Abstract
Heart failure is a leading cause of death among people worldwide. The cost of treatment can be prohibitive, and early prediction of heart failure would reduce treatment costs to patients and hospitals. Improved readmission prediction would also greatly help hospitals, allowing them to manage their treatment programs and budgets better. This literature review aims to summarize recent studies of predictive analytics models that have been constructed to predict heart failure risk, readmission, and mortality. Random forest, logistic regression, neural networks, and XGBoost were among the most common modeling techniques applied. Most selected studies leveraged structured electronic health record data, including demographics, clinical values, lifestyle, and comorbidities, with some incorporating unstructured clinical notes. Preprocessing through imputation and feature selection were frequently employed in building the predictive analytics models. The reviewed studies exhibit demonstrated promise for predictive analytics in improving early heart failure diagnosis, readmission risk stratification, and mortality prediction. This review study highlights rising research activities and the potential of predictive analytics, especially the implementation of machine learning, in advancing heart failure outcomes. Further rigorous, comprehensive syntheses and head-to-head benchmarking of predictive models are needed to derive robust evidence for clinical adoption.
Keywords: heart failure, mortality, predictive analytics, predictive models, readmission, risk prediction
Introduction and background
Heart disease is one of the deadliest diseases among people worldwide [1], with 50% of heart failure (HF) patients dying within five years [2]. Doctors use a variety of tests to diagnose heart failure, including physical examination, blood and laboratory tests, and family history of the disease [1]. Even though there is no cure for heart failure, delicate medical procedures and treatments improve quality of life [2]. Researchers have conducted various studies to build prediction models to assist with the diagnosis of heart failure: early diagnosis allows a patient to get the proper treatment and minimizes the seriousness of this disease [3].
Medical procedures and treatments are expensive, especially for hospitalized patients [3]. To help reduce the expense, researchers have done numerous studies to identify heart failure patients who will need readmission. As information technology has developed, especially artificial intelligence, researchers have begun developing better early prediction systems. Including machine learning in the models improves their performance and prediction quality. With the right dataset and suitable data processing techniques, a predictive model’s performance should improve [4]. Predictive analytics has become the most used approach by researchers who construct predictive models using machine learning. In this approach, researchers use data gleaned from electronic health records to predict hospital readmission and mortality. However, there are serious obstacles to the development of predictive models that use a predictive analytics approach. Problems with data presentation and addressing problems such as imbalance in data class create challenges for researchers.
In this literature review, we provide an overview of predictive analytics methods, especially machine learning approaches, for predicting heart failure risk, readmission, and mortality among patients with heart failure. This narrative review aims to synthesize and interpret findings from a broad range of studies, providing a comprehensive overview of the machine learning methodologies employed in this domain. While traditional statistical approaches have been extensively used in clinical settings, our review highlights the advancements and contributions of machine learning techniques, which offer enhanced predictive capabilities by leveraging large, complex datasets. Through this narrative approach, we contextualize the role of machine learning in improving clinical decision-making and patient outcomes, offering insight into the potential and limitations of these technologies for heart failure risk prediction.
This article was previously posted to the Preprints.org preprint server on January 23, 2024, with doi https://doi.org/10.20944/preprints202401.1671.v1.
Review
Methodology of literature selection
Literature Exploration
Before starting our search for research articles on predictive analytics in heart failure, it was necessary to define the targets, topics, and themes necessary for a comprehensive search. Five such targets were defined: predictive analytics in heart failure, heart failure risk prediction, heart failure readmission prediction, heart failure mortality prediction, and machine learning implementation in heart failure research. Furthermore, we chose closely related keywords that would be critical to our Google Scholar and PubMed search and limited it to the years from 2000 to 2023 to include only the most recent studies conducted in this field. Quotation mark- (“ ”) and Boolean operators (AND and OR) were used to search for titles and abstracts that were closely related to the defined topic. The final search query was: ("machine learning" AND ("heart failure" OR "heart failure prediction" OR "heart failure risk" OR "heart failure risk prediction")) AND ("predictive analytics" AND ("heart failure" OR "heart failure prediction" OR "heart failure risk" OR "heart failure risk prediction")). After doing this wide search, the next step was to select the studies to be included. The search stage resulted in 3,270 publications.
Study Selection and Screening
In the study selection step, several considerations went into pairing down the articles found in the preliminary search, as follows: 1. A paper must have been published in a journal or conference booklet, or book series to be selected; 2. It must be a research paper, not a review, a meta-analysis, or a literature review; 3. We considered the credibility and quality of the publisher. We cross-checked the publisher and journal with Scimago/Scopus and Clarivate/Web of Science to do this; 4. The published papers should include the full text. However, our university’s limited access to journal subscriptions may be problematic in terms of the selection of papers. Having access to the major publications but not all possible publications may have resulted in the exclusion of potentially relevant studies, thereby limiting the comprehensiveness of our review. Despite these constraints, we endeavored to mitigate this limitation by accessing open-access journals and utilizing interlibrary loan services whenever possible.
To eliminate papers that varied from the topic and to select those that met our conditions, we read the full titles and abstracts of the selected papers, paying careful attention to the considerations we set above, giving us 173 published papers. All were read with careful consideration given to the availability of data analysis and processing techniques, the use of machine learning or other approaches to build the predictive analytics model, and the data specification described in the articles. It was also important to ensure that the paper was related to the prediction of heart failure diagnosis, mortality, and readmission of heart failure patients. Papers meeting the above conditions in the screening stages were extracted for this review.
Data Extraction
Only 65 papers that met our specifications. The items extracted included the author’s name, year of publication, study objective, origin of data, dataset specifications, machine learning algorithm(s), methodology, and the evaluation of the model.
Literature Review Diagram Flow
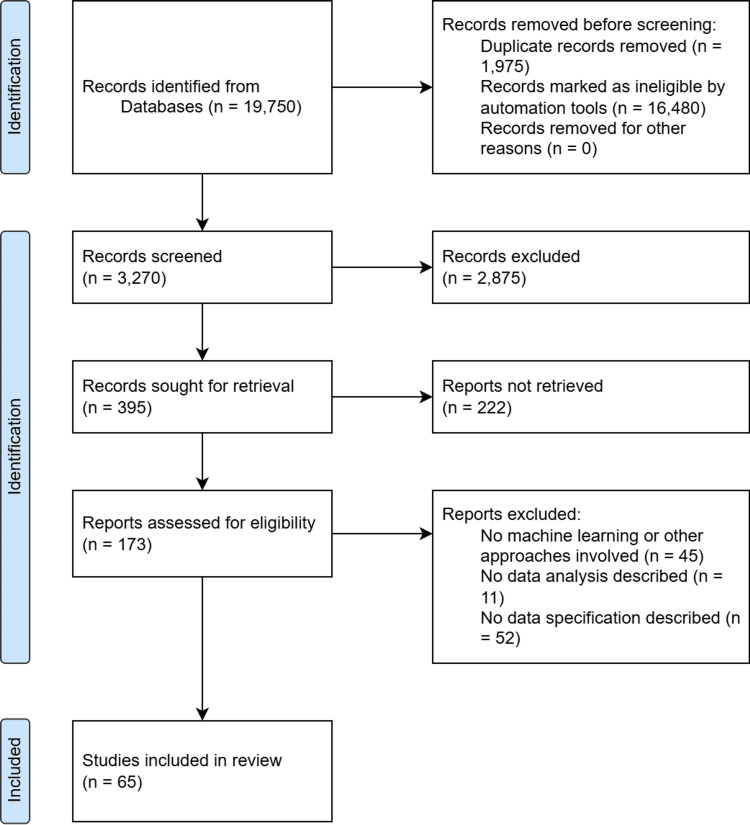
Figure 1 below shows the flow of this review process.
Figure 1. Literature review flow diagram.
Classification of the Papers
The 65 papers extracted were classified into two categories. The first includes 26 papers that mainly aim to predict or diagnose heart failure or risk of heart failure using statistical or machine learning approaches to build a predictive analytics model for heart failure prediction, using either their own or open-accessed datasets. The second category includes 39 papers that aimed to predict readmission or mortality among patients with heart failure. They were divided into three subcategories: readmission (17), mortality (14), or both (8).
Results
Table 1 summarizes the studies on predictive analytics for heart failure risk, readmission, and mortality prediction. It includes diverse data sources like hospitals, clinics, and national databases, with sample sizes ranging from small cohorts to over a million patients. The studies focus on predicting heart failure diagnosis, readmission, and mortality using models such as random forest, neural networks, support vector machines (SVM), and logistic regression. Performance metrics like accuracy and AUC are used, with notable results including high accuracy and area under the curve (AUC) values, highlighting the potential of machine learning to enhance predictive analytics in heart failure.
Table 1. Summary of the included studies.
Abbreviations:
NN:Neural Networks; SVM:Support Vector Machine; RF:Random Forest; DT:Decision Tree; CART:Classification and Regression Tree; kNN:k-Nearest Neighbor; LR:Logistic Regression; CNN:Convolutional Neural Network; LMT:Logistic Model Tree; ROT:Rotation Forest; XGBoost:eXtreme Gradient Boosting; Adaboost:Adaptive Boosting; LEBoosting:Least Error Boosting; LPBoosting:Linear Programming Boosting; NB:Naive Bayes; LightGBM:Light Gradient-Boosting Machine; MLP:Multilayer Perceptron; DNN:Deep Neural Networks; GB:Gradient Boosting; VFI:Voting Feature Intervals; GLMN:Generalized Linear Model Net; NLP:Natural Language Processing; LSTM:Long Short-Term Memory; SVC:Support Vector Classifier; BRF:Boosted Random Forest; ANN:Artificial Neural Networks; LASSO:Least Absolute Shrinkage and Selection Operator; PAR:Potentially Avoidable Readmissions; CMS:Centers for Medicare & Medicaid; PPR:Potentially Preventable Readmissions; SMOTE:Synthetic Minority Over-sampling Technique; KS-Test:Kolmogorov-Smirnov Test; PCA:Principal Component Analysis; RFE:Recursive Feature Elimination; AUC:Area Under the ROC curve; AUROC:Area Under Receiver Operating Characteristic Curve; PPV:Positive Predictive Values; NPV:Negative Predictive Values; SHAP:Shapley Additive Explanations; LR+:Positive Likelihood Ratio; NNT:Number Needed to Treat; PR-AUC:Precision Recall Aread under curve; MCC:Matthew's Correlation Coefficient; HF:Heart Failure; ECG:Electrocardiogram; BNP:Brain Natriuretic Peptide; KSUMC:King Saud University Medical City; NMMC:Northern Mindanao Medical Center; MARKER-HF:Machine Learning Assessment of Risk and Early Mortality in Heart Failure; UCI:University of California Irvine; UCSD:University of California San Diego; BIOSTAT-CHF:Biology Study to Tailored Treatment in Chronic Heart Failure; GWTG-HF:Get With The Guidelines-Heart Failure; ADHERE:Acute Decompensated Heart Failure National Registry; SHFM:Seattle Heart Failure Model; GISC:Gestione Integrata dello Scompenso Cardiaco
| Study | Data Source | Sample Size | Prediction Target | Model(s) Used | Key Techniques | Performance Metrics | Result |
| Heart failure prediction studies that include diagnosis and risk prediction | |||||||
| Guidi et al. [5] | St. Maria Nuova Hospital | 136 | HF severity | NN, SVM, Fuzzy genetic, CART, Random Forest | Developing CDSS for analysis HF patients | Accuracy | NN=84.73% SVM=85.2% Fuzzy genetic=85.9% CART=87.6% Random forest=85.6% |
| Yajuan et al. [6] | Geisinger Clinic | 400,000+ | HF diagnosis | Random Forest | Combining unstructured and structured data | AUC | Random forest=83% |
| Ng et al. [7] | Geisinger Clinic | 400,000+ | HF diagnosis | Logistic regression, Random Forest, SVM, kNN, Decision tree (Only L-1 logistic regression and Random forest were selected for superior predictive performance) | Processing longitudinal electronic health records data through feature extraction techniques | AUC | Both=0.74 - 0.80 |
| Rammal [8] | KSUMC | 100 | HF diagnosis | Random Forest, Logistic regression | Big data environment and PCA | Accuracy, recall, precision, AUC | Random forest (%)=93.3, 93.3, 94.3, 94.2 Logistic regresstion (%)=93.3, 93.3, 93.3, 94.3 |
| Nagrecha et al. [9] | Medicare USA | 1 million+ | HF diagnosis | Trajectory-based (Directed Acyclic Graph/DAG) | Disease progression | AUROC | DAG Depth: 1=0.5; 2=0.84; 3=0.82; 4=0.8 |
| Krittayaphon et al. [10] | COOL-AF | 3,461 | HF risk factors | Cox Hazard proportional model | Calculating risk factors and incidence rate using Cox-proportional model | C-index, D-statistic, calibration plot, brier test, and survival analysis | C-index=0.756; D-statistic=1.503; R-square of the calibration plot=0.933; brier test=0.056 |
| Austin et al. [11] | EFFECT Study | 9,943 | HFpEF prediction | Random Forest, Bagged decision tree, Boosted decision tree, SVM, Logistic regression | Comparison of predictive ability of different regression and classification methods | c-statistic, brier score, sensitivity, and specificity | classification tree=0.683, 0.2152, 0.462, 0.820; bagged tree=0.733, 0.2079, 0.451, 0.849; random forest=0.751, 0.1959, 0.378, 0.897; boosted tree (depth 1)=0.752, 0.2049, 0.453, 0.876; boosted tree (depth 2)=0.768, 0.1962, 0.491, 0.847; boosted tree (depth 3)=0.772, 0.1933, 0.492, 0.828; boosted tree (depth 4)=0.780, 0.1861, 0.500, 0.820; SVM=0.766, 0.1914, 0.401, 0.887 |
| Blecker et al. [12] | Tisch Hospital | 37,229 | ADHF identification | Logistic regression, L1-regularization model | Comparing various approaches in identifying patients with ADHF | AUC, sensitivity, PPV | algorithm 1=n/a, 0.98, 0.14; algorithm 2=0.96, 0.98, 0.15; algorithm 3=0.99, 0.98, 0.30; algorithm 4=0.99, 0.98, 0.34 |
| Plati et al. [13] | UCD and Ioannina Hospital | 487 | HF classification | Decision tree, random forest, rotation forest, naïve bayes, kNN, SVM, logistic model tree, and bayes network | Comparing five models with various sets of features including the implementation of pre-processing like balancing | Accuracy, sensitivity, specificity | Clinical features+LMT(%)=84.12, 82.10, 85.38; Clinidal features and BNP+LMT(%)=88.15, 85.80, 89.62; Clinical and ECG features+ROT(%)=90.76, 93.21, 89.23; ECG features+ROT(%)=87.91, 90.74, 86.15; All features+ROT(%)=91.23, 93.83, 89.62 |
| Wang et al. [14] | Medical University Hospital in Shanxi Province | 5,004 | HF prediction | LR, kNN, SVM, RF, XGBoost | SHAP for model interpretation | AUC | LR=0.7819; kNN=0.6481; SVM=0.6963; RF=0.7983; XGBoost=0.8010 |
| Quesada et al. [15] | ESCARVAL RISK | 32,527 | Cardiovascular risk | 15 machine learning methods | Risk scale comparison | AUC, accuracy, error rate, sensitivity, specificity, PPV, NPV, LR+, and NNT | Top-3 best performed ML algorithm: QDA=0.7086, 0.570, 0.430, 0.736, 0.559, 0.0.093, 0.972, 1.669, 10.7; NB=0.7084, 0.591, 0.409, 0.718, 0.583, 0.096, 0.971, 1.722, 10.4; NN=0.7042, 0.559, 0.441, 0.743, 0.548, 0.092, 0.972, 1.644, 10.9 |
| Kolukula et al. [16] | UCI Database | 918 | HF diagnosis | Logistic regression, SVM, random forest | Applying exploratory data analysis before model building | Accuracy, precision, recall, F1 score | LR=0.85, 0.82, 0.84, 0.845; SVM=0.84, 0.83, 0.83, 0.840; RF=0.986, 0.978, 0.981, 0.984 |
| Sornsuwit et al. [17] | UCI Database | 299 | HF diagnosis | kNN, naïve bayes, decision tree, adaboost, bagging, logistic regression, LEBoosting | Developing a new classifier by modifying adaboost m1 classifier using naïve bayes, kNN, and decision tree | Accuracy | kNN=62.22; NB=68.89; decision tree=67.78; Adaboost=95.69; bagging=67.78; logistic regression=68.89; LPBoost=70; LEBoosting=98.89 (%) |
| Ahmed et al. [18] | UCI Database | 500 | HF diagnosis | Logistic regression, kNN, gaussian naïve bayes, multinomial naïve bayes, SVM | Combining public dataset with local dataset | Accuracy | Logistic regression (82.76%), SVM (67.24%), KNN (60.34%), GNB (79.51%), and MNB (79.51%). |
| Praveena et al. [19] | UCI Database | 918 | HF diagnosis | kNN, SVM, and random forest | Removing missing values | Sensitivity, precision, specificity, accuracy | kNN=0.7963, 0.7818, 0.7073, 0.75789; SVM=0.8, 0.8, 0.725, 0.76842; RF=0.8654, 0.8182, 0.7674, 0.82105 |
| Alotaibi [20] | UCI Database | 918 | HF diagnosis | Decision tree, logistic regression, random forest, naïve bayes, SVM | Imputation implementation to public dataset | Accuracy | DT=93.19; LR=87.36; RF=89.14; NB=87.27; SVM=92.30 (%) |
| Mamun et al. [21] | UCI Database | 299 | HF diagnosis | LightGBM, XGBoost, Logistic regression, bagging, SVM, decision tree | SMOTE implementation of imbalance public dataset | Accuracy, AUC | LR(%)=80, 89.44; SVM(%)=73.22, 86.30; XGBoost(%)=84.00, 92.00; LightGBM(%)=85.00, 93.00; DT(%)=73.21, 73.20; bagging(%)=82.00, 89.05 |
| Nishat et al. [22] | UCI Database | 299 | HF diagnosis | Decision tree, logistic regression, naïve bayes, random forest, kNN, SVM | SMOTE with normalization of imbalance public dataset | Accuracy, precision, F1 score, recall, AUC | DT=0.733, 0.756, 0.795, 0.838, 0.720; LR=0.800; 0.878, 0.857, 0.837, 0.755; GNB=0.683, 1.000, 0.812, 0.683, 0.500; RF=0.800, 0.854, 0.854, 0.854, 0.769; kNN=0.667, 0.902, 0.787, 0.698, 0.530; SVM=0.683, 1.000, 0.812, 0.683, 0.500 |
| Senan et al. [23] | UCI Database | 299 | HF diagnosis | SVM, kNN, decision tree, random forest, logistic regression | SMOTE implementation of imbalance public dataset | Accuracy, precision, recall, F1 score | SVM(%)=90.00, 93.02, 93.02, 93.02; kNN(%)=93.33, 93.33, 97.67, 95.45; DT(%)=95.00, 93.48, 100, 96.63; RF(%)=95.00, 97.62, 05.35, 96.47; LR(%)=88.33, 93.00, 90.90, 91.93 |
| Al-Yarimi et al. [24] | UCI Database | 918 | HF diagnosis | Decision tree, kNN, SVM | KS-Test for feature selection | Precision, accuracy, sensitivity, specificity, F-measure, Matthews correlation coefficient | FODW=0.89912, 0.911102, 0.954829, 0.872451, 0.88852, 0.823278; HRFLM=0.86574, 0.888546, 0.931869, 0.815411, 0.84294, 077306; HIFS=0.84712, 0.86347, 0.91806, 0.80536, 0.82544, 0.736158 |
| Bharti et al. [25] | UCI Database | 918 | HF diagnosis | Logistic regression, kNN, SVM, random forest, decision tree, deep learning | Lasso for feature selection | Accuracy, specificity, sensitivity | LR(%)=83.3, 82.3, 86.3; kNN(%)=84.8, 77.7, 85.0; SVM(%)=83.2, 78.7, 78.2; RF(%)=80.3, 78.7, 78.2; DT(%)=82.3, 78.9, 78.5; Deep Learning(%)=94.2, 83.1, 82.3 |
| Kanagarathinam et al. [26] | UCI Database | 918 | HF diagnosis | Naïve bayes, XGBoost, kNN, SVM, MLP, CatBoost | Pearson's correlation for feature selection | Accuracy, AUC | Accuracy only (%) Naïve Bayes=85.98; XGBoost=86.91; kNN=85.98; SVM=85.98; MLP=85.98; CatBoost=87.85 |
| Venkatesh et al. [27] | UCI Database | 918 | HF diagnosis | Naïve bayes | Big data environment | Precision, recall, F1-score | NB=0.80, 0.82, 0.81 |
| Alsubai et al. [28] | UCI Database | 918 | HF diagnosis | Decision tree, SVM, random forest | Quantum computing | Accuracy, precision, recall, F1 score, AUC | DT=0.62, 0.65, 0.63, 0.61, 0.633; SVM=0.70, 0.71, 0.70, 0.70, 0.699; RF=0.79, 0.79, 0.79, 0.79, 0.787; QDL=0.98, 0.98, 0.98, 0.98, 0.98 |
| Botros et al. [29] | MIT-BIH and BIDMC | 18 | HF diagnosis | CNN, CNN-SVM | ECG signal analysis | Accuracy, sensitivity, specificity | CNN(%)=99.31, 99.50, 99.11; CNN-SVM(%)=99.17, 99.74, 98.61 |
| Alsinglawi et al. [30] | MIMIC-III | 1,592 | HF patient's LOS prediction | Random forest, gradient boosting, stacking regression, DNN | Predicting length of stay using EHR-based dataset | R-squared, mean average error | RF=0.8, 1.98; GB=0.81, 2.0; stacking regression=0.81, 1.92; DNN=0.77, 2.30 |
| Readmission Prediction | |||||||
| Shameer et al. [31] | Mouth Sinai Hospital | 1,068 | Hospital readmission | Naïve bayes | Applying five different data modalities based on feature's type | Accuracy and AUC score | NB=83.9% and 0.780 |
| Bat-Erdene et al. [32] | KAMIR-NIH registry | 13,104 | Rehospitalization prediction | Logistic regression, SVM, gradient boosting, AdaBoost, random forest, proposed DNN | Modifying deep neural network and comparing with other ML-based models | Accuracy, AUC, precision, recall, specificity, F1-score | LR(%)=94.37, 95.82, 88.51, 75.82, 97.30, 78.51; SVM(%)=98.53, 99.60, 99.32, 89.88, 99.90, 94.27; gradient boosting(%)=97.58, 98.73, 94.94, 86.91, 99.27, 90.70; AdaBoost(%)=95.89, 97.73, 86.01, 83.21, 97.88, 84.56; RF(%)=97.98, 98.75, 99.41, 85.67, 99.91, 91.98; DNN(%)=99.37, 99.90, 96.86, 98.61, 99.49, 97.73 |
| Rizinde et al. [33] | Seven hospitals in Rwanda | 4,083 | Risk of hospitalization prediction | Random forest, SVM, kNN, MLP, logistic regression, decision tree | Applying various pre-processing techniques including balancing and comparing with various models | Accuracy, precision, recall, F1 score, AUC | RF(%)=87, 84, 89, 87, 94; SVM_RBF(%)=79, 77, 82, 79, 88; SVM_Linear(%)=74, 73, 76, 74, 88; kNN(%)=85, 80, 91, 85, 88; MLP(%)=82, 79, 86, 82, 88; LR(%)=75, 74, 75, 74, 81; DT(%)=52, 50, 96, 66, 57 |
| Landicho et al. [34] | NMMC | 322 | Readmission of HF patients | Logistic regression, SVM, random forest, neural network | Applying cost-senstive classification approach to the model | Accuracy, sensitivity, specificity, precision, F-measure, AUC | LR=0.585, 0.250, 0.904, 0.714, 0.370, 0.577; RF=0.561, 0.150, 0.952, 0.750, 0.250, 0.551; SVM=0.610, 0.300, 0.905, 0.750, 0.428, 0.602; NN=0.512, 0.000, 1.000, -, -, 0.500 |
| Sohrabi et al. [35] | Iran Medical Centers | 230 | Re-hospitalization of HF patients | Decision tree, artificial neural networks, SVM, logistic regression | Data mining approach for data analytics | Accuracy, AUC | Re-hospitalization 1 month DT=88.1%, 0.76; ANN=86.5%, 0.68; SVM=86.4%, 0.62; LR=80.1%, 0.61 |
| AbdelRahman et al. [36] | University of Utah Health Care | 2,787 | Readmission of CHF patients | Voting feature intervals classifier, logistic regression, combination VFI and logistic regression | Utilizing three-step approaches | AUC, accuracy, sensitivitiy, specificity, PPV, NPV | Voting classifier(%)=86.8, 91.5, 62.5, 94.2, 50, 96.4 |
| Vedomske et al. [37] | University of Virginia CDR | 1 million+ | 30-day readmission of CHF patients | Random forest | Processing administrative data | AUC | RF-based models with prior weighting on the response based on divided variables: Base=0.67; Procedure=0.68; Diagnosis=0.77; Both=0.8 |
| Hilbert et al. [38] | California State Inpatient Database of the Healthcare Cost and Utilization Project | 78,091 | Readmission of HF patients | Decision tree | Transparant analysis of decision tree models | AUC | Training=0.594; Testing=0.583 |
| Zolbanin and Delen [39] | CHSI at OSU | 32,350 | Readmission of HF patients | Naïve bayes, neural network, gradient boosting | Conducting database-wide data processing in model building | AUC | Naïve Bayes=0.733; neural network=0.728; gradient boosting= 0.722 |
| Golas et al. [40] | Partners Healthcare System | 28,031 | 30-day hospital readmission of HF patients | Modifiend deep learning, logistic regression, gradient boosting, neural network | Designing deep unified networks (DUNs) to avoid overfitting in predictive model | AUC, accuracy, precision, recall, f1 | LR=0.664, 0.626, 0.336, 0.616, 0.435; gradient boosting=0.650, 0.612, 0.325, 0.615, 0.425; maxout networks=0.695, 0.645, 0.354, 0.631, 0.454; DUN=0.705, 0.646, 0.360, 0.652, 0.464 |
| Mortazavi et al. [41] | Tele-HF | 1,653 | 30-day hospital readmission of HF patients | Random forest, boosting, SVM, logisitc regression | Involving telemedicine-based data | Discrimination c-statistic | 180 days heart failure readmission: LR=0.566; boosting=0.678; RF=0.669; RF into SVM=0.657 |
| Lorenzoni et al. [42] | GISC | 380 | Hospitalization of HF patients | GLMN, logistic regression, CART, random forest, adaboost, logitboost, SVM, neural network | Involving three different approaches (complete case, kNN imputed, median imputed) in handling missingness | Sensitivity, PPV, NPV, specificity, accuracy, AUC | Only best performed algorithm, GLMN (%): Complete Case: 77.8, 87.5, 75, 85.7, 81.2, 80.6; Mean-imputation: 26.5, 66.0, 59.5, 68.1, 60.3, 62.8; kNN-imputation: 24.1, 64.8, 59.4, 89.5, 60.3, 62.4 |
| Sundararaman et al. [43] | MIMIC-III | 11,318 | Readmission of HF patients | Logistic regression | Employing four model approaches involving structured and unstructured data | Accuracy and AUC score | structured data=0.91, 0.68; unstructured data=0.92, 0.63; feature selection=0.98, 0.96; structured + unstructured data=0.92, 0.64; structured + feature selection=0.98, 0.97 |
| Liu et al. [44] | MIMIC-III | 58,000+ | Readmission of HF patients | NLP CNN | Employing only clinical notes | Accuracy | General readmission: CNN=0.759, 0. 754, 0.756, 75.70%; RF=0.720, 0.633, 0.674, 69.35% |
| Sharma et al. [45] | Alberta Health Services | 10,641 | Readmission of HF patients | 12 ML models | Comparing 12 ML-based with LaCE score | AUC | Validation set: XGBoost=0.654; GBM=0.650; AdaBoost=0.646; CatBoost=0.642; LightGBM=0.641; Linear SVC=0.639; NB=0.624; RF=0.617; DT=0.597; LR=0.596; NN=0.578; LSTM=0.624; LaCE=0.570 |
| Shams et al. [46] | Veteran Health Administration | 7,200 | Readmission of HF patients | PAR | Proposing PAR approach and comparing with CMS and PPR | AUC-ROC | PAR=0.836 |
| Ben-Assuli et al. [47] | Sheba Medical Center | 10,763 | 30-day readmission of HF patients | XGBoost | Involving expert cardiologists for feature selection | AUC | Machine superlist=0.8156; Expert list=0.7116; Human-Machine collaboration=0.8289 |
| Mortality Prediction | |||||||
| Jing et al. [48] | Geisinger EHR | 270,000 | Mortality | Logistic regression, random forest, XGBoost | Performing split-by-year training scheme and simulating care gap closure to the predictive models | AUC | Logistic regression=0.74; Random forest= 0.76, XGBoost=0.77 |
| Kamio et al. [49] | Tokushukai Medical Database | 1,416 | In-hospital mortality for ICU-admitted patients with AHF | SVM, XGBoost, neural network | Proposing three types of data: static, time-seris, and a combination | F1-score, precision, recall, ROC AUC, PR AUC | Time-series & static data: LSVC=0.49, 0.38, 0.69, 0.74, 0.47; XGB=0.49, 0.42, 0.64, 0.73, 0.48; Multi-neural network=0.37, 0.41, 0.52, 0.65, 0.37 |
| Adler et al. [50] | UCSD | 5,822 | Mortality risk | Boosted decision tree | Performing MARKER-HF score | AUC | Results for each cohort study: UCSD (all variables)=0.88; UCSD (RDW imputed)=0.87; UCSF=0.81; BIOSTAT-CHF=0.84 |
| Lagu et al. [51] | HealthFacts data of Cerner Corporation | 13,163 | Mortality of patients with ADHF | Logistic regression | Comparing seven mortality prediction models | AUC | Results for each model: Premier + =0.81; LAPS2=0.80; Premier=0.76; EFFECT=0.70; GWTG-HF-Eapen=0.70; GWTG-HF-Peterson=0.69; ADHERE=0.68 |
| Panahiazar et al. [52] | Mayo Clinic | 119,749 | Mortality | Decision tree, random forest, Adaboost, SVM, logistic regression | Comparing SHFM with ML-based models | AUC | Results of 1-year mortality prediction: SHFM-based: DT=0.60; RF=0.62; AdaBoost=0.59; SVM=0.56; LR=0.68 Proposed model: DT=0.66; RF=0.80; AdaBoost=0.74; SVM=0.46; LR=0.81 |
| Almazroi et al. [53] | UCI Heart Failure | 299 | Mortality | Decision tree, SVM, ANN, LR | Comparing three ML-based model to predict death event of HF patients | Accuracy, precision, recall, F1-score | LR(%)=78.34, 91.67, 47.82, 62.8; DT(%)=80, 78.94, 65.21, 71.4; SVM(%)=66.67, 80, 17.39, 28.57; ANN(%)=60, 40, 8.69, 14.28 |
| Karakuş et al. [54] | UCI Heart Failure | 299 | Mortality | Logistic regression, naïve bayes, SVM, kNN, decision tree, random forest, neural network | Utilizing PCA to determine the effectiveness in prediction performance | Accuracy, AUC | Accuracy was achieved between 69 and 100% AUC: Gaussian SVM (1.00) and Coarse kNN (0.87) |
| Zaman et al. [55] | UCI Heart Failure | 299 | Mortality | Decision tree, random forest, XGBoost | Implementing SMOTE for balancing and two learners: base and meta | Accuracy, precision, recall, F1-score, AUC | DT(%)=80.49, 16.19, 84.21, 80.00; RF(%)=92.68, 88.09, 97.36, 92.50; XGBoost(%)=91.46, 87.80, 94.73, 91.13 |
| Newaz et al. [56] | UCI Heart Failure | 299 | Mortality | SVM, kNN, logistic regression, adaboost, random forest | Presenting a robust BRF for imbalance problems | Senstivity, specificity, G-mean, accuracy, MCC, AUC | BRF + RFE(%)=78.21, 70.51, 74.26, 72.93, 46.33, 74.36; BRF + Chi2(%)=80.21, 74.45, 76.83, 76.25, 52.53, 77.33 |
| Kedia et al. [57] | UCI Heart Failure | 299 | Mortality | Logistic regression, naïve bayes, decision tree, random forest, SVM | Performing feature selection RFE with SMOTE for balancing | Accuracy, precision, recall, F1-score | LR=85.56%, 0.82, 0.81, 0.81; GNB=85.56%, 0.83, 0.78, 0.80; DT=80.0%, 0.75, 0.77, 0.76; RF=88.89%, 0.85, 0.84, 0.84; Linear SVM=87.78%, 0.85, 0.84, 0.84; Stack model=90.00%, 0.88, 0.87, 0.87 |
| Chicco et al. [58] | UCI Heart Failure | 299 | Mortality | Random forest, decision tree, gradient boosting, linear regression, ANN, naïve bayes, SVM, kNN | Combining ML- and biostatistical-based feature selection | Accuracy, F1-score, MCC, PR-AUC, ROC-AUC | Results of model after feature selection: RF=0.585, 0.754, +0.418, 0.541, 0.698; gradient boosting=0.585, 0.750, +0.414, 0.673, 0.792; SVM radial=0.543, 0.720, +0.348, 0.494, 0.667 |
| Li et al. [59] | MIMIC-III | 1,177 | Mortality of ICU-admitted HF patients | XGBoost, LASSO, logistic regression | Utilizing ML-based model for screning independent risk factors for in-hospital mortality | AUC and calibration c-statistic test | AUC score of each model: XGBoost=0.8416; LASSO=0.8562; GWTG-HF=0.7747 |
| Luo et al. [60] | MIMIC-III | 5,676 | Mortality of ICU-admitted HF patients | XGBoost, logistic regression | Utilizing imputation and ML-based feature selection | AUC, calibration plot, decision curve analysis | AUC score of each model: XGBoost=0.831; ElasticNet=0.817; SAPS-II=0.719; GWTG-HF=0.662 |
| Chen et al. [61] | MIMIC-IV and eICU | 20,878 and 15,483 | Mortality of ICU-admitted HF patients | Xgboost, logistic regression | Utilizing an updated of MIMIC dataset and testing the proposed model with eICU dataset | AUC | XGBoost=0.771; Logistic=0.725; GWTG-HF=0.649 |
| Readmission and Mortality Prediction | |||||||
| Awan et al. [62] | Hospital Morbidity Data Collection and Mortality Database | 248,387 | Readmission and Mortality | logistic regression, random forest, decision tree, SVM, MLP | Comparing proposed models with LaCE score | AUC, PR-AUC, accuracy, sensitivity, specificity | LR=0.576, 0.455, 62.26%, 48.37%, 66.85%; Weighted-RF=0.548, 0.386, 76.22%, 21.71%, 88.07%; Weighted-DT=0.528, 0.379, 64.18%, 31.44%, 74.16%; Weighted-SVM=0.535, 0.377, 65.36%, 31.39%, 75.78%; MLP=0.628, 0.461, 64.93%, 48.42%, 70.01% |
| Awan et al. [63] | Hospital Morbidity Data Collection and Mortality Database | 248,387 | Readmission and Mortality | MLP | Leveraging feature selection and dimension reduction | sensitivitiy, specificity, AUC | Based on feature selection methods: forward selection=32.2%, 85.3%, 0.56; backward selection=44.2%, 66.6%, 0.57; mRMR=58.7% 60.6%, 0.62 |
| Sarijaloo et al. [64] | EPIC EHR and the McKesson Change ECG Reporting System | 3,189 | 90-day AHF readmission and mortality | SVM, random forest, gradient boosting, LASSO, logistic regression | Comparing ML-based models with traditional statistical analysis for risk assessment | AUC and 95% CI | LR=0.744, (0.732-0.755); ML-LASSO=0.748, (0.745-0.751); ML-GBM=0.750, (0.743-0.756); ML-RF=0.570, (0.545-0.594); ML-SVM=0.718, (0.703-0.733); Combined ML-LASSO+LR=0.760, (0.752-0.767) |
| Tian et al. [65] | Shanxi Province | 1,011 | Death, readmission, and MACEs | Logistic regression, random forest, XGBoost, LightGBM, naïve bayes, MLP | Developing chronic heart failure measure (CHF-PROM) | AUC | Final models for HF-readmission: XGBoost=0.718; LightGBM=0.704; RF=0.707; Logistic=0.693; NB=0.673; MLP=0.690 Final models for all-cause death: XGBoost=0.754; LightGBM=0.733; RF=0.709; Logistic=0.742; NB=0.658; MLP=0.746 |
| Lv et al. [66] | Hospital of Dalian Medical University | 13,602 | 1-year in hospital mortality and 1 year readmission | Logistic regression, SVM, ANN, random forest, XGBoost | Presenting calibration plots of each predictive models for comparison | calibration plot, AUC | All-cause mortality: LR=0.91; RF=1.00; SVM=0.99; ANN=0.99; XGBoost=0.99 All-cause readmission: LR=0.63; RF=0.91; SVM=0.96; ANN=0.82; XGBoost=0.92 |
| Eapen et al. [67] | GWTH-HF | 33,349 | Readmission and Mortality | CMS tool | Demonstrating the use of CMS tool | C-statistics | Validation cohort: 30-day mortality=0.75; 30-day hospitalization=0.59; 30-day mortality or rehospitalization=0.62 |
| Zhao et al. [68] | TOPCAT trial dataset | 3,445 | Readmission and Mortality of HFmrHF patients | Random forest, logistic regression, LASSO, RIDGE, gradient boosting, SVM | Presenting DeLong test to assess discrimination and its improvement | AUC, c-index | Death 1-year: RF=0.68, 0.67; LASSO=0.75, 0.77; Logistic=0.46, 0.53; Ridge=0.52, 0.51; GBT=0.77, 0.76; SVM=0.62, 0.64 HF-hospitalization 1-year: RF=0.84, 0.85; LASSO=0.47, 0.49; Logistic=0.58, 0.43; Ridge=0.39, 0.63; GBT=0.81, 0.81; SVM=0.40, 0.70 |
| Beecy et al. [69] | CLEVER-HEART | 3,774 | 30-day unplanned readmission and mortality | XGBoost | Comparing proposed models with HOSPITAL score | AUC | 30-day outcomes: Index Admission=0.723; Index Discharge=0.754; Feature Aggregated=0.756; HOSPITAL score=0.666; Index Admission BNP=0.5046 |
Predictive Analytics for Heart Failure Prediction
The selected studies highlighted the important role of machine learning in predicting HF from electronic medical records. This approach may greatly help the clinical decision-making process and diagnose patients with heart failure. Guidi et al. designed a clinical decision support system (CDSS) by implementing machine learning in decision-making to evaluate the severity of HF among patients with HF [5]. The study underscored the readability and accessibility of machine learning-based CDSS to non-cardiologist users.
Involving more than 400,000 primary care patients, two studies built machine learning-based predictive analytics models for the early diagnosis of heart failure in primary care patients collected by the Geisinger Clinic [6,7]. By using unstructured and structured electronic health record (EHR) data, these studies were able to predict heart failure in different time windows and showed good performance. Similarly, Ramal and Emam utilized the data of 100 patients from King Saud University Medical City (KSUMC) in a big data environment [8]. Involving principal component analysis (PCA)’s pre-processing and feature reduction techniques, the study obtained a promising result in predicting heart failure
Aside from machine learning in building its predictive models, Nagrecha et al. involved more than one million elderly patients from Medicare USA and built predictive models using a trajectory-based disease progression model to predict heart failure among unseen patients [9]. Another study used the Cox Hazard proportional model to predict heart failure risk factors by patients collected by COOL-AF Thailand [10]. The proposed study performed well in predicting heart failure by calculating the model’s C-index, D-statistics, calibration plot, brier test, and survival analysis.
Austin et al. described the use of a machine learning-based predictive analytics approach to predict Heart Failure with preserved Ejection Fraction (HFpEF), a subtype of heart failure [11]. The study assessed the proposed models using c-statistic, brier score, sensitivity, and specificity and accurately predicted the presence of HFpEF in heart failure patients. Similarly, Blecker et al. used machine learning-based predictive analytics models to identify acute decompensated heart failure (ADHF) in patients collected by Tisch Hospital, USA [12]. The proposed study found that a machine learning-based predictive model best predicted ADHF.
Plati et al. involved 487 patients provided by the University College of Dublin (UCD) and the Department of Cardiology of the Hospital University Ioannina to build machine learning-based predictive analytics to classify the type of heart failure [13]. By performing pre-processing techniques and class balancing to obtain an ideal dataset, the study generated promising results for classifying heart failure by dividing the main dataset into a sub-dataset for each type.
Furthermore, Wang et al. underscored the interpretability of predictive analytics models [14]. The study described the use of model interpretation and feature importance explanation using the Shapley additive explanation (SHAP) approach to give physicians an understanding of the models. The study used the SHAP approach for the best-performed model to interpret the model and its feature importance. Additionally, comparing machine learning-based with commonly used risk scales showed the effectiveness of their predictive analytics approach [15]. The study showed that machine learning-based predictive analytics outperformed other risk scales like SCORE and REGICOR.
While various previously discussed studies rely on hospital data, there are selected studies that build predictive models that leverage open, public data from repositories such as Physionet and University of California Irvine (UCI). Three selected studies used heart disease datasets from the UCI machine learning database to build predictive models for diagnosing heart failure or heart disease [16-18]. By directly processing the dataset, the studies employed various machine learning algorithms. However, challenges arose due to missing values that existed in the dataset. Removing all the missing values was shown to be fundamental to obtaining a dataset without missing values [19]. However, this might result in biased prediction results. By implementing an imputation technique, Alotaibi showed better performance [20].
In other cases, an imbalance problem may have caused a reduction in prediction performance. Mamun et al. showed that the SMOTE technique successfully addresses the imbalance in the UCI heart disease dataset, and it improved the predictive performance [21]. However, the number of UCI heart disease patients differed slightly in each class, so this technique was unnecessary. Nishat et al. and Senan et al. utilized the SMOTE technique to solve the imbalance problem in the UCI heart failure dataset [22,23]. Furthermore, by implementing normalization techniques, Nishat et al. showed improved prediction performance compared to models without balancing and normalization, as Senan et al. did.
Selecting important features may lead to predictive performance improvement. Leveraging datasets from the UCI database, Al-Yarimi et al. adopted the KS-Test to select the optimal attributes for their dataset and build a decision tree-based predictive model [24]. However, according to Mathew’s correlation test to evaluate the predictive model, the model's performance was not significantly improved. Bharti et al. used Lasso for feature selection, resulting in better classification performance than was seen with the KS-Test approach [25]. Differing from those studies, Kanagarathinam et al. performed feature selection using Pearson’s correlation to obtain an ideal version of the UCI heart disease dataset [26]. This study shows improved predictive performance compared to previously mentioned studies. However, the result might be biased because the study discarded variables with missing values.
Unlike other studies, Venkatesh et al. used a big data approach and the UCI heart disease dataset to analyze and predict heart status [27]. The study improved the prediction effectiveness with high CPU utilization and low processing time by utilizing a clustering technique to filter unnecessary data. Alsubai et al. implemented quantum computing in machine learning and deep learning algorithms, resulting in better predictive performance than conventional machine learning algorithms [28].
Utilizing datasets from Physionet, Botros et al. used datasets from the MIT-BIH and BIDMC databases to predict heart failure by analysis of ECG signals [29]. By leveraging a deep learning algorithm, the study was able to build a predictive model with good performance in terms of accuracy, sensitivity, and specificity. Using the MIMIC-III dataset, Alsinglawi et al. were able to predict the length of stay for patients with heart failure using machine learning-based predictive analytics [30].
The selected studies underscore the benefit of implementing predictive analytics in heart failure prediction, offering valuable insights for clinicians and physicians.
Predictive Analytics for the Prediction of Readmission or Mortality
Patients with heart failure face a high risk of mortality and hospital readmission. As with the implementation of predictive analytics to predict heart failure and its risk, this approach can be applied to predicting the hospitalization, readmission, and mortality of patients with heart failure.
Readmission: With a dataset collected by Mount Sinai Hospital in 2014, Shameer et al. used the naïve Bayes model to build a predictive analytics model [31]. Reaching an AUC score of 0.78 with an accuracy of 83.19%, the study was able to predict hospital readmission among heart failure patients. Bat-Erdene et al. developed predictive analytics models involving 52 hospitals from the Korea Acute Myocardial Infarction-National Institute of Health (KAMIR-NIH) registry to predict rehospitalization of patients with acute myocardial infarction (AMI) [32]. This study used a deep learning algorithm to build the predictive model, which resulted in better performance than traditional machine learning models.
Rizinde et al. built machine learning-based predictive analytics models for predicting the risk of hospitalization of patients with heart failure from the medical records of seven hospitals in Rwanda [33]. Their findings showed that their approach was able to predict a high risk of hospitalization with good performance. In a study from the Philippines, Landicho et al. were able to predict the readmission of 322 patients with heart failure from NMMC in Cagayan de Oro City using machine learning-based predictive analytics [34]. Sohrabi et al. built machine learning-based predictive models to predict one- and three-month hospitalization of 230 patients with heart failure collected by medical centers in Iran [35]. The study used a data mining approach to build machine learning-based predictive models, and their models reached up to a 0.73 AUC score.
By utilizing a database from the University of Utah Health Care, AbdelRahman et al. built a machine learning-based predictive model to predict the readmission of patients with chronic heart failure (CHF) [36]. By dividing the dataset used into a three-step approach, this study produced a high-performance predictive analytics model that can be applied in various healthcare areas to improve the effectiveness of patient care. Involving the data of more than 1 million patients from the University of Virginia Clinical Database Repository (CDR), Vedomske et al. built a Random Forest-based predictive analytics model to predict unplanned, all-cause, 30-day readmission of patients with CHF [37]. By processing administrative data, the proposed model reached a 0.8 AUC in their model’s evaluation. Similarly, Hilbert et al. described the implementation of their predictive analytics by demonstrating how their decision tree algorithm produced a transparent analysis of their readmission prediction, resulting in a good AUC score [38].
Zolbanin and Delen proposed a novel data processing approach to extract data from medical records for improved readmission prediction in a sample of heart failure patients [39]. Utilizing data from the Center for Health Systems Innovation (CHSI) at Oklahoma State University (OSU), the proposed model was able to demonstrate competitive advantages in predicting readmission among heart failure patients. Utilizing data from Partners Healthcare System, Golas et al. implemented predictive analytics for the prediction of 30-day hospital readmission of patients with heart failure [40]. By processing structured and unstructured data, the study was able to showcase optimal performance in predicting 30-day readmission through the use of their modified deep learning algorithm.
Two long-term projects, the Tele-HF project and the Gestione Integrata dello Scompenso Cardiaco (GISC) study, built predictive analytics models for readmission [41,42]. The Tele-HF project, involving 1,653 patients, produced a 17.8% improvement in predicting 30-day all-cause readmission. The GISC study compared various machine learning algorithms to predict the hospitalization of patients with heart failure. The study demonstrated various levels of performance in predicting readmission.
Differing from other studies, Sundararaman et al. and Liu et al. utilized open, public data from the MIMIC-III dataset for the predictive analytics of their heart failure readmission prediction [43,44]. Sundararaman et al. employed structured and unstructured data to produce logistic regression-based predictive models. By dividing the dataset into five types based on their proposed iteration, they achieved high accuracy and AUC scores. Attempting a different approach, Liu et al. used only unstructured data (clinical notes) to build their NLP CNN-based predictive models, achieving an accuracy of over 70% for predicting readmission.
To validate predictive analytics models for predicting readmission, Sharma et al. developed predictive analytics models with 12 different machine learning algorithms and compared the performance of each model with the LaCE score, a common method used for predicting patient readmission [45]. They demonstrated that most machine learning-based predictive analytics models provided better performance in predicting readmission than did the LaCE score. Shams et al. compared their proposed approach, the Potentially Avoidable Readmission (PAR) approach, with other methods, such as 3M of the Centers for Medicare and Medicaid Services (CMS) and Potentially Preventable Readmission (PPR) [46]. The study showed efficacy in avoiding patient readmission within two weeks post-hospital discharge.
Involving expert cardiologists in the feature selection of the heart failure data collected from Sheba Medical Center, Ben-Assuli et al. implemented three approaches to building their predictive models categorized by the feature selection method: machine-based, human expert-based, and collaboration-based [47]. The study generated better performance in predicting the 30-day readmission of patients with heart failure with collaboration-based feature selection, providing a different perspective on developing predictive hospital readmission analytics.
Mortality: Improved prediction of the risk of death for individual patients with heart failure is invaluable to healthcare professionals and help reverse the trend toward high cost. By leveraging clinical data from Geisinger EHR, nearly 27,000 patients with heart failure and 276,819 episodes, Jing et al. constructed machine learning-based predictive models using a split-by-year training scheme from 2013 to 2018. They presented good accuracy in predicting all-cause mortality for this large cohort [48]. In a study done between 2013 and 2019 that involved data from 70 Japanese hospitals that contributed to the Tokushukai Medical Database, Kamio et al. generated machine learning-based predictive models that included data for in-hospital mortality by intensive care unit-admitted patients with acute heart failure [49]. By proposing three types of data: static, time-series, and a combination, the study produced varying prediction performances for different outcomes.
Utilizing the electronic medical records of 5,822 patients collected by the University of California San Diego (UCSD), Adler et al. generated a new mortality risk predictive analytics model they named MARKER-HF [50]. The proposed model was able to predict the mortality risk separately for high- and low-risk groups based on the MARKER-HF score. Similarly, Lagu et al. compared seven different mortality prediction models to predict the inpatient mortality of hospitalized patients with ADHF collected from the HealthFacts data of Cerner Corporation [51]. Although the study did not generate a new predictive model, it highlighted the performance of existing approaches from various datasets and showcased their effectiveness in predicting mortality. In contrast, Panahiazar et al. demonstrated that the Seattle Heart Failure Model (SHFM) could predict mortality using the Mayo Clinic dataset [52]. However, their study also generated an approach to building predictive models that resulted in better performance than the SHFM approach, with improved prediction accuracy.
The use of open, public data, such as that from the UCI repository and Physionet, benefits researchers in building mortality risk predictive analytics models. Without performing pre-processing, machine learning-based predictive analytics models were built that predicted the survival possibility of heart failure patients in the UCI heart failure dataset [53]. Utilizing the PCA method to determine the effectiveness of dimension reduction in prediction performance, Karakuş and Er used various machine learning algorithms with the UCI heart failure dataset and showed an improvement in classification accuracy [54]. However, because the dataset contains an imbalance class, the study did not perform a balancing method, which may have led to biased prediction results. Therefore, Zaman et al. addressed the imbalance class by employing the SMOTE technique. The proposed models performed better than previous studies. However, Newaz et al. claimed that the SMOTE technique might cause information loss and presented a robust Random Forest classifier, BRF, that could handle imbalance problems with fine prediction performance [56].
Two studies employed feature selection methods for the UCI heart failure dataset [57,58]. While Kedia and Bhushan experienced prediction performance reduction after employing recursive feature elimination, Chicco and Jurman showcased improvement by combining machine learning-based and biostatistical-based feature selection, leading to better performance metrics.
Leveraging the MIMIC-III dataset, Li et al. developed an XGBoost-based predictive analytics model for all-cause in-hospital mortality of ICU-admitted patients with heart failure [59]. With data from 1,177 patients, the study predicted mortality with good AUC-ROC and calibration c-statistic test performance. Similarly, Luo et al. predicted in-hospital mortality among ICU-admitted heart failure patients using the MIMIC-III dataset and the XGBoost algorithm [60]. The study incorporated imputation and feature selection techniques, resulting in a better predictive model with better in-hospital mortality prediction performance than Li et al. Using the updated MIMIC-IV dataset, Chen et al. constructed a predictive analytics model for in-hospital all-cause mortality of ICU-admitted patients with heart failure [61]. The study demonstrated excellent performance by building predictive models using 17 important features selected by LASSO regression.
Both Readmission and Mortality
Various studies have implemented predictive analytics to predict hospital readmission and mortality risk for heart failure patients. Awan et al. aimed to develop machine learning-based predictive analytics models for 30-day heart failure readmission or mortality by extracting datasets from the Hospital Morbidity Data Collection and Mortality Database of the Western Australian Data Linkage System [62]. The study, which was based on data from over 200,000 patients, showed good performance compared to the LaCE score. Awan et al. also proposed different types of predictive model-building by leveraging feature selection and dimension reduction techniques [63]. The study showed improved sensitivity and feature efficiency compared to their previous approach. Similarly, through the use of a core data system, Sarijaloo et al. extracted datasets from EPIC EHR and the McKesson Change ECG Reporting System to build machine learning-based models for the prediction of 90-day acute heart failure readmission or all-cause mortality [64]. By assessing their models with the AUC and 95% CI, the study generated predictive analytics to identify high-risk heart failure patients for either readmission or mortality.
Using a dataset from Shanxi Province, China, Tian et al. generated predictive analytics models to predict death, readmission, and Major Adverse Cardiovascular Events (MACEs) for heart failure patients [65]. The proposed models were able to calculate the risk of death, readmission, and MACEs hospital outpatients with heart failure. Similarly, using electronic health records (EHR) data from the First Affiliated Hospital of Dalian Medical University, China, Lv et al. developed predictive analytics models to predict one-year in-hospital mortality, the use of positive inotropic agents, and one-year all-cause readmission, resulting in good discrimination and predictive performances [66].
The GWTH-HF in-hospital program derived and validated risk-prediction tools from a large nationwide registry [67]. Utilizing the Center for Medicare and Medicaid Services (CMS) tool for predicting mortality and rehospitalization, the study claimed improved mortality and rehospitalization prediction, demonstrating fair discriminative capacity in predicting rehospitalization.
Unlike previously discussed studies, Zhao et al. processed the TOPCAT trial dataset to predict mortality and readmission risk among HFmrEF patients [68]. Their models showed promising prediction performance. Similarly, Beecy et al. used the dataset from the CLEVER-HEART study dataset to build machine learning-based predictive analytics models for predicting 30-day unplanned readmission and all-cause mortality [69]. The proposed models outperformed the HOSPITAL score, indicating superior predictive capabilities.
Discussion
Machine Learning Algorithms for Building Predictive Models
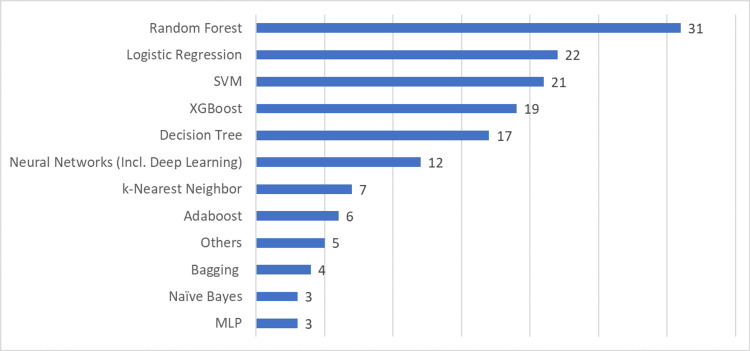
Machine learning algorithms are essential to building and developing predictive analytics models. The objective of implementing machine learning algorithms is to make the decision-making of predictive analytics more accurate in predicting specific diseases. The selected papers in this literature review present diverse machine-learning algorithms that can be used to predict heart failure, readmission, and patient mortality. As shown in Figure 2, the Random Forest algorithm appeared in 31 articles. It was useful for predicting heart failure, readmission, and mortality. Notably, two studies demonstrated the effectiveness of a Random Forest algorithm when applied to the EHR-based dataset [37,56]. However, an extensive examination of its performance across diverse healthcare datasets is required to measure its trustworthiness in clinical practice. Guidi et al. showed that although the Random Forest algorithm provided the best accuracy in prediction, it provided a less understandable model compared to other algorithms [5]. The implications of this for clinical acceptance need to be carefully considered.
Figure 2. Machine learning algorithms used in selected research articles.
SVM:Support Vector Machine; XGBoost:eXtreme Gradient Boosting; MLP:Multilayer Perceptron
Logistic regression, a traditional machine learning and statistics approach, also improves the robustness of predictive analytics models. Sundararaman et al. indicated a deeper analysis of its predictive potential and its appropriateness for complex clinical settings [43]. Although this algorithm provides simplicity and interpretability, compared to ensemble learning like XGBoost, logistic regression produces lower predictive performance [61].
SVM and Decision Tree (DT) have demonstrated effectiveness in heart failure prediction [34,53]. SVM is known to be an effective nonparametric classification tool, especially for high-dimensional data. However, its complexity results in a lack of interpretability and difficulty in evaluating feature importance [70]. Unlike SVM, DT provides understandable information for identifying risk factors [38]. Nonetheless, a more thorough examination of their results across diverse datasets and potential biases will improve the overall critical discourse. Furthermore, a more comprehensive evaluation is required to confirm their effectiveness in practical applications.
When compared to statistical approach-based predictive models, XGBoost yields superior predictive performance [48]. This algorithm can reduce the likelihood of overfitting when attempting to predict heart failure [60]. However, the intricacy of XGBoost can make interpretation difficult, requiring different approaches for experts looking for clear insights into the decision-making process [14]. Despite its potency, XGBoost has a complexity that provides challenges for real-world deployment and interpretability, which may limit its practical use.
Various selected studies have used neural networks and deep learning to build their predictive analytics. Deep learning algorithms would produce far better performance than conventional machine learning algorithms [32,64]. However, they require massive data, which risks overfitting to narrow datasets [44]. Furthermore, it can be challenging to interpret them due to their “black box” nature [40]. Therefore, their applicability may be limited in real-world clinical settings.
Five of the studies in this literature review used algorithms that were different from those previously mentioned. AbdelRahman et al. used a voting feature classifier model in building their predictive analytics [36]. Lagu et al. modified existing classifier models to predict heart failure mortality [51]. Nagrecha et al. introduced a novel perspective on predicting heart failure based on the unseen disease history of patients by using a directed acyclic graph-based model [9]. Krittayaphong et al. used the Cox Proportional Hazard model to predict heart failure [10]. Eapen et al. used the CMS approach to predict the rehospitalization and mortality of heart failure patients [67]. These five studies highlight the versatility of predictive analytics and demonstrate how various methodologies may predict heart failure risk. Various approaches for building mortality and readmission predictive analytics models, such as CMS, LACE, 3M PPR, and HOSPITAL, are worth considering and evaluating.
Data Pre-processing Implementation in Building Predictive Models
In predictive analytics, obtaining an ideal dataset is a process that allows the predictive models to achieve satisfactory performance in predicting heart failure risk. As a crucial part of building a predictive model, the pre-processing step leads to much better results predicting heart failure than models without pre-processing. Most selected studies implemented a pre-processing stage. Their results showed differences and improvements compared to studies without pre-processing. Table 2 shows the studies that used pre-processing techniques.
Table 2. Pre-processing techniques used in selected articles.
Data cleaning includes removing missing values and performing imputation techniques. Data transformation includes normalization, standardization, and feature selection.
Abbreviations:
PCA:Principal Component Analysis; SMOTE:Synthetic Minority Over-sampling Technique; ADASYN:Adaptive Synthetic
| Pre-processing Step | Article | Frequent method |
| Data Cleaning | [7,13,19,20,23,26,28,32-36,39-42,45-47,54,55,60,64-66,68,69] | Mean imputation, predictive mean matching, median imputation, random forest imputation, kNN imputation, XGBoost imputation, and missForest |
| Data Transformation | [13,14,22-26,30,31,33,34,36,39,41-43,47,54,55,58,60,62-65,69] | Recursive feature elimination, SelectKBest, Chi-Square, Pearson's correlation, KS-Test, T-Test |
| Data Reduction | [47,50,54] | PCA |
| Data Balancing | [13,14,21-23,32,33,39,43,55,62,65,66] | SMOTE, Under-sampling, Over-sampling, ADASYN |
Mean imputation frequently appears in various studies, while the machine learning-based imputation technique obtained better results than statistical-based imputation. Although removing missing values by employing imputation methods is commonplace, a more critical evaluation is necessary to determine their impact on predictive model performance [39].
Only a few of the selected studies used normalization and standardization. However, feature selection appears in many selected studies. Various studies used machine learning-based feature selection in post-processing to describe and present the most important features that influenced their prediction [41]. Although most studies did not compare feature selection methods, comparing them will allow the determination of optimal techniques for use in the pre-processing stage [63]. Furthermore, a comprehensive evaluation is essential to comprehend whether feature selection genuinely improves prediction performance or introduces potential biases. Feature selection risks optimizing models to the specifics of training data rather than generalizable patterns.
PCA was the most frequently used data reduction technique in the selected articles. Nevertheless, a more in-depth analysis is required to understand the implication of dimensionality reduction in preserving essential information. Data balancing was done in studies with imbalanced classes to obtain the ideal dataset. The most used data balancing technique was the SMOTE method. However, a more comprehensive evaluation is needed to determine its effectiveness across different datasets and potential consequences, such as information loss.
Dataset Specification Used in Building Predictive Models
Understanding the dataset specification is essential to building the ideal and optimal predictive analytics models for heart failure prediction with satisfactory performance. Demographic data, prominently age, and sex, frequently appear in selected studies and are used in their predictive analytics building. Ben-Assuli et al. reported that sex and age; expert-recommended demographical data; were the features most closely related to the risk of heart failure [47]. Two studies found age to be the leading feature for predicting 30-day readmission or death [62,63]. These studies give evidence of the importance of the use of demographical data. Through the use of the UCI database involving the feature selection technique, Senan et al. showed that sex and age impacted heart failure in patients [23]. While demographic data is undoubtedly crucial, there is a need for a more nuanced exploration of how each demographic variable contributes to prediction performance. Furthermore, considering factors like lifestyle or comorbidities, a deep analysis could improve prediction performance.
Similarly, the physical and clinical values were essential to building an effective predictive analytics model for heart failure prediction [41]. Physical and clinical values such as weight, diastolic and systolic blood pressure, and heart rate are patient data that clinicians routinely collect. Various studies indicate the importance of physical and clinical values in improving the prediction performance of heart failure predictive analytics models [7,13,41]. Although these values have been shown to be indicators of heart conditions, a more profound exploration is needed to understand the specific relationship between these values and prediction outcomes.
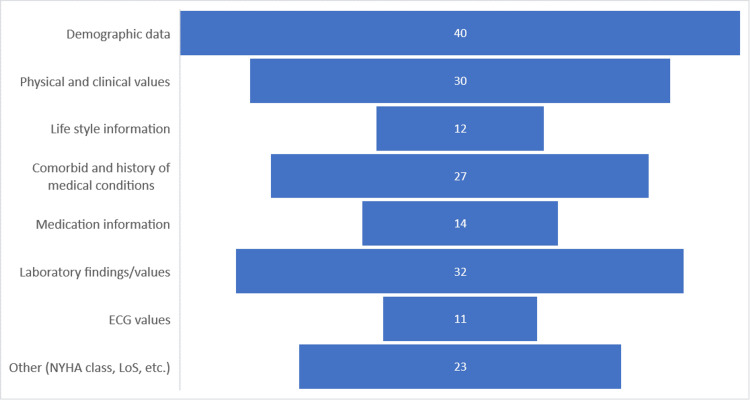
Lifestyle variables, such as smoking and alcohol consumption, introduce a layer of complexity that necessitates a holistic understanding of patient health [6,13]. However, these studies lack a comprehensive investigation of how lifestyle variables contribute to heart failure prediction. In various studies, comorbidities have been essential to building predictive models for the prediction of heart failure or readmission. Panahiazar et al. showed that including comorbidity data improved prediction performance compared to models without comorbidity data [52]. Nevertheless, a deeper exploration of the specific comorbid conditions and their varying impacts on heart failure prediction is warranted. Figure 3 summarizes the data categories described in the selected papers.
Figure 3. Data categories reported in the selected papers.
NYHA:New York Heart Association; LoS:Length of Stay
According to Figure 3, various studies used data like medication information and ECG values in building their models [29,64]. However, a few studies used unstructured data, such as doctor's notes. By processing patient data, such as patient descriptions, clinical notes, discharge summaries, diagnoses and procedures, and admission events, Sundararaman et al. were able to generate well-performance predictive models [43]. Processing unstructured data to build a heart failure predictive model is suitable for in-house applications like telemedicine, where the clinician collects patient data by phone [41]. Nevertheless, integrating between unstructured and structured variables could unlock richer insights into heart failure risk prediction [40].
Publication by Year
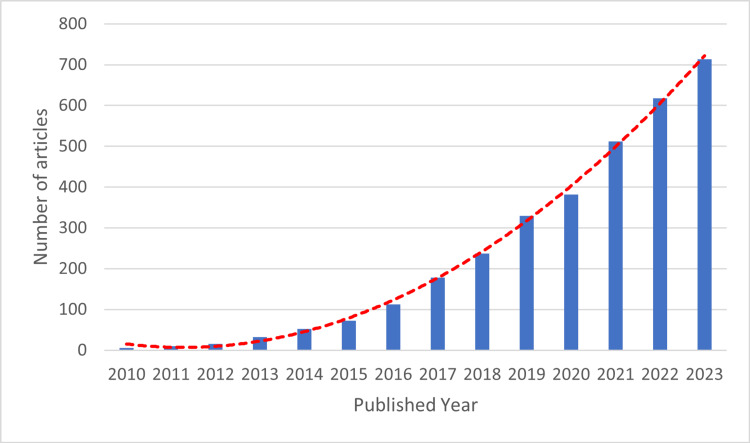
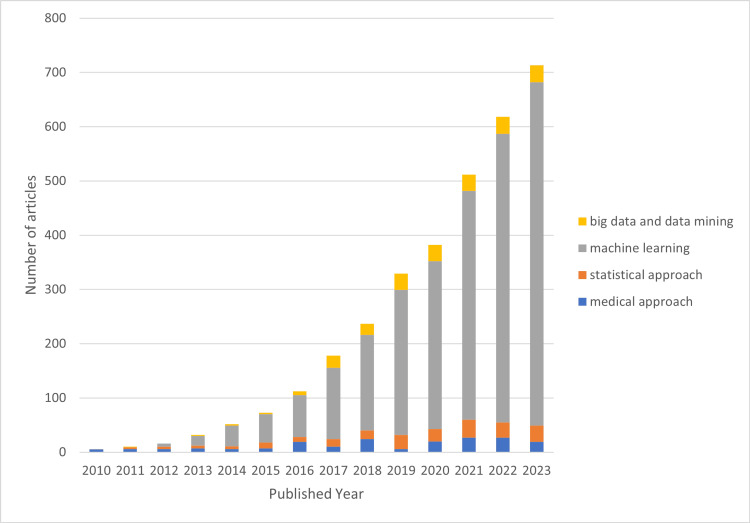
Although our search was initially set for publications between 2000 and 2023, our refined search mainly generated papers from 2010, as shown in Figure 4.
Figure 4. Publication by year based on refined-search results.
We broke down the publications into four well-known methodologies for building predictive models, as presented in Figure 5. As can be seen in the graph, researchers first utilized the machine learning approach in 2012. Its use has increased over time and peaked in 2023 when about 633 studies utilized machine learning to build a predictive model. This approach is currently widespread among researchers, especially for developing predictive analytics models.
Figure 5. Methodology used in the refined search papers.
The medical approach, commonly used by medical field experts like doctors and clinicians in presenting their predictive model by using medical or clinical approaches like physical examination and laboratory tests. The statistical approach or statistical analysis approach including Cox hazard proportional approach. The machine learning approach, the artificial intelligence-based approach. The big data and data mining approach, where researchers use various big data applications or software in building predictive models.
Limitation of the study
This review has various limitations, including a restricted search strategy and databases, a lack of dual independent screening and data extraction, and the inability to synthesize results quantitatively across various studies. In the study selection, it is important to acknowledge the subjectivity in the process, which may have biased the results. Although the selected studies utilized datasets from various countries, the transferability of predictive models across diverse populations and settings remains unclear. Future research should encompass quality appraisal, bias assessment, and meta-analysis to derive definitive conclusions about the role and effectiveness of predictive analytics in improving heart failure prognosis. Despite these limitations, this review delivers a foundation for progress toward higher-quality evidence synthesis in this field.
Conclusions
Predictive analytics and machine learning techniques have demonstrated promising potential for improving early diagnosis of heart failure, stratification of readmission risk, and prediction of mortality for heart failure patients. Various machine learning algorithms like random forest, logistic regression, neural networks, and XGBoost have been applied to structured data from electronic health records and unstructured clinical notes. Appropriate data preprocessing through imputation, feature selection, and the handling of class imbalance have emerged as crucial for developing high-performing predictive models. While the reviewed studies highlight the rising research interest and show initial success, the authors would like to emphasize the need for more rigorous evaluation, head-to-head benchmarking, and quality synthesis to derive more robust evidence that supports the clinical adoption of these predictive analytics approaches for improving heart failure outcomes.
Disclosures
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following:
Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work.
Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work.
Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Author Contributions
Acquisition, analysis, or interpretation of data: Qisthi A. Hidayaturrohman
Drafting of the manuscript: Qisthi A. Hidayaturrohman
Concept and design: Eisuke Hanada
Critical review of the manuscript for important intellectual content: Eisuke Hanada
Supervision: Eisuke Hanada
References
- 1.Survival of patients with chronic heart failure in the community: a systematic review and meta-analysis. Jones NR, Roalfe AK, Adoki I, Hobbs FD, Taylor CJ. Eur J Heart Fail. 2019;21:1306–1325. doi: 10.1002/ejhf.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heart failure: preventing disease and death worldwide. Ponikowski P, Anker SD, AlHabib KF, et al. ESC Heart Fail. 2014;1:4–25. doi: 10.1002/ehf2.12005. [DOI] [PubMed] [Google Scholar]
- 3.Rehospitalizations among patients in the Medicare fee-for-service program. Jencks SF, Williams MV, Coleman EA. N Engl J Med. 2009;360:1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 4.Disease prediction by machine learning over big data from healthcare communities. Chen M, Hao Y, Hwang K, Wang L, Wang L. IEEE Access. 2017;5:8869–8879. [Google Scholar]
- 5.A machine learning system to improve heart failure patient assistance. Guidi G, Pettenati MC, Melillo P, Iadanza E. IEEE J Biomed Health Inform. 2014;18:1750–1756. doi: 10.1109/JBHI.2014.2337752. [DOI] [PubMed] [Google Scholar]
- 6.Early detection of heart failure with varying prediction windows by structured and unstructured data in electronic health records. Wang Y, Ng K, Byrd RJ, et al. Annu Int Conf IEEE Eng Med Biol Soc. 2015;2015:2530–2533. doi: 10.1109/EMBC.2015.7318907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Early detection of heart failure using electronic health records: practical implications for time before diagnosis, data diversity, data quantity, and data density. Ng K, Steinhubl SR, deFilippi C, Dey S, Stewart WF. Circ Cardiovasc Qual Outcomes. 2016;9:649–658. doi: 10.1161/CIRCOUTCOMES.116.002797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heart failure prediction models using big data techniques. Rammal HF. IJACSA. 2018;9 [Google Scholar]
- 9.Nagrecha S, Thomas PB, Feldman K, Chawla NV. Machine Learning and Knowledge Extraction. New York: Springer International Publishing; 2017. Predicting chronic heart failure using diagnoses graphs. [Google Scholar]
- 10.Predicting heart failure in patients with atrial fibrillation: a report from the prospective COOL-AF Registry. Krittayaphong R, Chichareon P, Komoltri C, Sairat P, Lip GY. J Clin Med. 2023;12 doi: 10.3390/jcm12041265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Using methods from the data-mining and machine-learning literature for disease classification and prediction: a case study examining classification of heart failure subtypes. Austin PC, Tu JV, Ho JE, Levy D, Lee DS. J Clin Epidemiol. 2013;66:398–407. doi: 10.1016/j.jclinepi.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Early identification of patients with acute decompensated heart failure. Blecker S, Sontag D, Horwitz LI, Kuperman G, Park H, Reyentovich A, Katz SD. J Card Fail. 2018;24:357–362. doi: 10.1016/j.cardfail.2017.08.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.A machine learning approach for chronic heart failure diagnosis. Plati DK, Tripoliti EE, Bechlioulis A, et al. Diagnostics (Basel) 2021;11 doi: 10.3390/diagnostics11101863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Improving risk identification of adverse outcomes in chronic heart failure using SMOTE+ENN and machine learning. Wang K, Tian J, Zheng C, et al. Risk Manag Healthc Policy. 2021;14:2453–2463. doi: 10.2147/RMHP.S310295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Machine learning to predict cardiovascular risk. Quesada JA, Lopez-Pineda A, Gil-Guillén VF, Durazo-Arvizu R, Orozco-Beltrán D, López-Domenech A, Carratalá-Munuera C. Int J Clin Pract. 2019;73:0. doi: 10.1111/ijcp.13389. [DOI] [PubMed] [Google Scholar]
- 16.Predictive analytics of heart disease presence with feature importance based on machine learning algorithms. Kolukula NR, Pothineni PN, Chinta VM, Boppana VG, Kalapala RP, Duvvi S. IJEECS. 2023;32:1070. [Google Scholar]
- 17.A new efficiency improvement of ensemble learning for heart failure classification by least error boosting. Sornsuwit P, Jundahuadong P, Pongsakornrungsilp S. Emerg Sci J. 2022;7:135–146. [Google Scholar]
- 18.Prediction of cardiovascular disease on self-augmented datasets of heart patients using multiple machine learning models. Ahmed S, Shaikh S, Ikram F, Fayaz M, Alwageed HS, Khan F, Jaskani FH. J Sensors. 2022:1–21. [Google Scholar]
- 19.Predictive analytics for heart disease detection: a machine learning approach. Praveena Rachel Kamala S, Gayathri S, Pillai NM, Anto Gracious LA, Varun CM, Siva Subramanian R. 2023 4th International Conference on Electronics and Sustainable Communication Systems (ICESC) IEEE: Coimbatore, India. 2023;1583:9. [Google Scholar]
- 20.Implementation of machine learning model to predict heart failure disease. Alotaibi FS. IJACSA. 2019;10 [Google Scholar]
- 21.Mamun M, Farjana A, Mamun MA, Ahammed MS, Rahman MM. Seattle: IEEE; 2022. Heart Failure Survival Prediction Using Machine Learning Algorithm: Am I Safe From Heart Failure? [Google Scholar]
- 22.A comprehensive investigation of the performances of different machine learning classifiers with SMOTE-ENN oversampling technique and hyperparameter optimization for imbalanced heart failure dataset. Nishat MM, Faisal F, Ratul IJ, et al. Sci Program. 2022 [Google Scholar]
- 23.Score and correlation coefficient-based feature selection for predicting heart failure diagnosis by using machine learning algorithms. Senan EM, Abunadi I, Jadhav ME, Fati SM. Comput Math Methods Med. 2021;2021:8500314. doi: 10.1155/2021/8500314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feature optimization by discrete weights for heart disease prediction using supervised learning. Al-Yarimi FA, Munassar NM, Bamashmos MH, Ali MY. Soft Comput. 2021;25:1821–1831. [Google Scholar]
- 25.Prediction of heart disease using a combination of machine learning and deep learning. Bharti R, Khamparia A, Shabaz M, Dhiman G, Pande S, Singh P. Comput Intell Neurosci. 2021;2021:8387680. doi: 10.1155/2021/8387680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Machine learning-based risk prediction model for cardiovascular disease using a hybrid dataset. Kanagarathinam K, Sankaran D, Manikandan R. Data Knowledge Engineer. 2022;140:102042. [Google Scholar]
- 27.Development of big data predictive analytics model for disease prediction using machine learning technique. Venkatesh R, Balasubramanian C, Kaliappan M. J Med Syst. 2019;43:272. doi: 10.1007/s10916-019-1398-y. [DOI] [PubMed] [Google Scholar]
- 28.Heart failure detection using instance quantum circuit approach and traditional predictive analysis. Alsubai S, Alqahtani A, Binbusayyis A, Sha M, Gumaei A, Wang S. Mathematics. 2023;11:1467. [Google Scholar]
- 29.CNN and SVM-based models for the detection of heart failure using electrocardiogram signals. Botros J, Mourad-Chehade F, Laplanche D. Sensors (Basel) 2022;22 doi: 10.3390/s22239190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Predicting length of stay for cardiovascular hospitalizations in the intensive care unit: machine learning approach. Alsinglawi B, Alnajjar F, Mubin O, Novoa M, Alorjani M, Karajeh O, Darwish O. Annu Int Conf IEEE Eng Med Biol Soc. 2020;2020:5442–5445. doi: 10.1109/EMBC44109.2020.9175889. [DOI] [PubMed] [Google Scholar]
- 31.Predictive modeling of hospital readmission rates using electronic medical record-wide machine learning: a case-study using Mount Sinai Heart failure cohort. Shameer K, Johnson KW, Yahi A, et al. Pac Symp Biocomput. 2017;22:276–287. doi: 10.1142/9789813207813_0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deep learning-based prediction of heart failure rehospitalization during 6, 12, 24-month follow-ups in patients with acute myocardial infarction. Bat-Erdene BI, Zheng H, Son SH, Lee JY. Health Informatics J. 2022;28:14604582221101529. doi: 10.1177/14604582221101529. [DOI] [PubMed] [Google Scholar]
- 33.Comparing machine learning classifiers for predicting hospital readmission of heart failure patients in Rwanda. Rizinde T, Ngaruye I, Cahill ND. J Pers Med. 2023;13 doi: 10.3390/jpm13091393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Comparison of predictive models for hospital readmission of heart failure patients with cost-sensitive approach. Landicho JA, Esichaikul V, Sasil RM. Int J Healthc Manag. 2021;14:1536–1541. [Google Scholar]
- 35.Predicting the readmission of heart failure patients through data analytics. Sohrabi B, Vanani IR, Gooyavar A, Naderi N. J Info Know Mgmt. 2019;18:1950012. [Google Scholar]
- 36.A three-step approach for the derivation and validation of high-performing predictive models using an operational dataset: congestive heart failure readmission case study. AbdelRahman SE, Zhang M, Bray BE, Kawamoto K. BMC Med Inform Decis Mak. 2014;14:41. doi: 10.1186/1472-6947-14-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vedomske MA, Brown DE, Harrison JH. IEEE: Miami, FL. Miami: 12th International Conference on Machine Learning and Applications; 2013. Random Forests on Ubiquitous Data for Heart Failure 30-Day Readmissions Prediction. [Google Scholar]
- 38.Using decision trees to manage hospital readmission risk for acute myocardial infarction, heart failure, and pneumonia. Hilbert JP, Zasadil S, Keyser DJ, Peele PB. Appl Health Econ Health Policy. 2014;12:573–585. doi: 10.1007/s40258-014-0124-7. [DOI] [PubMed] [Google Scholar]
- 39.Processing electronic medical records to improve predictive analytics outcomes for hospital readmissions. Zolbanin HM, Delen D. Decision Support Systems. 2018;112:98–110. [Google Scholar]
- 40.A machine learning model to predict the risk of 30-day readmissions in patients with heart failure: a retrospective analysis of electronic medical records data. Golas SB, Shibahara T, Agboola S, et al. BMC Med Inform Decis Mak. 2018;18:44. doi: 10.1186/s12911-018-0620-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Analysis of machine learning techniques for heart failure readmissions. Mortazavi BJ, Downing NS, Bucholz EM, et al. Circ Cardiovasc Qual Outcomes. 2016;9:629–640. doi: 10.1161/CIRCOUTCOMES.116.003039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Comparison of machine learning techniques for prediction of hospitalization in heart failure patients. Lorenzoni G, Sabato SS, Lanera C, et al. J Clin Med. 2019;8 doi: 10.3390/jcm8091298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Novel approach to predict hospital readmissions using feature selection from unstructured data with class imbalance. Sundararaman A, Valady Ramanathan S, Thati R. Big Data Res. 2018;13:65–75. [Google Scholar]
- 44.Liu X, Chen Y, Bae J, Li H, Johnston J, Sanger T. San Diego: 2019 IEEE International Conference on Bioinformatics and Biomedicine (BIBM); 2019. Predicting Heart Failure Readmission from Clinical Notes Using Deep Learning. [Google Scholar]
- 45.Predicting 30-day readmissions in patients with heart failure using administrative data: a machine learning approach. Sharma V, Kulkarni V, McAlister F, et al. J Card Fail. 2022;28:710–722. doi: 10.1016/j.cardfail.2021.12.004. [DOI] [PubMed] [Google Scholar]
- 46.A predictive analytics approach to reducing 30-day avoidable readmissions among patients with heart failure, acute myocardial infarction, pneumonia, or COPD. Shams I, Ajorlou S, Yang K. Health Care Manag Sci. 2015;18:19–34. doi: 10.1007/s10729-014-9278-y. [DOI] [PubMed] [Google Scholar]
- 47.Human-machine collaboration for feature selection and integration to improve congestive heart failure risk prediction. Ben-Assuli O, Heart T, Klempfner R, Padman R. Decision Support Systems. 2023;172:113982. [Google Scholar]
- 48.A machine learning approach to management of heart failure populations. Jing L, Ulloa Cerna AE, Good CW, et al. JACC Heart Fail. 2020;8:578–587. doi: 10.1016/j.jchf.2020.01.012. [DOI] [PubMed] [Google Scholar]
- 49.Machine learning-based prognostic modeling of patients with acute heart failure receiving furosemide in intensive care units. Kamio T, Ikegami M, Machida Y, Uemura T, Chino N, Iwagami M. Digit Health. 2023;9:20552076231194933. doi: 10.1177/20552076231194933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Improving risk prediction in heart failure using machine learning. Adler ED, Voors AA, Klein L, et al. Eur J Heart Fail. 2020;22:139–147. doi: 10.1002/ejhf.1628. [DOI] [PubMed] [Google Scholar]
- 51.Validation and comparison of seven mortality prediction models for hospitalized patients with acute decompensated heart failure. Lagu T, Pekow PS, Shieh MS, et al. Circ Heart Fail. 2016;9 doi: 10.1161/CIRCHEARTFAILURE.115.002912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Panahiazar M, Taslimitehrani V, Pereira N, Pathak J. MEDINFO 2015. Vol. 216. Amsterdam: IOS Press; 2015. Using EHRs and machine learning for heart failure survival analysis; pp. 40–44. [PMC free article] [PubMed] [Google Scholar]
- 53.Survival prediction among heart patients using machine learning techniques. Almazroi AA. Math Biosci Eng. 2022;19:134–145. doi: 10.3934/mbe.2022007. [DOI] [PubMed] [Google Scholar]
- 54.A comparative study on prediction of survival event of heart failure patients using machine learning algorithms. Özbay Karakuş M, Er O. Neural Comput Applic. 2022;34:13895–13908. [Google Scholar]
- 55.Zaman SM, Qureshi WM, Raihan MS, Shams AB, Sultana S. IEEE International Women in Engineering (WIE) Conference on Electrical and Computer Engineering (WIECON-ECE) Dhaka: IEEE; 2021. Survival prediction of heart failure patients using stacked ensemble machine learning algorithm. [Google Scholar]
- 56.Survival prediction of heart failure patients using machine learning techniques. Newaz A, Ahmed N, Shahriyar Haq F. Inform Med Unlocked. 2021;26:100772. [Google Scholar]
- 57.Kedia S, Bhushan M. 2022 2nd International Conference on Emerging Frontiers in Electrical and Electronic Technologies (ICEFEET) Patna: IEEE; 2022. Prediction of mortality from heart failure using machine learning; pp. 1–6. [Google Scholar]
- 58.Machine learning can predict survival of patients with heart failure from serum creatinine and ejection fraction alone. Chicco D, Jurman G. BMC Med Inform Decis Mak. 2020;20:16. doi: 10.1186/s12911-020-1023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Prediction model of in-hospital mortality in intensive care unit patients with heart failure: machine learning-based, retrospective analysis of the MIMIC-III database. Li F, Xin H, Zhang J, Fu M, Zhou J, Lian Z. BMJ Open. 2021;11:0. doi: 10.1136/bmjopen-2020-044779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.A machine learning-based risk stratification tool for in-hospital mortality of intensive care unit patients with heart failure. Luo C, Zhu Y, Zhu Z, Li R, Chen G, Wang Z. J Transl Med. 2022;20:136. doi: 10.1186/s12967-022-03340-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Machine learning-based in-hospital mortality risk prediction tool for intensive care unit patients with heart failure. Chen Z, Li T, Guo S, Zeng D, Wang K. Front Cardiovasc Med. 2023;10:1119699. doi: 10.3389/fcvm.2023.1119699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Machine learning-based prediction of heart failure readmission or death: implications of choosing the right model and the right metrics. Awan SE, Bennamoun M, Sohel F, Sanfilippo FM, Dwivedi G. ESC Heart Fail. 2019;6:428–435. doi: 10.1002/ehf2.12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Feature selection and transformation by machine learning reduce variable numbers and improve prediction for heart failure readmission or death. Awan SE, Bennamoun M, Sohel F, Sanfilippo FM, Chow BJ, Dwivedi G. PLoS One. 2019;14:0. doi: 10.1371/journal.pone.0218760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Predicting 90 day acute heart failure readmission and death using machine learning-supported decision analysis. Sarijaloo F, Park J, Zhong X, Wokhlu A. Clin Cardiol. 2021;44:230–237. doi: 10.1002/clc.23532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Machine learning prognosis model based on patient-reported outcomes for chronic heart failure patients after discharge. Tian J, Yan J, Han G, et al. Health Qual Life Outcomes. 2023;21:31. doi: 10.1186/s12955-023-02109-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Machine learning-driven models to predict prognostic outcomes in patients hospitalized with heart failure using electronic health records: retrospective study. Lv H, Yang X, Wang B, et al. J Med Internet Res. 2021;23:0. doi: 10.2196/24996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Validated, electronic health record deployable prediction models for assessing patient risk of 30-day rehospitalization and mortality in older heart failure patients. Eapen ZJ, Liang L, Fonarow GC, Heidenreich PA, Curtis LH, Peterson ED, Hernandez AF. JACC Heart Fail. 2013;1:245–251. doi: 10.1016/j.jchf.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 68.Machine learning models in heart failure with mildly reduced ejection fraction patients. Zhao H, Li P, Zhong G, et al. Front Cardiovasc Med. 2022;9:1042139. doi: 10.3389/fcvm.2022.1042139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Utilizing electronic health data and machine learning for the prediction of 30-day unplanned readmission or all-cause mortality in heart failure. Beecy AN, Gummalla M, Sholle E, et al. Cardiovasc Digit Health J. 2020;1:71–79. doi: 10.1016/j.cvdhj.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Interpretable support vector machines for functional data. Martin-Barragan B, Lillo R, Romo J. Eur J Operation Res. 2014;232:146–155. [Google Scholar]