1(3):162–165. doi: 10.1002/eji.1830010304. [DOI] [PubMed] [Google Scholar]
- Resch K., Ferber E. Phospholipid metabolism of stimulated lymphocytes. Effects of phytohemagglutinin, concanavalin A and anti-immunoglobulin serum. Eur J Biochem. 1972 May;27(1):153–161. doi: 10.1111/j.1432-1033.1972.tb01821.x. [DOI] [PubMed] [Google Scholar]
- STOKER M., MACPHERSON I. SYRIAN HAMSTER FIBROBLAST CELL LINE BHK21 AND ITS DERIVATIVES. Nature. 1964 Sep 26;203:1355–1357. doi: 10.1038/2031355a0. [DOI] [PubMed] [Google Scholar]
- Sell S., Rowe D. S., Gell P. G. Studies on rabbit lymphocytes in vitro. 3. Proteins, RNA, and DNA synthesis by lymphocyte cultures after stimulation with phytohaemagglutinin, with staphylococcal filtrate, with antiallotype serum, and with heterologous antiserum to rabbit whole serum. J Exp Med. 1965 Oct 1;122(4):823–839. doi: 10.1084/jem.122.4.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon N., Lis H. Lectins: cell-agglutinating and sugar-specific proteins. Science. 1972 Sep 15;177(4053):949–959. doi: 10.1126/science.177.4053.949. [DOI] [PubMed] [Google Scholar]
- Young N. M., Leon M. A., Takahashi T., Howard I. K., Sage H. J. Studies on a phytohemagglutinin from the lentil. 3. Reaction of Lens culinaris hemagglutinin with polysaccharides, glycoproteins, and lymphocytes. J Biol Chem. 1971 Mar 25;246(6):1596–1601. [PubMed] [Google Scholar]


