Abstract
Severe combined immunodeficient (SCID) mice lack functional lymphocytes and therefore develop Pneumocystis carinii pneumonia. However, when infected SCID mice are immunologically reconstituted with congenic spleen cells, a protective inflammatory cascade is initiated. Proinflammatory cytokines are produced, and lymphocytes and macrophages are recruited specifically to alveolar sites of infection. Importantly, uninfected regions of the lung remain free from inflammatory involvement, suggesting that there are specific mechanisms that limit inflammation in the infected lung. Therefore, to determine whether chemokines are involved in targeting the P. carinii-driven inflammatory response, steady-state mRNA levels of several chemokines were measured in the lungs of both reconstituted and nonreconstituted P. carinii-infected SCID mice. Despite significant organism burdens in the lungs of 8- and 10-week-old SCID mice, there was no evidence of elevated chemokine gene expression, which is consistent with the lack of an inflammatory response in these animals. However, when 8-week-old infected SCID mice were immunologically reconstituted, signs of focal pulmonary inflammation were observed, and levels of RANTES, MCP-1, lymphotactin, MIP-1α, MIP-1β, and MIP-2 mRNAs were all significantly elevated. Chemokine mRNA abundance was elevated at day 10 postreconstitution (PR), was maximal at day 12 PR, and returned to baseline by day 22 PR. In situ hybridization demonstrated that during the peak of inflammation, RANTES gene expression was localized to sites of inflammatory cell infiltration and P. carinii infection. Thus, these observations indicate that chemokines play a role in the focal targeting of inflammatory cell recruitment to sites of P. carinii infection after the passive transfer of lymphocytes to the host.
Pneumocystis carinii is an opportunistic pulmonary pathogen that causes life-threatening pneumonia in patients suffering from a variety of immunocompromising conditions, including AIDS (34, 46). Despite the dramatic increase in prevalence of P. carinii pneumonia (PCP) during the past 25 years, the specific mechanisms of pulmonary defense contributing to host resistance against this organism remain vaguely understood. The SCID mouse model of PCP has provided a valuable tool for defining specific lymphocyte populations and host factors that are critical for resistance (7, 35). SCID mice lack functional CD4+ T lymphocytes and therefore develop noticeable P. carinii infections by 3 weeks of age. Initially, focal infection of scattered alveoli distal to the terminal airways is observed. Despite the presence of functional macrophages and granulocytes, the infection progresses with minimal host response against the organism (7). However, if infected SCID mice are immunologically reconstituted with congenic spleen cells or fractionated CD4+ T lymphocytes, they mount an intense pulmonary inflammatory response and resolve the infection (7, 36). This P. carinii-driven inflammatory response is dependent on CD4+ T lymphocytes (36), interleukin-1 (IL-1) (8), and tumor necrosis factor alpha (TNF-α) (9) and is characterized by the expression of proinflammatory cytokines and the accumulation of T lymphocytes and activated macrophages in the lung (7, 47). Recently we have reported that inflammatory cell recruitment is specifically targeted to alveolar sites of P. carinii infection, while uninfected alveoli remain free from inflammation (47). Thus, signals generated at sites of P. carinii-epithelial cell interactions must function to initiate a focal inflammatory response.
Differential recruitment of appropriate inflammatory cell populations is an important initial step in the host defense against pulmonary infections of bacterial (12), fungal (16), and viral (39) origin. Recent studies have identified a growing superfamily of chemotactic peptides, termed chemokines, which play a role in the recruitment and activation of specific leukocyte populations (1). These molecules have been implicated in the initiation and amplification of a variety of pulmonary inflammatory responses, including those associated with infection (41), inhaled toxicants (10), irradiation (18), and autoimmune disorders (32). The chemokines have been subdivided into three classes based on target cell specificity and the organization of the conserved N-terminal cysteine motif (1, 14). The C-X-C or α chemokines include IL-8, macrophage inflammatory protein 2 (MIP-2), and interferon-inducible protein 10 (IP-10) and are generally chemoattractant for neutrophils, but not monocytes. The C-C or β chemokines include RANTES (regulated on activation normal T cell expressed and secreted), monocyte chemotactic protein 1 (MCP-1), T-cell activation gene 3 (TCA3), macrophage inflammatory proteins 1α and 1β (MIP-1α and -1β), and are generally chemoattractant for monocytes and certain lymphocyte subsets, but not neutrophils. Additionally, the one recently characterized C chemokine, lymphotactin, is apparently a potent chemoattractant for T lymphocytes (19). Thus, depending upon the nature of the injury and the cell types injured, a specific type of inflammatory response may be mounted based on which chemokines are secreted at the site of insult.
The focal, cell-specific nature of the P. carinii-driven inflammatory response in reconstituted SCID mice suggests that chemokines may play a role in this model of pulmonary inflammation. In particular, the C-C or β chemokines are attractive candidates for mediating P. carinii-induced inflammatory cell recruitment, because this infection is associated with a predominantly mononuclear cell infiltration (21). Certain β chemokines are chemoattractant for CD4+ T lymphocytes and monocytes/macrophages, the main cellular infiltrates in the lungs of reconstituted SCID mice (1). β chemokines can be secreted by alveolar epithelial cells (20, 30), macrophages (6, 25), and CD4+ T lymphocytes (38), three cell populations that interact with P. carinii in the lung. Finally, β chemokine expression and secretion by alveolar epithelial cells and macrophages is upregulated in response to IL-1 and TNF-α stimulation, proinflammatory cytokines that are required for induction of the P. carinii-driven inflammatory response in reconstituted SCID mice (6, 20, 25, 40). Therefore, pulmonary chemokine expression in P. carinii-infected SCID mice was examined before and after the passive transfer of critical CD4+ T lymphocytes to the host.
MATERIALS AND METHODS
Animals.
Male CB.17+/+ and CB.17 scid/scid mice were obtained from a colony at the Trudeau Institute Animal Breeding Facility (Saranac Lake, N.Y.). The mice were bred and housed in microisolator cages and were free of common murine pathogens. The foundation stock was originally obtained from Leonard Schultz of The Jackson Laboratory (Bar Harbor, Maine). In order to induce infection, 3-week-old SCID mice were cohoused with P. carinii-infected SCID mice for 5 weeks. At 8 weeks of age, 30 P. carinii-infected SCID mice were immunologically reconstituted as previously described (8). Briefly, spleens were asceptically removed from 6-week-old male CB.17+/+ donor mice, gently pushed through stainless steel screens into Hanks balanced salt solution, and then triturated with a Pasteur pipette. The cells were washed twice with phosphate-buffered saline (pH 7.2), counted, and then resuspended in phosphate-buffered saline at a concentration of 3.5 × 107 splenocytes/ml. P. carinii-infected SCID mice were reconstituted with a 1-ml tail vein injection of the cell suspension.
Lung tissue preparation.
At predetermined time points, mice were sacrificed by cervical dislocation. The chest cavity and surrounding connective tissue were cut open to expose the lungs and trachea. The lower left lung lobe was tied off at the bronchial airway with surgical string and then removed with sterile scissors. The isolated lung tissue was immediately quick-frozen in liquid nitrogen and stored at −80°C for subsequent RNA isolation. For tissue fixation, a 20-gauge, 1 1/4 in. intravenous catheter unit was then inserted into the trachea and tied in place with surgical string. The lungs were inflated with 30-cm gravity flow pressure of 2% gluteraldehyde–100 mM cacodylic acid fixative (Sigma, St. Louis, Mo.). The lungs were fixed for 10 min under gravity flow pressure and then removed from the animal and placed in fixative for 16 h at 4°C. The lungs were stored at 4°C in 100 mM cacodylic acid, pH 7.4. Prior to embedding, the fixed lungs were dehydrated in sequential 15-min washes of 30, 50, 70, 80, 90, 95, and 99% ethanol. At this time the lower right lung lobe of each animal was removed, snapped into a tissue cassette, and then placed in xylene. Each lung lobe was embedded in parafin, and 4-μm-thick sections were cut from the tissue blocks.
RPA.
Total RNA was isolated from lung tissue with TRIzol reagent (Life Technologies, Grand Island, N.Y.) according to the manufacturer’s instructions. Each frozen lung lobe (50 to 100 mg) was homogenized in 1 ml of TRIzol reagent. Each final RNA pellet was resuspended in 50 μl of diethylpyrocarbonate-treated water. The RNA concentration and purity were quantified with the GeneQuant RNA/DNA calculator (Pharmacia Biotech, Piscataway, N.J.). Quantitation of steady-state cytokine mRNA levels was performed by a previously described multicytokine RNase protection assay (RPA) (15, 47). The mCK-5 template set (PharMingen) was used to transcribe radiolabelled, antisense riboprobes for murine lymphotactin, RANTES, eotaxin, MIP-1β, MIP-1α, MIP-2, IP-10, MCP-1, TCA-3, the murine ribosomal protein L32, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The protected RNA duplexes were purified by phenol-chloroform extraction and ethanol precipitation, and the pellets were resuspended in 5-μl portions of RPA loading buffer (80% formamide, 0.5× TBE [Tris-borate-EDTA], 0.05% bromphenol blue [Sigma]). The protected, radiolabelled RNA fragments were electrophoresed on a 5% acrylamide–8 M urea sequencing gel, and the dried gel was used to expose X-AR film (Kodak, Rochester, N.Y.). For quantitation, the dried gels were placed against PhosphorImager screens (Molecular Dynamics, Sunnyvale, Calif.). The intensity of each specific chemokine band was measured with a computer-linked PhosphorImager and ImageQuant software (Molecular Dynamics). To correct for RNA loading, each intensity score was normalized to the intensity of hybridization for the L32 gene. A one-way analysis of variance was performed with the SigmaStat 2.0 software (Jandel, San Rafael, Calif.) to determine the confidence intervals of observed variations in chemokine mRNA levels in the experimental animals. The Student-Newmann-Keuls method was used for all pairwise multiple comparisons of experimental groups.
In situ hybridization.
A cDNA clone for murine RANTES was obtained from E. G. Neilson at the University of Pennsylvania, Philadelphia (28). The RANTES cDNA was subcloned into the plasmid vector pBluescript II SK+ (Stratagene, La Jolla, Calif.) for the in vitro transcription of RNA (27). Sense and antisense orientations were confirmed by DNA sequencing. RANTES antisense RNA was transcribed from 1 μg of linearized plasmid template by the procedure described above. Full-length transcripts for RANTES were approximately 0.5 kb long. Prior to hybridization, limited alkaline hydrolysis was performed to create riboprobes ranging in length from 0.1 to 0.3 kb. Hydrolyzed transcripts were sized by denaturing agarose gel electrophoresis.
Tissue sections were treated by the method of Angerer et al. (2) with modifications. In situ hybridization was performed as previously described (47). Slides were counterstained with hematoxylin and eosin to visualize lung architecture. After photodocumentation of the in situ hybridization slides, the coverslips were removed by xylene treatment. The slides were then subjected to Gomori’s methenamine silver staining with fast green counterstain to visualize P. carinii organisms. Microscope coordinates were recorded for all photographs taken, and identical lung regions were examined for hybridization signal and P. carinii infection.
RESULTS
Pulmonary chemokine gene expression in SCID mice.
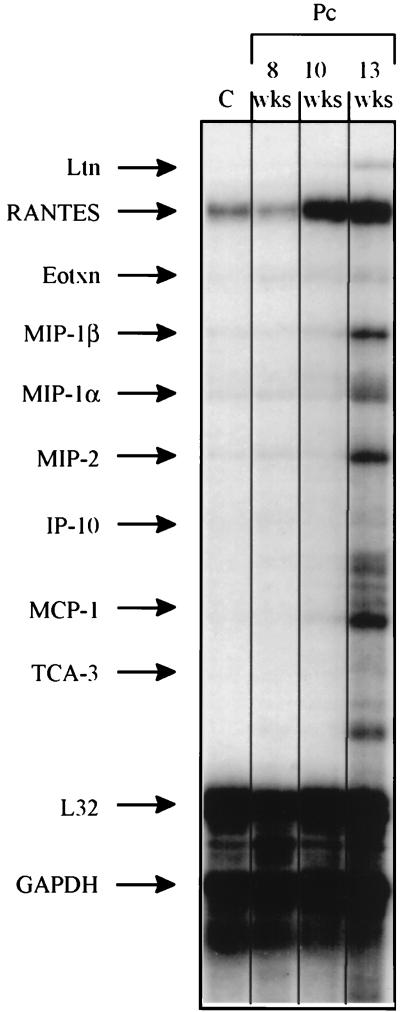
Groups of three P. carinii-infected SCID mice were sacrificed at 8, 10, and 13 weeks of age. In addition, three pathogen-free SCID mice were sacrificed as controls. Lung sections from these animals were stained with Gomori’s methenamine silver. The control animals were found to be free of infection, while the exposed animals demonstrated increasing P. carinii infection with increasing age (data not shown). To determine whether P. carinii infection induces elevated pulmonary chemokine expression in the absence of CD4+ T cells, steady-state levels of chemokine mRNAs were measured in these SCID mice by a RPA (Fig. 1). Despite the presence of P. carinii organisms in the lungs of 8-, 10-, and 13-week-old SCID mice, there was no statistically significant increase in the steady-state mRNA levels of any of the chemokines measured (Table 1). However, the mRNA levels for RANTES (2.4-fold), MIP-1α (2.4-fold), and MIP-2 (3.2-fold) were elevated in 13-week-old P. carinii-infected SCID mice compared to those in the P. carinii-free SCID controls (Table 1). These increases did not reach statistical significance at this sample size but may be relevant observations (Table 1). Furthermore, it appears that RANTES mRNA levels were elevated in the lungs of 10-week-old SCID mice (Fig. 1). However, when the mean RANTES mRNA levels of three mice at this time point were normalized to L32 mRNA levels, there was no difference between the control and experimental group (Table 1).
FIG. 1.
Pulmonary chemokine mRNA abundance in P. carinii-infected SCID mice. Steady-state mRNA levels in the lungs of infected 8-, 10-, and 13-week-old SCID mice and P. carinii-free SCID mice were measured by a multitemplate RPA. The migration position of each chemokine-specific protected fragment, as determined from a standard curve based on the migration of RNA of known molecular weight, is denoted to the left. Samples from the lungs of a P. carinii-free SCID control (C) mouse and SCID mice infected with P. carinii (Pc) (age in weeks [wks]) were used. Each lane contains a representative sample of three mice studied at each time point. Ltn, lymphotactin; Eotxn, eotaxin.
TABLE 1.
Relative chemokine mRNA abundance in P. carinii-infected SCID mice
| Mouse and age (wk) | Relative chemokine mRNA abundancea (mean ± SEM [n = 3])
|
|||||
|---|---|---|---|---|---|---|
| Ltnb | RANTES | MIP-1α | MIP-1β | MIP-2 | MCP-1 | |
| Controlc | 0.30 ± 0.06 | 1.20 ± 0.38 | 0.53 ± 0.15 | 0.53 ± 0.15 | 0.53 ± 0.15 | 0.50 ± 0.12 |
| Infected | ||||||
| 8 | 0.37 ± 0.07 | 1.37 ± 0.48 | 0.57 ± 0.03 | 0.53 ± 0.07 | 0.7 ± 0.06 | 0.57 ± 0.07 |
| 10 | 0.4 ± 0.10 | 1.60 ± 0.46 | 0.6 ± 0.06 | 0.6 ± 0.06 | 0.67 ± 0.07 | 0.63 ± 0.13 |
| 13 | 0.47 ± 0.12 | 2.57 ± 0.90 | 1.27 ± 0.37 | 1.0 ± 0.25 | 1.7 ± 0.65 | 1.33 ± 0.32 |
mRNA values are expressed as ratio of each chemokine band to the murine L32 band divided by 0.1.
Ltn, lymphotactin.
Control mice are P. carinii-free SCID mice.
Pulmonary chemokine expression in immunologically reconstituted P. carinii-infected SCID mice.
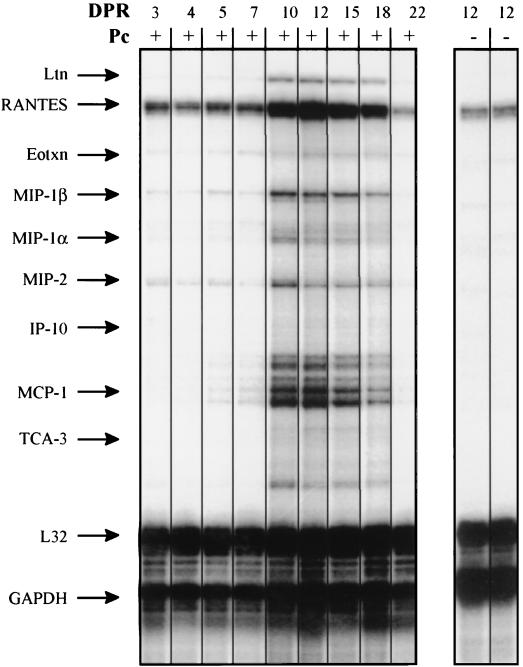
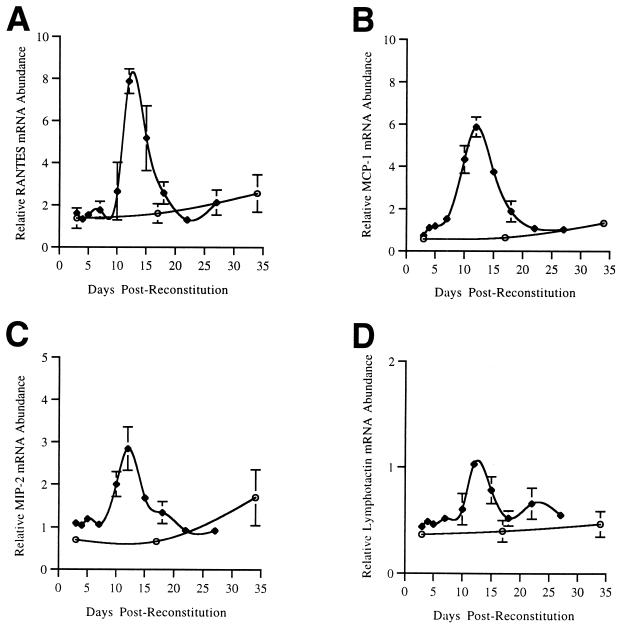
P. carinii-infected, 8-week-old SCID mice were immunologically reconstituted with a single tail vein injection of unfractionated, congenic spleen cells from an immunocompetent donor animal. Groups of three mice were sacrificed at 3, 4, 5, 7, 10, 12, 15, 18, 22, and 27 days postreconstitution (PR). In addition, P. carinii-free SCID mice were also immunologically reconstituted to study the effects of reconstitution alone on chemokine expression. Gomori’s methenamine silver staining of infected mice demonstrated a moderate P. carinii infection up to 12 days PR, but no organisms were detected at days 22 and 27 PR (data not shown). To determine whether chemokines played a role in inflammatory cell recruitment and the resolution of P. carinii infection in reconstituted SCID mice, RPAs were performed on RNA isolated from the lungs of these mice (Fig. 2). No significant changes in the mRNA levels of any of the chemokines assayed were observed prior to day 10 PR. However, levels of RANTES, MCP-1, MIP-1β, MIP-1α, and MIP-2 mRNAs were all elevated at day 10 PR, was maximal at day 12 PR, and returned to control levels by day 22 PR when the P. carinii organisms had been cleared (Fig. 2 and Table 2). Lymphotactin mRNA levels were not elevated at day 10 PR but were significantly elevated at days 12 and 15 PR. MCP-1, MIP-1α, MIP-1β, and MIP-2 mRNA levels were increased approximately fivefold, and RANTES and lymphotactin levels were increased approximately threefold over control mRNA levels at day 12 PR (Table 2). In contrast, at 12 days PR, the immunologically reconstituted P. carinii-free SCID mice demonstrated no significant increases in the pulmonary expression of any of the chemokines studied. A comparison of chemokine mRNA expression profiles in immunologically reconstituted and nonreconstituted P. carinii-infected SCID mice was depicted graphically in Fig. 3. The time course data demonstrated that after reconstitution SCID mice mounted a sharp chemokine response against P. carinii, while nonreconstituted SCID mice with similar organism burdens did not respond to infection with chemokine expression.
FIG. 2.
Pulmonary chemokine mRNA abundance in immunologically reconstituted, P. carinii-infected SCID mice. Eight-week-old, infected SCID mice were reconstituted with spleen cells from a healthy (CB.17+/+) donor, and steady-state mRNA levels in the lungs were measured at various times PR by a multitemplate RPA. As controls for the effects of reconstitution on chemokine expression, reconstituted P. carinii-free SCID mice were also examined. The number of days postreconstitution (DPR) and the infection status (infected [+] or not infected [−] with P. carinii [Pc]) of the mice are indicated above each lane. Each lane contains a representative sample of three mice studied at each time point. The migration position of each cytokine-specific protected fragment, as determined from a standard curve based on the migration of RNA of known molecular weight, is denoted to the left. Ltn, lymphotactin; Eotxn, eotaxin.
TABLE 2.
Relative chemokine mRNA abundance in immunologically reconstituted P. carinii-infected SCID mice
| Days PR | Relative chemokine mRNA abundancea (mean ± SEM [n = 3])
|
|||||
|---|---|---|---|---|---|---|
| Ltnb | RANTES | MIP-1α | MIP-1β | MIP-2 | MCP-1 | |
| 3 | 0.44 ± 0.015 | 1.60 ± 0.06 | 0.83 ± 0.063 | 0.73 ± 0.054 | 1.09 ± 0.01 | 0.73 ± 0.042 |
| 4 | 0.49 ± 0.067 | 1.32 ± 0.22 | 0.92 ± 0.14 | 0.80 ± 0.093 | 1.04 ± 0.13 | 1.10 ± 0.33 |
| 5 | 0.46 ± 0.01 | 1.53 ± 0.29 | 1.09 ± 0.08 | 0.96 ± 0.084 | 1.19 ± 0.06 | 1.18 ± 0.04 |
| 7 | 0.52 ± 0.06 | 1.76 ± 0.41 | 0.97 ± 0.088 | 0.87 ± 0.067 | 1.06 ± 0.08 | 1.52 ± 0.10 |
| 10 | 0.61 ± 0.15 | 2.65 ± 1.4 | 1.8 ± 0.29c | 1.5 ± 0.31c | 2.0 ± 0.29c | 4.3 ± 0.65c |
| 12 | 1.0 ± 0.01c | 7.9 ± 0.59c | 2.5 ± 0.28c | 2.5 ± 0.22c | 2.8 ± 0.51c | 5.9 ± 0.47c |
| 15 | 0.78 ± 0.13c | 5.2 ± 1.5c | 1.8 ± 0.03c | 1.7 ± 0.4c | 1.68 ± 0.12 | 3.8 ± 0.27c |
| 18 | 0.52 ± 0.07 | 2.57 ± 0.52 | 1.23 ± 0.20 | 1.12 ± 0.19 | 1.34 ± 0.26 | 1.88 ± 0.50 |
| 22 | 0.66 ± 0.15 | 1.29 ± 0.16 | 0.88 ± 0.13 | 0.85 ± 0.14 | 0.92 ± 0.14 | 1.07 ± 0.21 |
| 27 | 0.55 ± 0.014 | 2.13 ± 0.60 | 0.95 ± 0.084 | 0.87 ± 0.079 | 0.92 ± 0.044 | 1.03 ± 0.08 |
mRNA values are expressed as ratio of each chemokine band to the murine L32 band divided by 0.1.
Ltn, lymphotactin.
Statistically significantly different from the values obtained with the controls (P < 0.05).
FIG. 3.
Time course of RANTES, MCP-1, MIP-2, and lymphotactin mRNA abundance in the lungs of immunologically reconstituted and nonreconstituted P. carinii-infected SCID mice. Eight-week-old P. carinii-infected SCID mice were either left nonreconstituted (open circles) or were reconstituted with spleen cells from a congenic donor (closed diamonds). The time points are expressed as days postreconstitution for both groups of mice. The relative mRNA abundance at each time point is expressed as a ratio of each chemokine mRNA to the murine L32 mRNA divided by 0.1.
Localization of RANTES mRNA expression in situ.
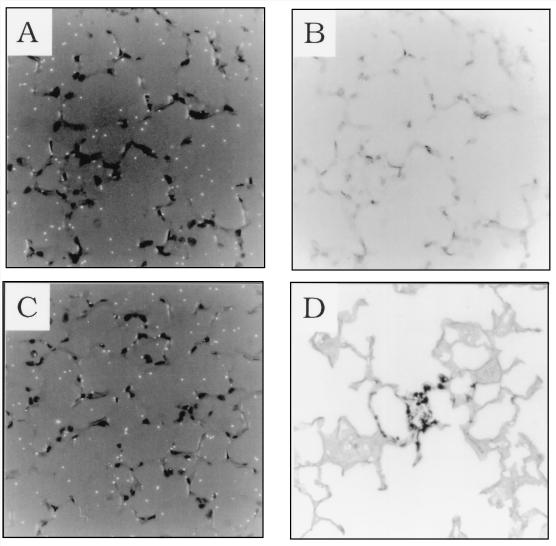
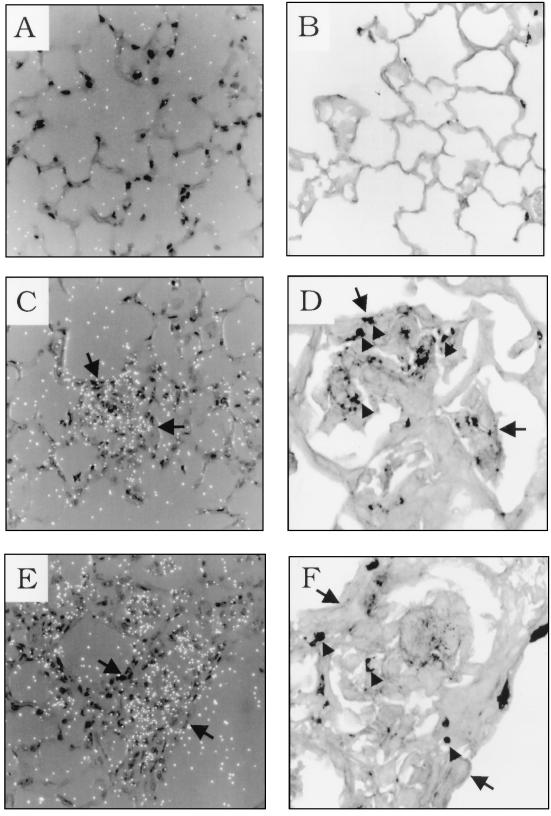
To identify regions of elevated chemokine mRNA expression in the lungs of immunologically reconstituted, P. carinii-infected SCID mice, in situ RNA-RNA hybridization was performed with a radiolabelled RANTES antisense riboprobe. Lung sections from pathogen-free SCID mice, 8-week-old P. carinii-infected SCID mice, and 8-week-old reconstituted P. carinii-infected SCID mice (12 days PR) were analyzed. In the P. carinii-free control and P. carinii-infected 8-week-old SCID mice, light background hybridization of the probe was observed throughout the lung (Fig. 4A and C). This finding was consistent with the RPA data demonstrating that there are only minimal background levels of RANTES mRNA in the lungs of P. carinii-free, 8- and 10-week-old infected SCID mice (Fig. 1). Gomori’s methenamine silver staining of the same lung sections posthybridization confirmed the absence of infection in the P. carinii-free control mice and demonstrated the presence of focal P. carinii organisms in the alveoli of 8-week-old SCID mice (Fig. 4D). In contrast, focal regions of alveolar inflammation corresponded to regions of intense RANTES hybridization in 8-week-old reconstituted P. carinii-infected mice (Fig. 5C and E). Gomori’s methenamine silver staining of the identical lung sections posthybridization indicated that these focal regions of elevated chemokine expression and inflammatory cell recruitment corresponded to sites of P. carinii infection (Fig. 5D and F). It was difficult to conclusively identify the cell types responsible for RANTES expression in the inflammatory regions because of the dense cellular infiltration. However, cells with morphology consistent with that of macrophages, lymphocytes, and alveolar epithelial cells were present at sites of inflammation and may contribute to RANTES gene expression. Neither the airway epithelium nor the endothelium appeared to be involved in P. carinii-induced RANTES expression. Furthermore, not all of the alveoli were involved in the response. Certain regions of the alveoli appeared normal and devoid of inflammation. These regions were not sites of RANTES expression, and no P. carinii organisms were detected by Gomori’s methenamine silver staining (Fig. 5A and B).
FIG. 4.
RANTES mRNA expression in the lungs of P. carinii-infected SCID mice. In situ hybridization with a RANTES antisense riboprobe was performed on lung sections from P. carinii-free (A and B), and 8-week-old P. carinii-infected SCID mice (C and D). Dark-field microscopy showing only background levels of RANTES hybridization is shown in panels A and C. Panel B is the same field shown in panel A but stained with hematoxylin and eosin. Panel D is the same tissue section shown in panel C but treated with Gomori’s methenamine silver stain, demonstrating P. carinii infection. Original magnification of all panels, ×400.
FIG. 5.
RANTES gene expression in the lungs of immunologically reconstituted P. carinii-infected SCID mice. In situ hybridization with a RANTES antisense riboprobe was performed on lung sections from reconstituted 8-week-old P. carinii-infected SCID mice at 12 days PR (A, C, and E). Background RANTES hybridization was observed in alveolar regions that were devoid of inflammation (A). Gomori’s methenamine silver staining revealed that these regions of the lung were also devoid of P. carinii infection (B). Panels C and E demonstrate elevated RANTES expression in regions of inflammatory cell infiltration. Panels D and F show Gomori’s methenamine silver staining of the identical microscope fields, in panels C and E, respectively, demonstrating that regions of cellular recruitment and RANTES expression correspond to regions of P. carinii infection. Arrows serve as landmarks to identify the regions of panels C and E shown in panels D and F, respectively. Arrowheads denote P. carinii cysts and portions of organisms present in inflammatory regions. Original magnification of panels A, B, C, and E, ×400; original magnification of panels D and F, ×1,000.
DISCUSSION
When P. carinii-infected SCID mice are immunologically reconstituted, distinct populations of inflammatory cells are recruited specifically to infected alveoli, while uninfected regions of the lung remain relatively free from the effects of inflammation (47). Therefore, given the differential chemoattractant properties of chemokines and the potential of T lymphocytes, alveolar macrophages, and alveolar epithelial cells to produce chemokines, their role in host defense against P. carinii in the reconstituted SCID mouse model was examined. In 8- and 10-week-old infected SCID mice, no inflammatory response was mounted against the organism, and pulmonary chemokine mRNA abundance was not elevated above control levels. However, when 8-week-old infected SCID mice were immunologically reconstituted, focal inflammatory cell infiltration was observed specifically at sites of infection. The onset and duration of the P. carinii-driven inflammatory response correlated temporally with increased chemokine mRNA levels. The chemokines lymphotactin, RANTES, MCP-1, MIP-1α, MIP-1β, and MIP-2 were all significantly elevated during the peak inflammatory stage PR (10 to 15 days PR). Pulmonary chemokine mRNA levels returned to baseline levels coincident with clearance of P. carinii from the lungs and the resolution of pulmonary inflammation. In addition, in situ hybridization demonstrated that RANTES mRNA expression was restricted to regions of P. carinii-induced inflammatory cell infiltration. Uninfected alveoli were not sites of elevated in situ RANTES gene expression. Thus, the presence of T lymphocytes initiates an inflammatory cascade that includes the pulmonary expression of chemokines in reconstituted SCID mice. Chemokines may play a role in specifically targeting the host inflammatory response to infected alveoli, thereby providing a mechanism for clearing infected alveoli, while preserving the critical function of the uninfected regions of the lung.
T lymphocytes, alveolar epithelial cells, and alveolar macrophages all interact with P. carinii (29, 31), and all are capable of secreting chemotactic cytokines. However, because of the intense focal nature of the P. carinii-driven inflammatory response in immunologically reconstituted SCID mice, it was difficult to definitively identify the cells responsible for chemokine production. Previous studies have demonstrated that the macrophage-derived proinflammatory mediators IL-1 and TNF-α are required for initiation of the T-lymphocyte-dependent inflammatory response against P. carinii and the clearance of organisms from the lung (8, 9). Interestingly, IL-1 and TNF-α induce the secretion of several chemokines from a variety of cell populations, including macrophages (6) and pulmonary epithelial cells (20). Roles for IL-1 and/or TNF-α in the induction of RANTES, MCP-1, MIP-1α, MIP-1β, and MIP-2 expression have been demonstrated (11, 20, 37, 40), and mRNA levels for all of these chemokines were significantly elevated in the lungs of reconstituted SCID mice coincident with IL-1 and TNF-α expression and inflammatory cell recruitment. Thus, T lymphocytes at sites of infection may (i) secrete chemokines themselves (RANTES and lymphotactin), (ii) activate macrophages to secrete chemokines (RANTES, MCP-1, MIP-1α, MIP-1β, and MIP-2), (iii) activate macrophages to secrete IL-1 and TNF-α which in turn cause alveolar epithelial cells to secrete chemokines (RANTES, MCP-1, and MIP-2), or (iv) all of the above. Together, these mechanisms of chemokine induction may cooperate to recruit the appropriate inflammatory cells specifically to sites of P. carinii infection in reconstituted SCID mice.
Since the main cellular infiltrates in response to P. carinii infection in immunologically reconstituted SCID mice are T lymphocytes and macrophages, the expression pattern of C-C chemokines was of particular interest. Pulmonary mRNA levels of RANTES, MCP-1, MIP-1α, and MIP-1β were all elevated during the acute inflammatory stage following immune reconstitution. Each of these chemokines can be secreted by one or more of the cell populations that interact with P. carinii in the lung, and each are chemoattractant for CD4+ T lymphocytes, monocytes/macrophages or both. The C-C chemokines have distinct but partially overlapping roles in the generation of antigen-specific T-cell responses by exhibiting direct effects on T lymphocytes, antigen-presenting cells, and macrophage effector functions (22, 43, 44). RANTES, MCP-1, MIP-1α, and MIP-1β are involved in the recruitment of CD4+ T lymphocytes by promoting their adhesion to extracellular matrix (ECM) proteins and inducing their directional migration across a chemokine concentration gradient (16, 24, 42). RANTES, MCP-1, MIP-1α, and MIP-1β also augment antigen-specific CD4+ T cell proliferation and lymphokine production through direct costimulatory effects on T cells and through the induction of the costimulatory molecule B7 on the surfaces of antigen-presenting cells (43, 44). In addition, C-C chemokines exert direct effects on monocyte/macrophage populations. RANTES, MCP-1, MIP-1α, and MIP-1β all induce the directional migration of monocytes/macrophages (1), and RANTES and MCP-1 also facilitate macrophage adhesion to ECM proteins by inducing expression of the β integrins CD11b/CD18 and CD11c/CD18 on the macrophage surface (17, 45). RANTES and MIP-1β activate macrophages for the uptake and intracellular destruction of parasitic organisms (22), and MCP-1 induces proinflammatory cytokine production in macrophages (17). Thus, C-C chemokines may play a role in the targeting of T lymphocytes and macrophages specifically to sites of P. carinii infection in reconstituted SCID mice. In addition, they may also facilitate antigen-specific T-cell responses and macrophage effector functions against P. carinii.
The C chemokine lymphotactin and the C-X-C chemokine MIP-2 were also examined. The recently identified chemokine lymphotactin is unique among the chemokine superfamily in that its chemotactic effects appear limited to lymphocyte subsets, with no discernible effects on macrophages or neutrophils (19). Lymphotactin mRNA levels were elevated during the acute inflammatory response in immunologically reconstituted SCID mice. Lymphotactin is secreted by activated T lymphocytes and is also chemotactic for T lymphocytes (19). Thus, activated T cells at sites of infection may secrete lymphotactin to amplify the T-cell-mediated inflammatory response. MIP-2 is a C-X-C chemokine that is a murine functional homologue of human and rabbit IL-8 (11). MIP-2 contains a glutamic acid-leucine-arginine (ELR) motif and is therefore a potent chemoattractant and activator of neutrophils (13, 33). Despite the lack of noticeable neutrophil infiltration in reconstituted P. carinii-infected SCID mice, there was a statistically significant increase in MIP-2 mRNA levels at 10 and 12 days PR. The functional significance of this is unknown, although it has been suggested that MIP-2 and IL-8 may exhibit T-lymphocyte chemoattractant properties (3). In addition, a threefold increase in MIP-2 mRNA abundance was observed in the 13-week-old SCID mice suffering from severe PCP. Although this value did not reach statistical significance at this sample size, it is consistent with previous observations regarding its human homologue, IL-8, in PCP patients. Increased bronchoalveolar lavage fluid IL-8 levels correlated with increased BALF neutrophil counts and a poorer patient prognosis (4, 23). These data suggest that in the late stages of PCP, neutrophil recruitment independent of CD4+ T cells may occur.
Although T lymphocytes are required for P. carinii-induced chemokine mRNA expression at sites of infection in SCID mice, we did observe elevated chemokine mRNA levels in the lungs of heavily infected SCID mice in the absence of T cells. However, these animals were nearing death, and severe lung injury had occurred. It is unknown whether elevated chemokine mRNA levels at this time are in response to P. carinii or are a consequence of lung injury. Lung injury can cause the breakdown of ECM proteins into biologically active fragments that can activate alveolar macrophages and induce the expression of several chemokine genes (26). Even though SCID mice lack functional lymphocytes, they do have functional alveolar macrophages. Thus, lung injury incurred during the latter stages of PCP may result in the generation of biologically active ECM fragments that bind receptors on alveolar macrophages and induce chemokine gene expression. Furthermore, a surface glycoprotein of P. carinii directly stimulates IL-8 and TNF-α release from human monocytes in vitro (5), and elevated TNF-α gene expression in the absence of T lymphocytes has been observed during the latter stages of PCP in a SCID mouse model of infection (47). TNF-α induces chemokine gene expression in a variety of cell types, including alveolar macrophages and pulmonary epithelial cells (20, 25, 40). Together, these mechanisms may contribute to increased chemokine mRNA levels in heavily infected SCID mice lacking CD4+ T cells.
In summary, the results of this study indicate that in the absence of lymphocytes, moderately infected SCID mice demonstrate neither increased pulmonary chemokine expression nor detectable pulmonary inflammation in response to P. carinii infection. However, when SCID mice carrying a comparable burden of organisms were immunologically reconstituted, both elevated pulmonary chemokine mRNA levels and acute alveolar inflammation were observed. Alveolar sites of infection were identified as regions of focal RANTES expression and inflammation. Thus, lymphocytes play a critical role in the induction of pulmonary inflammation in response to P. carinii infection by inducing pulmonary chemokine expression at sites of infection.
ACKNOWLEDGMENTS
This work was supported in part by Public Health Service grant HL-59833-02 from the National Heart, Lung, and Blood Institute and by a grant from the Strong Children’s Research Center, Rochester, N.Y.
REFERENCES
- 1.Adams D H, Lloyd A R. Chemokines: leucocyte recruitment and activation cytokines. Lancet. 1997;349:490–495. doi: 10.1016/s0140-6736(96)07524-1. [DOI] [PubMed] [Google Scholar]
- 2.Angerer L M, Cox K H, Angerer R C. Demonstration of tissue-specific gene expression by in situ hybridization. Methods Enzymol. 1987;152:649–661. doi: 10.1016/0076-6879(87)52071-7. [DOI] [PubMed] [Google Scholar]
- 3.Bacon K B, Flores-Romo L, Life P F, Taub D D, Premack B A, Arkinstall S J, Wells T N C, Schall T J, Power C A. IL-8-induced signal transduction in T lymphocytes involves receptor-mediated activation of phospholipases C and D. J Immunol. 1995;154:3654–3666. [PubMed] [Google Scholar]
- 4.Benfield T L, Vestbo J, Junge J, Nielsen T L, Jensen A B, Lundgren J D. Prognostic value of interleukin-8 in AIDS-associated Pneumocystis carinii pneumonia. Am J Respir Crit Care Med. 1995;151:1058–1062. doi: 10.1164/ajrccm/151.4.1058. [DOI] [PubMed] [Google Scholar]
- 5.Benfield T L, Lundgren B, Levine S J, Kronborg G, Shelhamer J H, Lundgren J D. The major surface glycoprotein of Pneumocystis carinii induces release and gene expression of interleukin-8 and tumor necrosis factor alpha in monocytes. Infect Immun. 1997;65:4790–4794. doi: 10.1128/iai.65.11.4790-4794.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brieland J K, Flory J M, Jones M L, Miller G R, Remick D G, Warren J S, Fantone J C. Regulation of monocyte chemoattractant protein-1 gene expression and secretion in rat pulmonary alveolar macrophages by lipopolysaccharide, tumor necrosis factor-alpha, and interleukin-1 beta. Am J Respir Cell Mol Biol. 1995;12:104–109. doi: 10.1165/ajrcmb.12.1.7811465. [DOI] [PubMed] [Google Scholar]
- 7.Chen W, Mills J W, Harmsen A G. Development and resolution of Pneumocystis carinii pneumonia in severe combined immunodeficient mice: a morphological study of host inflammatory responses. Int J Exp Pathol. 1992;73:709–720. [PMC free article] [PubMed] [Google Scholar]
- 8.Chen W, Havell E A, Moldawer L L, McIntyre K W, Chizzonite R A, Harmsen A G. Interleukin 1: an important mediator of host resistance against Pneumocystis carinii. J Exp Med. 1992;176:713–718. doi: 10.1084/jem.176.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen W, Havell E A, Harmsen A G. Importance of endogenous tumor necrosis factor alpha and gamma interferon in host resistance against Pneumocystis carinii infection. Infect Immun. 1992;60:1279–1284. doi: 10.1128/iai.60.4.1279-1284.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D’Angio C T, Johnston C J, Wright T W, Reed C K, Finkelstein J N. Chemokine mRNA alterations in newborn and adult mouse lung during acute hyperoxia. Exp Lung Res. 1998;24:685–702. doi: 10.3109/01902149809099588. [DOI] [PubMed] [Google Scholar]
- 11.Driscoll K E. Macrophage inflammatory proteins: biology and role in pulmonary inflammation. Exp Lung Res. 1994;20:473–490. doi: 10.3109/01902149409031733. [DOI] [PubMed] [Google Scholar]
- 12.Greenberger M J, Strieter R M, Kunkel S L, Danforth J M, Laichalk L L, McGillicuddy D C, Standiford T J. Neutralization of macrophage inflammatory protein-2 attenuates neutrophil recruitment and bacterial clearance in murine Klebsiella pneumonia. J Infect Dis. 1996;173:159–165. doi: 10.1093/infdis/173.1.159. [DOI] [PubMed] [Google Scholar]
- 13.Gupta S, Feng L, Yoshimura T, Redick J, Man Fu S, Rose C E. Intra-alveolar macrophage-inflammatory peptide 2 induces rapid neutrophil localization in the lung. Am J Respir Cell Mol Biol. 1996;15:656–663. doi: 10.1165/ajrcmb.15.5.8918372. [DOI] [PubMed] [Google Scholar]
- 14.Haelens A, Wuyts A, Proost P, Struyf S, Opdenakker G, Van Damme J. Leukocyte migration and activation by murine chemokines. Immunobiology. 1996;195:499–521. doi: 10.1016/S0171-2985(96)80019-2. [DOI] [PubMed] [Google Scholar]
- 15.Hobbs M V, Weigle W O, Noonan D J, Torbett B E, McEvilly R J, Koch R J, Cardenas G J, Ernst D N. Patterns of cytokine gene expression by CD4+ T cells from young and old mice. J Immunol. 1993;150:3602–3614. [PubMed] [Google Scholar]
- 16.Huffnagle G B, Strieter R M, Standiford T J, McDonald R A, Burdick M D, Kunkel S L, Toews G B. The role of monocyte chemotactic protein-1 (MCP-1) in the recruitment of monocytes and CD4+ T cells during a pulmonary Cryptococcus neoformans infection. J Immunol. 1995;155:4790–4797. [PubMed] [Google Scholar]
- 17.Jiang Y, Beller D I, Frendl G, Graves D T. Monocyte chemoattractant protein-1 regulates adhesion molecule expression and cytokine production in human monocytes. J Immunol. 1992;148:2423–2428. [PubMed] [Google Scholar]
- 18.Johnston C J, Wright T W, Rubin P, Finkelstein J N. Alterations in the expression of chemokine mRNA levels in fibrosis-resistant and sensitive mice after thoracic irradiation. Exp Lung Res. 1998;24:321–337. doi: 10.3109/01902149809041538. [DOI] [PubMed] [Google Scholar]
- 19.Kelner G S, Kennedy J, Bacon K B, Kleyensteuber S, Largaespada D A, Jenkins N A, Copeland N G, Bazan J F, Moore K W, Schall T J, Zlotnik A. Lymphotactin: a cytokine that represents a new class of chemokine. Science. 1994;266:1395–1399. doi: 10.1126/science.7973732. [DOI] [PubMed] [Google Scholar]
- 20.Kwon O J, Jose P J, Robbins R A, Schall T J, Williams T J, Barnes P J. Glucocorticoid inhibition of RANTES expression in human lung epithelial cells. Am J Respir Cell Mol Biol. 1995;12:488–496. doi: 10.1165/ajrcmb.12.5.7537968. [DOI] [PubMed] [Google Scholar]
- 21.Lanken P N, Minda M, Pietra G G, Fishman A P. Alveolar response to experimental Pneumocystis carinii pneumonia in the rat. Am J Pathol. 1980;99:561–588. [PMC free article] [PubMed] [Google Scholar]
- 22.Lima M F, Zhang Y, Villalta F. Beta-chemokines that inhibit HIV-1 infection of human macrophages stimulate uptake and promote destruction of Trypanosoma cruzi by human macrophages. Cell Mol Biol. 1997;43:1067–1076. [PubMed] [Google Scholar]
- 23.Limper A H, Offord K P, Smith T F, Martin W J., II Pneumocystis carinii pneumonia: differences in lung parasite number and inflammation in patients with and without AIDS. Am Rev Respir Dis. 1989;140:1204–1209. doi: 10.1164/ajrccm/140.5.1204. [DOI] [PubMed] [Google Scholar]
- 24.Lloyd A R, Oppenheim J J, Kelvin D J, Taub D D. Chemokines regulate T cell adherence to recombinant adhesion molecules and extracellular matrix proteins. J Immunol. 1996;156:932–938. [PubMed] [Google Scholar]
- 25.Marfaing-Koka A, Maravic M, Humbert M, Galanaud P, Emilie D. Contrasting effects of IL-4, IL-10 and corticosteroids on RANTES production by human monocytes. Int Immunol. 1996;8:1587–1594. doi: 10.1093/intimm/8.10.1587. [DOI] [PubMed] [Google Scholar]
- 26.McKee C M, Penno M B, Cowman M, Burdick M D, Strieter R M, Bao C. Hyaluronan (HA) fragments induce chemokine gene expression in alveolar macrophages: the role of HA size and CD44. J Clin Invest. 1996;98:2403–2413. doi: 10.1172/JCI119054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Melton D A, Krieg P A, Rebagliati M R, Maniatis T, Zinn K, Green M R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984;12:7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neilson E G, Krensky A M, O’Farrell S C, Sun M J, Meyers C, Wolf G, Heeger P S. Isolation and characterization of a cDNA from renal tubular epithelium encoding murine RANTES: a small intercrine from the Scy superfamily. Kidney Int. 1992;41:220–225. doi: 10.1038/ki.1992.31. [DOI] [PubMed] [Google Scholar]
- 29.O’Riordan D M, Standing J E, Limper A H. Pneumocystis carinii glycoprotein A binds macrophage mannose receptors. Infect Immun. 1995;63:779–784. doi: 10.1128/iai.63.3.779-784.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paine R, III, Rolfe M W, Standiford T J, Burdick M D, Rollins B J, Strieter R M. MCP-1 expression by rat type II alveolar epithelial cells in primary culture. J Immunol. 1993;150:4561–4570. [PubMed] [Google Scholar]
- 31.Pottratz S T, Paulsrud J, Smith J S, Martin W J., II Pneumocystis carinii attachment to cultured lung cells by Pneumocystis gp120, a fibronectin binding protein. J Clin Invest. 1991;88:403–407. doi: 10.1172/JCI115318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Powell N, Humbert M, Durham S R, Assoufi B, Kay A B, Corrigan C J. Increased expression of mRNA encoding RANTES and MCP-3 in the bronchial mucosa in atopic asthma. Eur Respir J. 1996;9:2454–2460. doi: 10.1183/09031936.96.09122454. [DOI] [PubMed] [Google Scholar]
- 33.Rollins B J. Chemokines. Blood. 1997;90:909–928. [PubMed] [Google Scholar]
- 34.Rosen M J. Pulmonary complications of HIV infection. MSJM. 1992;59:263–270. [PubMed] [Google Scholar]
- 35.Roths J B, Marshall J D, Allen R D, Carlson G A, Sidman C L. Spontaneous Pneumocystis carinii pneumonia in immunodeficient mutant scid mice. Am J Pathol. 1990;136:1173–1186. [PMC free article] [PubMed] [Google Scholar]
- 36.Roths J B, Sidman C L. Both immunity and hyperresponsiveness to Pneumocystis carinii result from transfer of CD4+ but not CD8+ T cells into severe combined immunodeficiency mice. J Clin Invest. 1992;90:673–678. doi: 10.1172/JCI115910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sakai S, Ochiai H, Kawamata H, Kogure T, Shimada Y, Nakajima K, Terasawa K. Contribution of tumor necrosis factor alpha and interleukin-1 alpha on the production of macrophage inflammatory protein-2 in response to respiratory syncytial virus infection in a murine macrophage cell line, RAW 264.7. J Med Virol. 1997;53:145–149. [PubMed] [Google Scholar]
- 38.Schall T J, Jongstra J, Dyer B J, Jorgensen J, Clayberger C, Davis M M, Krensky A M. A human T-cell specific molecule is a member of a new gene family. J Immunol. 1988;141:1018–1025. [PubMed] [Google Scholar]
- 39.Sprenger H, Meyer R G, Kaufmann A, Bussfeld D, Rischkowsky E, Gemsa D. Selective induction of monocyte and not neutrophil-attracting chemokines after influenza A virus infection. J Exp Med. 1996;184:1191–1196. doi: 10.1084/jem.184.3.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Standiford T J, Kunkel S L, Phan S H, Rollins B J, Strieter R M. Alveolar macrophage-derived cytokines induce monocyte chemoattractant protein-1 expression from human pulmonary type II-like epithelial cells. J Biol Chem. 1991;266:9912–9918. [PubMed] [Google Scholar]
- 41.Standiford T J, Kunkel S L, Greenberger M J, Laichalk L L, Strieter R M. Expression and regulation of chemokines in bacterial pneumonia. J Leukoc Biol. 1996;59:24–28. doi: 10.1002/jlb.59.1.24. [DOI] [PubMed] [Google Scholar]
- 42.Tanaka Y, Adams D H, Hubscher S, Hirano H, Siebenlist U, Shaw S. T cell adhesion induced by proteoglycan-immobilized cytokine MIP-1β. Nature. 1993;361:79–82. doi: 10.1038/361079a0. [DOI] [PubMed] [Google Scholar]
- 43.Taub D D, Ortaldo J R, Turcovski-Corrales S M, Key M L, Longo D L, Murphy W J. β chemokines costimulate lymphocyte cytolysis, proliferation, and lymphokine production. J Leukoc Biol. 1996;59:81–89. doi: 10.1002/jlb.59.1.81. [DOI] [PubMed] [Google Scholar]
- 44.Taub D D, Turcovski-Corrales S M, Key M L, Longo D L, Murphy W J. Chemokines and T lymphocyte activation. I. β chemokines costimulate human T lymphocyte activation in vitro. J Immunol. 1996;156:2095–2103. [PubMed] [Google Scholar]
- 45.Vaddi K, Newton R C. Regulation of monocyte integrin expression by beta-family chemokines. J Immunol. 1994;153:4721–4732. [PubMed] [Google Scholar]
- 46.Walzer P D, Perl D P, Krogstad D J, Rawson P G, Schultz M G. Pneumocystis carinii pneumonia in the United States: epidemiologic, diagnostic and clinical features. Ann Intern Med. 1974;80:83–89. doi: 10.7326/0003-4819-80-1-83. [DOI] [PubMed] [Google Scholar]
- 47.Wright T W, Johnston C J, Harmsen A G, Finkelstein J N. Analysis of cytokine mRNA profiles in the lungs of Pneumocystis carinii-infected mice. Am J Respir Cell Mol Biol. 1997;17:491–500. doi: 10.1165/ajrcmb.17.4.2851. [DOI] [PubMed] [Google Scholar]