Abstract
Inferior vena cava aneurysms (IVCAs) are rare yet potentially lethal, especially if they are symptomatic or complicated. Among the IVCAs reported in the literature, only a few are associated with congenital vascular anomalies, including congenital IVC obstruction, tetralogy of Fallot, left-sided IVC, duplicated IVC, Ehlers–Danlos syndrome, blue rubber bleb nevus syndrome, and Klipper-Trenaunay syndrome. We present the case of an 8-cm symptomatic saccular IVCA in a patient with tetralogy of Fallot, treated successfully with surgical repair. Although rare venous pathologies can sometimes be managed with endovascular treatment, open surgical reconstruction remains the mainstay of durable and definitive repair.
Keywords: Inferior vena cava, Tetralogy of fallot, Vascular congenital abnormalities
INTRODUCTION
Inferior vena cava aneurysms (IVCAs) are rare but potentially serious conditions, particularly when complicated or underdiagnosed. IVCAs can be primary (idiopathic) or secondary (external or iatrogenic) to trauma. They often occur in conjunction with other embryonic anomalies, such as tetralogy of Fallot [1], or connective tissue disorders, such as Ehlers–Danlos syndrome [2]. Most aneurysms are incidental findings during ultrasound or computed tomography (CT) examination in asymptomatic patients [3]. Symptomatic patients usually present with abdominal pain or lower limb edema due to thrombosis in the iliocaval axis. Other serious thrombotic complications, including pulmonary embolism and paradoxical embolism, can also occur in cases with a patent foramen ovale [4].
Here, we report a successful open surgical management of a pararenal IVCA in a patient with tetralogy of Fallot. Informed consent was obtained from the patient.
CASE
We present the case of a 30-year-old Caucasian male with a 10-day history of progressively worsening epigastric pain, vomiting, and postprandial nausea. The patient had been diagnosed with tetralogy of Fallot during infancy. He underwent pacemaker-defibrillator implantation for arrhythmias in 2004 and pulmonary valve replacement for pulmonic valve insufficiency in 2008. His medical history included two episodes of acute endocarditis treated with antibiotics. Since cardiac surgery, the patient was on half a tablet of acenocoumarol 4 mg daily for anticoagulation.
On admission, his vital signs were within the normal limits, but laboratory tests showed elevated bilirubin (3.1 mg/dL, normal <1.2 mg/dL) and C-reactive protein (5.8 mg/dL, normal <1.0 mg/dL). Based on the clinical symptoms and test results, acute cholecystitis was initially suspected, and the patient was admitted to the internal medicine unit.
During hospitalization, cardiac ultrasonography showed a left ventricular ejection fraction of 60% and right ventricular systolic pressure of 60 mmHg, indicating a mild increase in the right ventricle and IVC. Abdominal ultrasonography showed normal gallbladder and biliary tract findings, but identified a retroperitoneal mass adjacent to the IVC with reduced flow velocity on color Doppler imaging (Fig. 1). CT confirmed the presence of an 83×75-mm retroperitoneal mass arising from the right side of the IVC, just below the level of the right renal vein (Fig. 2A, B). Differential diagnosis included a vascular tumor, soft tissue neoplasm, or IVCA. Venography was deemed essential for confirming the diagnosis and surgical planning. Using the Seldinger technique, interventional radiologists accessed the right femoral vein and inserted a 5Fr pigtail catheter to the level of the renal vessels. Venography with contrast injection revealed a large type III saccular aneurysm originating from the right side of the IVC just below the orifice of the right renal vein (Fig. 3).
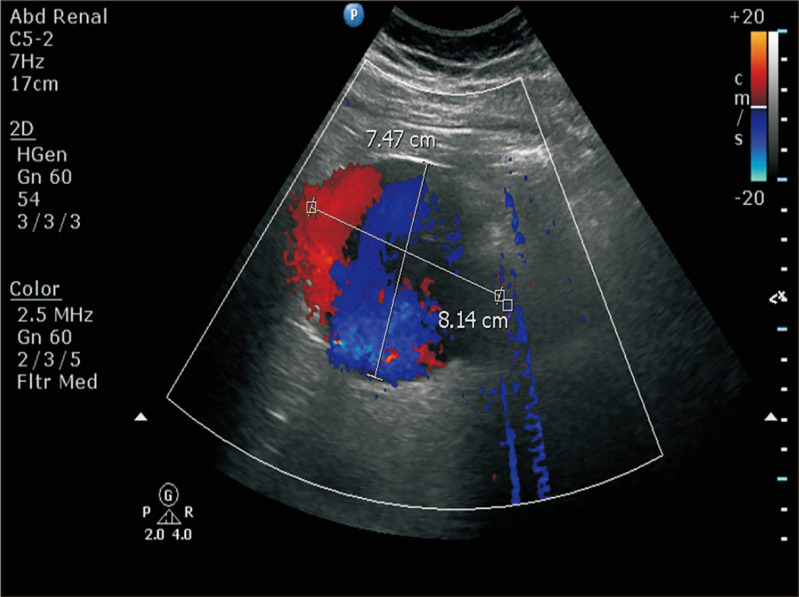
Fig. 1.
Color Doppler abdominal ultrasound showed a suspected inferior vena cava aneurysm with a diameter of 8.14 cm and slow velocity flow inside.
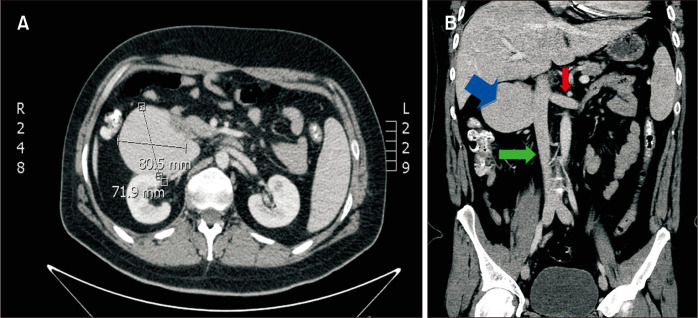
Fig. 2.
(A) Computed tomography (CT) scan with intravenous contrast demonstrated a mass near the inferior vena cava (IVC). (B) Coronal CT image with intravenous contrast demonstrated the saccular IVC aneurysm (blue arrow), IVC (green arrow) and left renal vein (red arrow).
Fig. 3.
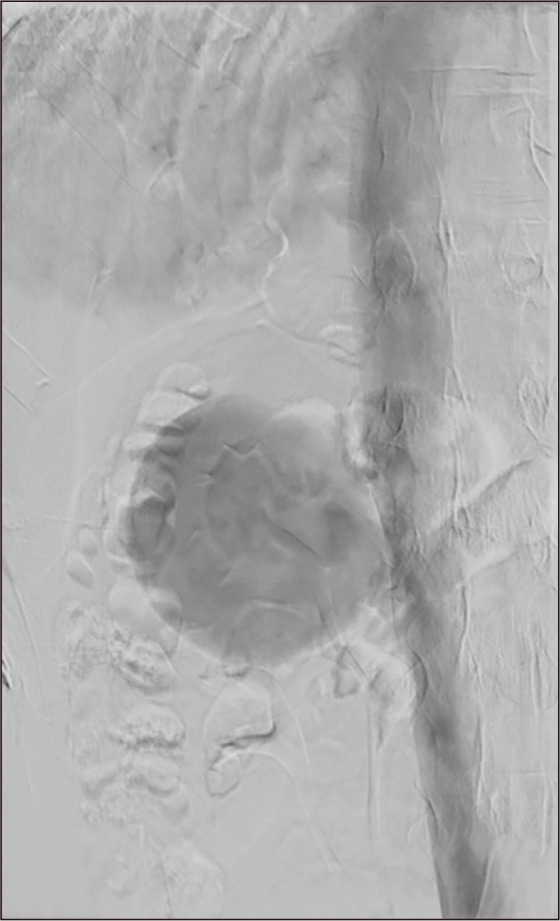
Venography revealed a giant saccular aneurysm (83×75 mm) originating from the right side of the inferior vena cava, just below the right renal vein.
The patient was transferred to the vascular surgery department for elective operative management. Acenocoumarol was temporarily discontinued and bridging therapy with a prophylactic dose of low-molecular-weight heparin was initiated.
Surgical reconstruction was chosen based on the patient’s age, anatomical location of the aneurysm, and associated comorbidities. An endovascular approach with an endograft was considered an inferior treatment option because of the aneurysmal neck’s close proximity to the right renal vein and the risk of inadvertent coverage of the renal vein ostia with an endograft.
A midline laparotomy was performed. Right medial visceral rotation was conducted via a Kocher maneuver by mobilization of the right colon and the IVCA was visualized retroperitoneally (Fig. 4A). No further mobilization of the liver was required. A saccular IVCA with a neck diameter of 3 cm was identified, positioned to the right of the IVC. The right renal vein was located just above the neck of aneurysm. A Satinsky clamp was applied to the base of the aneurysm neck (Fig. 4B). The aneurysm was excised entirely (Fig. 4C), and the aneurysm neck was closed with a continuous 4-0 polypropylene suture (Fig. 4D).
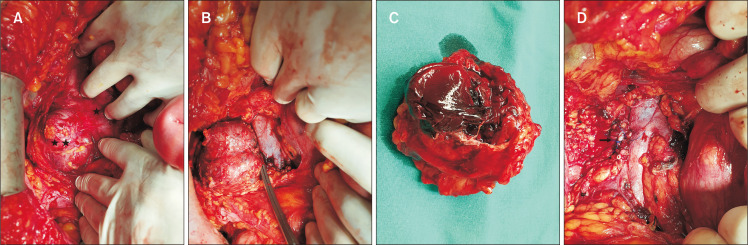
Fig. 4.
(A) Intraoperative picture of (*) IVC and (**) large saccular aneurysm of IVC. (B) Intraoperative picture of IVC and large saccular aneurysm of IVC with a clamp at the aneurysm’s neck before ligation. (C) Excised aneurysmal sac of IVCA with an approximate diameter of 7 cm. (D) Intraoperative picture showing lateral continuous venorrhaphy with 4-0 polypropylene sutures (arrow) at the aneurysm’s neck. IVCA, inferior vena cava aneurysm.
The patient was transferred to the intensive care for postoperative monitoring and recovered well. He was moved to the vascular surgery ward on the first postoperative day and discharged home on the 7th postoperative day. Oral anticoagulation therapy was resumed as recommended. Apart from an anterior abdominal wall hematoma, which was managed with conservatively, no other perioperative or postoperative complications occurred. At the 3-year follow-up, the patient remained asymptomatic, with no evidence of recurrence or additional complications.
DISCUSSION
Inferior vena cava aneurysms are rare pathologies that are usually discovered incidentally during evaluation in patients with clinical suspicion of iliofemoral venous thrombosis or atypical abdominal pain. The first case of a caval aneurysm was reported in the 1950s by Abbott and Leigh [5], and Oh et al. [1] described the first case of IVCA in 1973. IVCAs are typically saccular (diverticular) in shape, rarely fusiform, involve all three layers of the venous wall, and occur more frequently in men [3].
Thompson and Lindenauer [6] classified IVCAs into three types: congenital, acquired, and secondary to an arteriovenous fistula or malformation (congenital or acquired) [7]. Common etiologies include inflammation, trauma, and long-standing systemic venous hypertension secondary to right-sided heart failure or outflow obstruction. Other etiological factors include tricuspid valve lesions and cardiomyopathies [8].
Tetralogy of Fallot is a rare heart condition that is present at birth. It causes altered blood flow through the heart and to the rest of the body. Surgical intervention is indicated for these cases. However, there is no clear association between this pathology and IVCA. In the majority of cases, surgical intervention is considered in the cases of pulmonic valve insufficiency, right ventricular dysfunction, or arrhythmias, according to the existing literature [9].
Congenital vascular defects, such as Ehlers–Danlos syndrome [2], Klippel-Trenaunay Syndrome [4], tetralogy of Fallot [1], left-sided IVC [10], azygous agenesis [10], and membranous IVC obstruction [11] are often associated with IVCAs. It has been suggested that they develop in sites of anastomosis between the cardinal veins owing to failure of regression or abnormal fusion, particularly in the anastomosis sites between the subcardinal and supracardinal veins [8,12]. Gradman and Steinberg [13] anatomically classified IVCAs into four subtypes. Type I is confined to the suprahepatic IVC without venous obstruction; type II involves supra- or infrahepatic interruption of the IVC; type III involves the infrarenal IVC without congenital anomalies; and type IV is miscellaneous. So far, the most common type of IVCAs is type I according to recent literature.
Clinically, IVCAs frequently present with IVC syndrome (especially in children), characterized by lower extremity swelling, abdominal pain, and venous hypertension secondary to aneurysm thrombosis [14]. Other complications include Budd–Chiari syndrome, pulmonary embolism, paradoxical cerebral embolism, massive penile hemorrhage, and rupture [15,16]. In our case, the patient’s gastrointestinal symptoms were likely caused by bowel compression from the large IVCA, with no lower leg edema due to patent iliofemoral veins and IVC.
Imaging is critical for diagnosis, with CT being the most commonly used modality. Duplex ultrasonography, magnetic resonance imaging (MRI), and digital venography also provide useful anatomic details and play an important role in differential diagnosis. In cases of non-thrombotic IVCA, diagnosis is relatively straightforward, and a biopsy is generally unnecessary. However, in thrombotic IVCA, there is a risk of misdiagnosis as a retroperitoneal tumor, such as lymphoma, renal cell carcinoma, sarcoma, or other neurogenic tumors. In such cases, careful evaluation is essential, and tools like biopsy or MRI with the “layered gadolinium” sign can aid in distinguishing thrombotic IVCA from malignant retroperitoneal masses [16].
Montero-Baker et al. [12] reviewed 54 IVCA cases up to 2014. Among these, 23 were type I IVCAs and the second most common was type III (21 cases). Treatment approaches depend on the type, symptoms, size, and thrombotic risk of the IVCA. Asymptomatic patients with type I IVCAs are often managed conservatively (expectant with close monitoring unless they become symptomatic or increase in size). In cases with thrombosis in type I IVCA, anticoagulant therapy for at least 6-12 months or thrombolysis should be considered. If there is a clear contraindication to anticoagulation therapy, suprarenal IVC filter is indicated [14]. Surgical treatment is indicated for symptomatic type I IVCAs and type II-IV due to the high risk of thromboembolic complications and rupture [12].
The surgical approach involves excision and reconstruction. After resection of saccular (diverticular) aneurysms, simple continuous suturing, lateral venorrhaphy, aneurysmorrhaphy, and patch angioplasty using a vein or bovine patch of the IVC are feasible options. In cases of fusiform IVCAs, resection is followed by interposition with synthetic or bovine pericardium tube graft. However, in patients who are at high risk for open surgery with small type II-IV IVCAs (<5 cm), conservative management with close monitoring is considered [14].
Although surgical repair is currently considered the gold standard treatment, endovascular techniques, including embolization and stent graft implantation, have emerged as promising alternatives for treating IVCAs. Endovascular treatments are usually indicated in patients with increased surgical risk and suitable anatomy. Falkowski and Wiernicki [17] reported implantation of a stent graft to treat IVCA. An oversizing of at least 20% was suggested to prevent migration. Embolization using coils or Amplatzer vascular plugs has also been reported [7,18-20]. In our case, embolization of the sac aneurysm with coils may be an option, but it was excluded due to the necessity of many coils, patient age, 3 cm aneurysm neck, and the risk of distal embolization with coils to the pulmonary circulation.
In conclusion, we present a rare case of IVCA in a patient with tetralogy of Fallot, diagnosed via digital venography and successfully managed with surgical excision and primary IVC repair. While endovascular approach represent an attractive alternative owing to their minimally invasive nature, open reconstruction remains the gold standard for selected patients, providing durable long-term results, especially in challenging anatomical scenarios.
ACKNOWLEDGEMENTS
We would like to thank Dr. Deftereos Savvas, Professor and Director of Interventional Radiology, Department of University Hospital of Alexandroupolis for his valuable contribution in diagnosis.
Funding Statement
FUNDING None.
Footnotes
CONFLICTS OF INTEREST
The authors have nothing to disclose.
AUTHOR CONTRIBUTIONS
Concept and design: KD. Analysis and interpretation: KD, NT. Data collection: NP, SP. Writing the article: KD, NP, SP. Critical revision of the article: CA. Final approval of the article: CA, GG. Statistical analysis: none. Obtained funding: none. Overall responsibility: GG.
REFERENCES
- 1.Oh KS, Dorst JP, Haroutunian LM. Inferior vena caval varix. Radiology. 1973;109:161–162. doi: 10.1148/109.1.161. https://doi.org/10.1148/109.1.161. [DOI] [PubMed] [Google Scholar]
- 2.Wheen P, Mahon C, Sharma R, Rubens M, Nicol E. Inferior vena cava aneurysm in a patient with Ehlers-Danlos syndrome. J Cardiovasc Comput Tomogr. 2021;15:e94–e95. doi: 10.1016/j.jcct.2021.03.002. https://doi.org/10.1016/j.jcct.2021.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Moncada R, Demos TC, Marsan R, Churchill RJ, Reynes C, Love L. CT diagnosis of idiopathic aneurysms of the thoracic systemic veins. J Comput Assist Tomogr. 1985;9:305–309. doi: 10.1097/00004728-198503000-00014. https://doi.org/10.1097/00004728-198503000-00014. [DOI] [PubMed] [Google Scholar]
- 4.Machin M, Coupland A, Thapar A, Davies AH. An inferior vena cava aneurysm in a patient with Klippel-Trenaunay syndrome. Ann Vasc Surg. 2018;50:300.e1–300.e3. doi: 10.1016/j.avsg.2018.01.092. https://doi.org/10.1016/j.avsg.2018.01.092. [DOI] [PubMed] [Google Scholar]
- 5.Abbott OA, Leigh TF. Aneurysmal dilatations of the superior vena caval system. Ann Surg. 1964;159:858–872. doi: 10.1097/00000658-196406000-00004. https://doi.org/10.1097/00000658-196406000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thompson NW, Lindenauer SM. Central venous aneurysms and arteriovenous fistulas. Ann Surg. 1969;170:852–856. doi: 10.1097/00000658-196911000-00018. https://doi.org/10.1097/00000658-196911000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Vuuren TMAJ, Kurstjens RLM, Van Zandvoort C, De Graaf R, Wittens CHA, Van Laanen JHH. Endovascular treatment of a renocaval arteriovenous fistula induced inferior vena cava aneurysm. Ann Vasc Surg. 2017;45:269.e5–269.e9. doi: 10.1016/j.avsg.2017.07.014. https://doi.org/10.1016/j.avsg.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 8.Ayaon Albarrán A, Blázquez González JA, Domínguez Melcón FJ, Mesa García JM. Giant inferior vena cava aneurysm. J Card Surg. 2017;32:292. doi: 10.1111/jocs.13130. https://doi.org/10.1111/jocs.13130. [DOI] [PubMed] [Google Scholar]
- 9.Furukawa T, Yamada T, Mori Y, Shibakiri I, Fukakusa S, Jitsukawa K, et al. Idiopathic aneurysm of inferior vena cava: CT demonstration. J Comput Assist Tomogr. 1986;10:1076–1077. doi: 10.1097/00004728-198611000-00044. https://doi.org/10.1097/00004728-198611000-00044. [DOI] [PubMed] [Google Scholar]
- 10.Sullivan VV, Voris TK, Borlaza GS, Lampman RM, Sood M, Shanley CJ. Incidental discovery of an inferior vena cava aneurysm. Ann Vasc Surg. 2002;16:513–515. doi: 10.1007/s10016-001-0110-z. https://doi.org/10.1007/s10016-001-0110-z. [DOI] [PubMed] [Google Scholar]
- 11.Cakmakçi M, Abbasoglu O, Balkanci F, Akhan O, Ozenç A. Combined saccular aneurysm and proximal interruption of the inferior vena cava associated with Budd-Chiari syndrome. AJR Am J Roentgenol. 1992;158:635–636. doi: 10.2214/ajr.158.3.1739009. https://doi.org/10.2214/ajr.158.3.1739009. [DOI] [PubMed] [Google Scholar]
- 12.Montero-Baker MF, Branco BC, Leon LL, Jr, Labropoulos N, Echeverria A, Mills JL., Sr Management of inferior vena cava aneurysm. J Cardiovasc Surg (Torino) 2015;56:769–774. [PubMed] [Google Scholar]
- 13.Gradman WS, Steinberg F. Aneurysm of the inferior vena cava: case report and review of the literature. Ann Vasc Surg. 1993;7:347–353. doi: 10.1007/BF02002888. https://doi.org/10.1007/bf02002888. [DOI] [PubMed] [Google Scholar]
- 14.Davidovic L, Dragas M, Bozic V, Takac D. Aneurysm of the inferior vena cava: case report and review of the literature. Phlebology. 2008;23:184–188. doi: 10.1258/phleb.2008.008008. https://doi.org/10.1258/phleb.2008.008008. [DOI] [PubMed] [Google Scholar]
- 15.Michel LL, Alomari AI. Embolization of a large inferior vena cava aneurysm in a child. J Vasc Interv Radiol. 2008;19:1509–1512. doi: 10.1016/j.jvir.2008.07.012. https://doi.org/10.1016/j.jvir.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 16.de Bree E, Klaase JM, Schultze Kool LJ, van Coevorden Aneurysm of the inferior vena cava complicated by thrombosis mimicking a retroperitoneal neoplasm. Eur J Vasc Endovasc Surg. 2000;20:305–307. doi: 10.1053/ejvs.2000.1128. https://doi.org/10.1053/ejvs.2000.1128. [DOI] [PubMed] [Google Scholar]
- 17.Falkowski A, Wiernicki I. Stent-graft implantation to treat an inferior vena cava aneurysm. J Endovasc Ther. 2013;20:714–717. doi: 10.1583/13-4368R.1. https://doi.org/10.1583/13-4368r.1. [DOI] [PubMed] [Google Scholar]
- 18.Elliot A, Henn A, Pamuklar E, Rivero H, Hyslop WB, Semelka RC, et al. Aneurysm of the inferior vena cava: case report. Abdom Imaging. 2006;31:457–460. doi: 10.1007/s00261-005-0048-7. https://doi.org/10.1007/s00261-005-0048-7. [DOI] [PubMed] [Google Scholar]
- 19.Walsh K, O'Connor D, Wilderman M, Ratnathicam A, Simonian G, Napolitano MM. Balloon angioplasty for symptomatic inferior vena cava aneurysm. J Vasc Surg Venous Lymphat Disord. 2018;6:661–663. doi: 10.1016/j.jvsv.2018.04.008. https://doi.org/10.1016/j.jvsv.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 20.Wang M, Wang H, Huang XL, Chang GQ. Endovascular treatment of an inferior vena cava aneurysm in a patient with blue rubber bleb nevus syndrome. J Vasc Surg Cases Innov Tech. 2021;7:634–635. doi: 10.1016/j.jvscit.2021.07.008. https://doi.org/10.1016/j.jvscit.2021.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]