Abstract
Wheat germ agglutinin (WGA), a lectin with specificity for N-acetylglucosamine and sialic acid, was investigated with respect to its ability to activate the NADPH-oxidase of in vivo-exudated neutrophils (obtained from a skin chamber), and the activity was compared to that of peripheral blood neutrophils. The exudate cells responded to WGA, by both releasing reactive oxygen species into the extracellular milieu and producing oxygen metabolites intracellularly. The peripheral blood cells were unresponsive. To mimic the in vivo-exuded neutrophils with regards to receptor exposure, peripheral blood neutrophils were induced to mobilize their granules and vesicles to varying degrees (in vitro priming), prior to challenge with WGA. The oxidative response to WGA increased with increasing levels of granule mobilization, and the receptor(s) could be shown to reside in the secretory vesicles and/or the gelatinase granules in resting neutrophils. Several WGA-binding glycoproteins were detected in subcellular fractions containing these organelles. The extra- and intracellular NADPH-oxidase responses showed differences in sialic acid dependency, indicating that these two responses are mediated by different receptor structures.
Many neutrophil functions involve lectin-carbohydrate interactions, e.g., neutrophil adhesion to the endothelium initially relies on the action of selectins (carbohydrate-binding proteins that mediate the attachment of leukocytes to endothelial carbohydrates [46]) and recognition and phagocytosis of bacteria in the absence of opsonins most often involve microbial lectins binding to neutrophil glycoconjugate receptors, a process called lectinophagocytosis (41). Also plant lectins possess biological activities in relation to mammalian cells and have been used to study a wide variety of biological processes. Many plant lectins are toxic, some may aggregate erythrocytes of different blood groups (hemagglutination), and yet others function as mitogens of T and B lymphocytes (42, 45, 52). In relation to neutrophils, lectins with different carbohydrate specificities, e.g., concanavalin A binding to mannose and wheat germ agglutinin (WGA) binding to N-acetylglucosamine (GlcNAc) and sialic acid, have been shown to induce cellular responses (10, 22, 34, 39).
Human neutrophil granulocytes depend on many adhesion-related events in order to be able to fulfill their major task of eliminating foreign intruders by ingestion and killing. A prerequisite for the proper function of the neutrophil is thus their capability to mobilize new adhesion molecules and receptors to the cell surface in addition to releasing proteolytic enzymes and inflammatory mediators in the vicinity of the cell. The receptor delivery system consists of at least three different subcellular organelles: the secretory vesicles, the gelatinase granules, and the specific granules, which can be mobilized to the cell surface, merging with the plasma membrane, in a hierarchical manner. Among the membrane components of these intracellular organelles are adhesion molecules, receptors for opsonins and chemoattractants, and receptors for bacteria (4, 27, 29). Most studies on neutrophil activation have been conducted on cells isolated from peripheral blood. However, the neutrophils exert their function in vivo mainly after extravasation into the tissue. This process is accompanied by an increased surface expression of various receptors concomitant with a loss of other surface markers as well as granule matrix components (48). The fact that neutrophil extravasation is associated with quantitative as well as qualitative changes in the cellular responsiveness to external stimuli (6, 18, 28) further stresses the importance in neutrophil research of performing functional studies not only on resting peripheral blood neutrophils but also on cells that have mobilized their intracellular receptor stores. In the context of lectin-phagocyte interaction and the requirement for receptor mobilization, we have recently shown that galectin-3, a mammalian, lactose-specific lectin, activates the neutrophil respiratory burst in exudated cells, whereas no response is induced in peripheral blood cells (28). We could also demonstrate that the galectin-3-induced activation is dependent on granule mobilization. The present study was conducted to analyze the effect of granule mobilization on the ability of WGA to induce respiratory burst activity in neutrophils. This lectin has earlier been shown to be a potent activator of neutrophil oxidative burst, provided that cytochalasin B was present during the interaction (39), indicating that this lectin activates neutrophils, provided that the cells can degranulate. This fact encouraged the use of WGA as a model activator for studies of mobilizable receptor structures.
MATERIALS AND METHODS
Isolation of phagocytic cells.
Exuded neutrophils were harvested from skin chambers placed on unroofed skin blister lesions on the volar surfaces of the forearms of healthy human volunteers as previously described (6, 18). In each experiment, two chambers with three 0.6-ml wells covering the lesions were used. The chambers were filled with an autologous serum, and the neutrophils were allowed to accumulate in the chamber for 24 h. More than 95% of the cells harvested from the chambers were neutrophils.
Peripheral blood neutrophils were isolated from buffy coats obtained from the blood bank at Sahlgren’s University Hospital, Göteborg, Sweden. After dextran sedimentation at 1 × g and hypotonic lysis of the remaining erythrocytes, the neutrophils were separated from mononuclear leukocytes by centrifugation in a Ficoll-Paque gradient (5).
The neutrophils were washed twice and resuspended in Krebs-Ringer phosphate buffer containing glucose (10 mM), Ca2+ (1 mM), and Mg2+ (1.5 mM) (KRG; pH 7.3). The cells were stored on ice and used within 4 h.
Depletion of sialic acid.
Neutrophils were incubated with Clostridium perfringens neuraminidase (0.2 U/ml in KRG) on ice for 5 min to detach terminal sialic acid residues, after which the cells were washed once. The function of the neuraminidase was routinely controlled by measuring the cellular response to a GalNAcβ-binding Actinomyces naeslundii strain, a response that is dramatically increased after the removal of sialic acid on the neutrophils, due to the unmasking of a GalNAcβ-containing receptor (unpublished observation and reference 47).
Mobilization of subcellular organelles.
Three different protocols were used for the mobilization of neutrophil subcellular organelles. The secretory vesicles were partly mobilized by incubating peripheral blood cells at room temperature (22°) for 1 h without an additive (1). The secretory vesicles were fully mobilized by stimulation with the chemoattractant formyl-methionyl-leucyl-phenylalanine (fMLP). Cells were incubated at 15°C for 5 min, after which fMLP (10−7 M final concentration) was added and the incubation was continued for another 10 min. The cells were transferred to a heated water bath (37°C) and were allowed to incubate for 5 min. This treatment (referred to as fMLP 5′) results in degranulation without activating the NADPH-oxidase (38). The third protocol used the same fMLP-induced mobilization as described above, the only difference being a prolonged incubation at 37°C (10 min; referred to as fMLP 10′) which further increased the degranulation. All cell populations were sedimented by centrifugation, and the supernatants were collected for marker analysis. The cell pellets were suspended in KRG, washed once, resuspended to 107 cells/ml in KRG and stored on ice until use.
The mobilization of subcellular organelles was followed by measuring the exposure of complement receptors 1 and 3 (CR1 and CR3, respectively) on the neutrophil surface as well as determining the release of gelatinase and vitamin B12-binding protein into the supernatant. CR1 was measured by labeling the cells with mouse anti-human CD35 (M0710; 10 μl to a cell pellet of 106 cells; Dakopatts) and subsequent binding of fluorescein isothiocyanate-conjugated goat anti-mouse immunoglobulin (F0479; 1/2000; Dakopatts). To measure CR3 exposure, the cells were labeled with phycoerythrin-conjugated monoclonal antibodies specific for CD11b (M741; 10 μl to a cell pellet of 106 cells; Dakopatts). The cells were examined by a FACScan (Becton Dickinson, Mountain View, Calif. [35]). The level of vitamin B12-binding protein was determined with the cyanocobalamin technique as described by Gottlieb et al. (20), and gelatinase was measured by using an enzyme-linked immunosorbent assay (ELISA) method (31).
Neutrophil respiratory burst activity.
The NADPH-oxidase activity was measured by using luminol- or isoluminol-enhanced chemiluminescence (CL) systems (14, 36). The CL activity was measured in a six-channel Biolumat LB 9505 apparatus (Berthold Co., Wildbad, Germany), with disposable 4-ml polypropylene tubes. Samples (0.1 ml; 106 cells) were withdrawn from the neutrophil suspensions, added to the reaction mixture (0.8 ml; see below), and placed in the Biolumat apparatus to be equilibrated for 5 min at 37°C. The stimulus (0.1 ml of WGA in KRG; 20 μg/ml) was added, and the light emission was recorded continuously. In order to quantify intracellularly and extracellularly generated reactive oxygen species, two different reaction mixtures were used (for a review see reference 14). Tubes used for the measurement of the extracellular release of the reactive oxygen species contained neutrophils, horseradish peroxidase (HRP; a cell-impermeable peroxidase; 4 U), and isoluminol (a cell-impermeable CL substrate; 2 × 10−5 M) (36). Tubes used for the measurement of the intracellular generation of reactive oxygen species contained neutrophils, superoxide dismutase (SOD; a cell-impermeable scavenger for O2−; 50 U), catalase (a cell-impermeable scavenger for H2O2; 2,000 U), and luminol (a cell-permeable CL substrate; 2 × 10−5 M).
Subcellular fractionation.
Subcellular fractionation was performed according to the method described by Borregaard et al. (3) with some modifications. In short, neutrophils were disrupted by nitrogen cavitation (Parr Instrument Company, Moline, Ill.), and the postnuclear supernatant was centrifuged on Percoll gradients. Plasma membranes were separated from secretory vesicles by using a flotation gradient as previously described (13). Gelatinase granules were separated from the classical specific granules as described by Kjeldsen et al. (32). The gradients were collected in 1.5-ml fractions by aspiration from the bottom of the centrifuge tube, and the localization of subcellular organelles in the gradient was determined by marker analysis of the fractions. The level of vitamin B12-binding protein (marker for the specific granules) was determined with the cyanocobalamin technique as described by Gottlieb et al. (20). Gelatinase (marker for the specific and gelatinase granules) and myeloperoxidase (MPO; marker for the azurophil granules) levels were measured by using ELISA methods (31, 49). The alkaline phosphatase (marker for secretory vesicles and plasma membranes) level was measured by hydrolysis of p-nitrophenyl phosphate (2 mg/ml) in the presence or absence of Triton X-100 (0.4%) (15). The HLA class I antigen (marker for the plasma membrane) level was determined by a mixed ELISA (2).
SDS-PAGE, Western blotting, and WGA overlay.
Samples of the plasma membrane (γ2), the secretory vesicles (γ1), the gelatinase granules (β2), and the specific granules (β1) were diluted in a nonreducing sample buffer, boiled for 5 min, and applied to the gels in volumes corresponding to the fractionated content of 5 × 105 cells. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed according to the method of Laemmli (33) in 5 to 20% linear gradient polyacrylamide gels. After electrophoresis the proteins were transferred to polyvinylidene difluoride (PVDF) membranes by using a Tris-glycine buffer system (7). The PVDF replicas were blocked in phosphate-buffered saline (PBS)–Tween (0.05% Tween 20) containing gelatin (3% [wt/vol]) at 4°C overnight before being incubated with WGA-HRP (1 μg/ml) in PBS-Tween containing gelatin (1% [wt/vol]) at 4°C overnight. After the replicas were washed (6 times for 5 min each) in PBS-Tween the bound WGA-HRP was detected by using a peroxidase substrate (VIP kit; Vector). The reaction was stopped by extensive washing of the replicas in water.
Inhibitors.
With the exception of ethanol, the inhibitory substances were diluted in dimethyl sulfoxide and stored in a stock solution at −20°C. Fresh working solutions in KRG were prepared new for every experiment. When the inhibitors were used during measurements of superoxide anion, they were added to the samples during equilibration and were preincubated with the cells for appropriate times (see Table 1), after which WGA (2 μg/ml) was added.
TABLE 1.
Effects of different inhibitors on WGA-induced NADPH-oxidase activity in preactivated neutrophils
| Inhibitor (concn) | % (± SD) of control responsea
|
No. of expts | |
|---|---|---|---|
| Extracellular O2− production | Intracellular O2− production | ||
| Staurosporine (100 nM) | 2.7 ± 1.7 | 0.6 ± 0.5 | 3 |
| zLYCK (10 μM) | 22 ± 17 | 30 ± 27 | 3 |
| Ethanol (1%) | 36 ± 36 | 31 ± 20 | 4 |
| Okadaic acid (100 nM) | 414 ± 348 | 147 ± 64 | 4 |
| Calyculin A (300 nM) | 300 ± 108 | 22 ± 4 | 2 |
| Wortmannin (100 nM) | 8.5 ± 1.7 | 4.3 ± 0.7 | 3 |
| Pertussis toxin (0.5 μg/ml) | NDb | 33 ± 16 | 6 |
Primed neutrophils (fMLP 10′) were pretreated at 37°C with the inhibitors for 5 min (staurosporine, zLYCK, and ethanol), 15 min (calyculin A and wortmannin), 30 min (okadaic acid), or 120 min (pertussis toxin), after which WGA was added and the CL was recorded. The neutrophil response to WGA in the presence of the inhibitor is given as a percentage of the response in the control cells (incubated at 37°C for the corresponding time without the inhibitor) calculated from the peak or integral (calyculin A only) values of the responses.
ND, not determined (due to the heat sensitivity of the response).
Reagents.
The p-nitrophenyl phosphate, Triton X-100, fMLP, wortmannin, staurosporine, pertussis toxin, isoluminol, and luminol were obtained from Sigma (Sigma Chemical Co., St. Louis, Mo.). SDS was from Fluka Chemie AG, Buchs, Switzerland. Catalase, SOD, and HRP were purchased from Boehringer (Mannheim, Germany). Dextran, Ficoll-Paque, and Percoll were from Pharmacia (Uppsala, Sweden). WGA and griffonia simplicifolia II (GS-II) were obtained from EY Laboratories, San Mateo, Calif. The molecular weight standard proteins were from Bio-Rad Laboratories, Richmond, Calif. The [57Co]vitamin B12 was supplied by Amersham Laboratories (Little Chalfont, Buckinghamshire, England). Ionomycin and calyculin A were purchased from Calbiochem (La Jolla, Calif.). Carbobenzyloxy-leucine-tyrosine-chloromethylketone (zLYCK) originated from S. Schlegel, University of Geneva, Geneva, Switzerland, and is available from Bachem, Basel, Switzerland. Okadaic acid was a generous gift from Lars Edebo, University of Göteborg, Göteborg, Sweden. Antibodies for the gelatinase ELISA were a kind gift from Lars Kjeldsen and Niels Borregaard, Copenhagen, Denmark.
RESULTS
WGA-induced production of superoxide anion in exudate neutrophils.
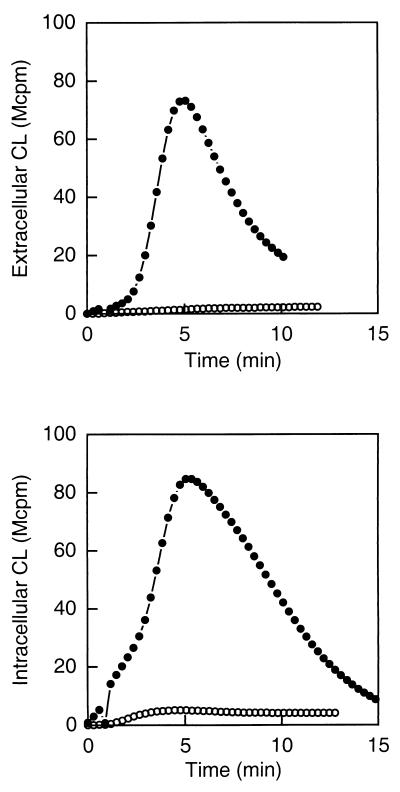
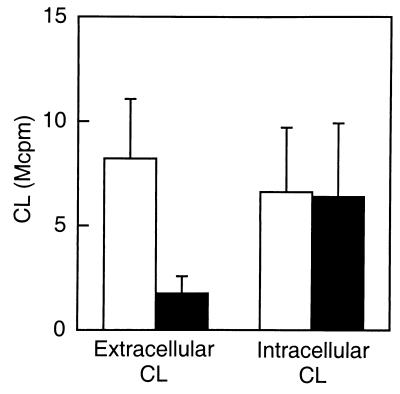
The WGA-induced NADPH-oxidase activity in exudate neutrophils was followed by luminol- or isoluminol-amplified CL. The terminal component of the NADPH-oxidase, the b cytochrome, resides both in the plasma membrane and in the membranes of subcellular granules and vesicles (9, 50). Superoxide anion produced by the plasma membrane-localized NADPH-oxidase will be released from the cell into the extracellular milieu and can be exclusively measured with isoluminol, a membrane-impermeable CL amplifier (36). The superoxide anion produced by the granule-localized NADPH-oxidase can be measured by using the membrane-permeable CL amplifier luminol in combination with SOD and catalase, which consume the extracellularly produced radicals. WGA induced an extensive oxidative response in exudate neutrophils giving rise to superoxide anion production both extra- and intracellularly, while no significant superoxide anion production was detected in the peripheral blood cells (Fig. 1). Thus, the extravasation process transferred the neutrophils from a nonresponding to a responding state with regard to WGA stimulation.
FIG. 1.
WGA-induced NADPH-oxidase activation in exudate and peripheral blood neutrophils. Shown is the time course of the CL response induced by WGA (2 μg/ml) in exudate (closed circles) and peripheral blood (open circles) neutrophils (106 cells). The extracellular responses were measured in the presence of isoluminol (5 × 10−5 M) and HRP (4 U), while the intracellular responses were measured in the presence of luminol (5 × 10−5 M), SOD (50 U), and catalase (2,000 U). The CL is given in increments of 106 cpm (Mcpm). The figure shows a representative experiment (n = 6).
Mobilization of neutrophil granules.
Most of the receptors in peripheral blood neutrophils are localized in mobilizable subcellular organelles such as the secretory vesicles or the gelatinase and specific granules (4). Exudate cells have mobilized their secretory vesicles and parts of their gelatinase granules and specific granules (48), suggesting that the WGA receptors mediating the NADPH-oxidase activation need to be mobilized from at least one of these subcellular organelles in order to be available for lectin binding. To investigate this hypothesis and make an attempt to disclose the identity of the storage organelle(s), the sequential mobilization of vesicles and granules was induced in peripheral blood neutrophils by the use of three different preactivation protocols. The mobilization of membrane proteins to the cell surface, the release of granule matrix proteins, and the ability of WGA to activate the NADPH-oxidase were determined in these preactivated cell populations.
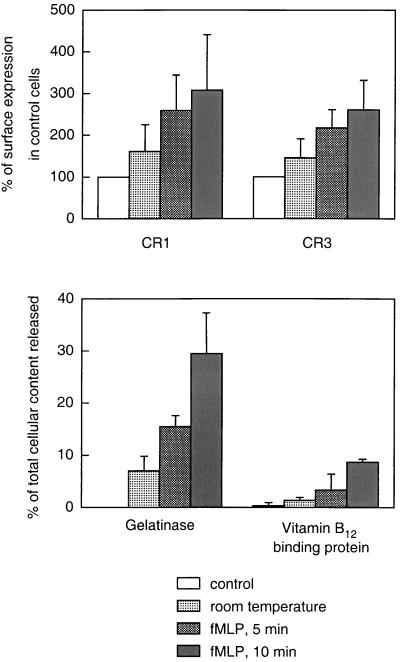
The extent to which neutrophil intracellular organelles were mobilized to the cell surface was assessed by determining the upregulation of CR1 and CR3 on the neutrophil cell surface. In resting cells, CR1 is localized in the secretory vesicles, the most easily mobilized neutrophil organelles. During incubation at room temperature for 1 h, the CR1 expression on the cell surface increased by 50% (Fig. 2). Pretreatment of the cells with fMLP at 15°C and heating to 37°C for 5 and 10 min, respectively, further increased the CR1 expression (around 150 and 200%, respectively). CR3 is localized in both the secretory vesicles, the gelatinase granules, and the specific granules. Thus, CR3 mobilization (Fig. 2) could reflect the degranulation of either of these three organelles. Therefore, the release of specific markers for the gelatinase and specific granules was measured in the extracellular fluid after incubation under the different conditions used. The mobilization of gelatinase granules was monitored by measuring the release of gelatinase (50% of which is localized to these organelles, the rest being localized in the specific granules). Incubation at room temperature for 1 h induced a release of around 7% of the cellular content of gelatinase (Fig. 2). Under these conditions, very little release of vitamin B12-binding protein could be detected (Fig. 2), indicating that the secreted gelatinase was almost entirely derived from the gelatinase granules and not from the specific granules. Thus, room temperature incubation of the cells induced a release of approximately 15% of the gelatinase granules. Incubation with fMLP and heating for 5 min further upregulated the gelatinase granules (around 30%), while the specific granules were almost entirely retained (Fig. 2). The treatment of the peripheral blood cells with fMLP and heating for 10 min mobilized a substantial part of the gelatinase granules together with around 10% of the specific granules, shown by the release of vitamin B12-binding protein (Fig. 2).
FIG. 2.
Effects of different mobilization protocols on the surface exposure of complement receptors and secretion of granule markers. Neutrophils were isolated, resuspended in KRG, and divided into four portions treated as follows: (i) incubated on ice (control), (ii) incubated at 22°C for 1 h, (iii) incubated with fMLP (10−7 M) at 15°C for 10 min and then at 37°C for 5 min, and (iv) incubated with fMLP (10−7 M) at 15°C for 10 min and then at 37°C for 10 min. The top panel shows the surface exposure of the membrane components CR1 (mobilized from the secretory vesicles) and CR3 (mobilized from secretory vesicles, gelatinase granules, and specific granules), calculated from the mean fluorescence value of each cell population and expressed as a percentage of the value obtained with control (resting) cells. The bottom panel shows the release into the medium of markers for gelatinase granules (gelatinase) and specific granules (vitamin B12-binding protein). The values are given as the percent released marker of the total amount in control cells. Data are means plus standard deviations (error bars) from three experiments.
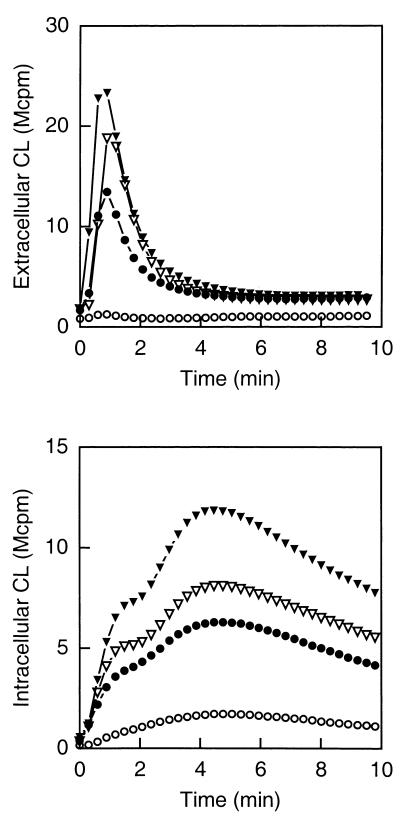
The WGA-induced NADPH-oxidase activity increased in parallel with the mobilization of subcellular organelles, i.e., after incubating the cells at room temperature for 1 h the activity was considerably increased compared to resting cells; after mobilization induced by fMLP and heating for 5 min the activity was markedly higher, and the response was raised even further in cells stimulated with fMLP and heated for 10 min (Fig. 3).
FIG. 3.
Effects of different mobilization protocols on the WGA-induced activation of peripheral blood neutrophils. Shown is the time course of the CL response induced by WGA (2 μg/ml) in peripheral blood neutrophils (105 cells) treated as follows: (i) incubated on ice (control; open circles), (ii) incubated at 22°C for 1 h (closed circles), (iii) incubated with fMLP (10−7 M) at 15°C for 10 min and then at 37°C for 5 min (open triangles), and (iv) incubated with fMLP (10−7 M) at 15°C for 10 min and then at 37°C for 10 min (closed triangles). The extracellular responses were measured in the presence of isoluminol (5 × 10−5 M) and HRP (4 U), while the intracellular responses were measured in the presence of luminol (5 × 10−5 M), SOD (50 U), and catalase (2,000 U). The CL is given in increments of 106 cpm (Mcpm). The figure shows a representative experiment (n = 5).
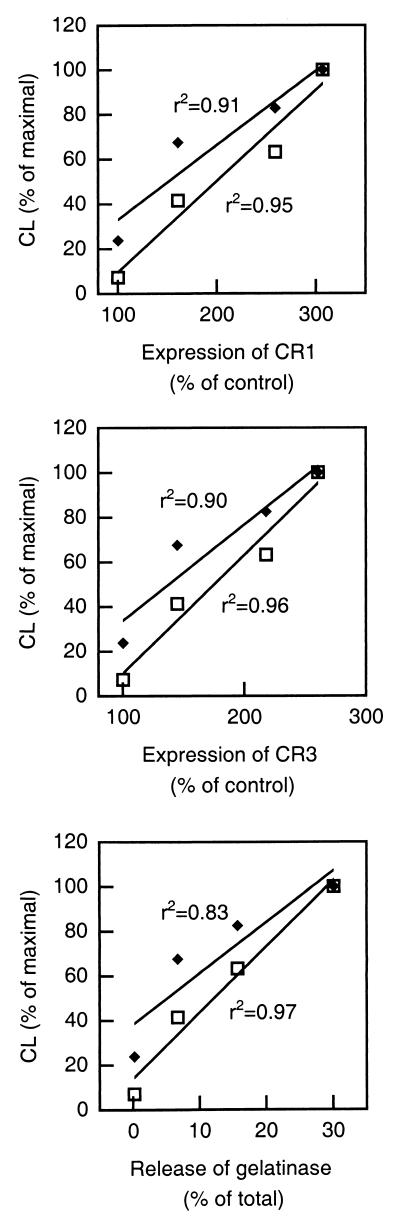
The increase in superoxide anion production in the different cell populations after WGA stimulation correlated with the mobilization of the secretory vesicles (CR1 expression) and the gelatinase granules (release of gelatinase) (Fig. 4), indicating that the receptor responsible for activation probably resides in either or both of these subcellular organelles in resting peripheral blood cells.
FIG. 4.
Correlation between neutrophil degranulation and NADPH-oxidase activity. Mean values (n = 5) of extracellular (open squares) and intracellular (filled diamonds) CL responses obtained with pretreated (22°C, fMLP 5′, and fMLP 10′) neutrophils are plotted against mean values (n = 5) of CR1 mobilization (top), CR3 mobilization (middle), and gelatinase release (bottom). The correlation coefficients (r2) for the calculated linear regressions are given.
Binding of WGA to proteins from subcellular fractions of human neutrophils.
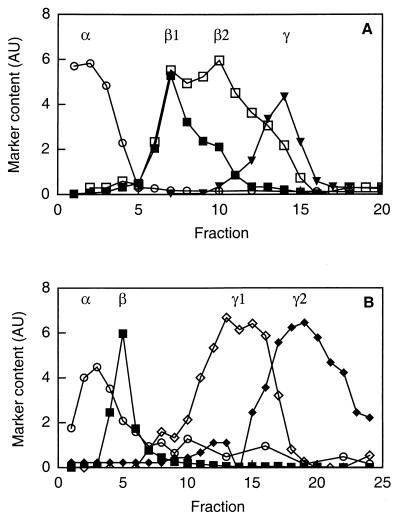
To investigate the presence of WGA-binding proteins in neutrophil subcellular organelles, cells were fractionated on two different Percoll gradients and subcellular fractions corresponding to the specific granules (β1), the gelatinase granules (β2), the secretory vesicles (γ1), and the plasma membrane (γ2) were collected (Fig. 5). Proteins from these fractions were separated by SDS-PAGE and blotted onto a PVDF membrane. WGA-binding glycoproteins were detected by incubation of the replicas with WGA-HRP and subsequent development with a peroxidase substrate.
FIG. 5.
Subcellular fractionation of human neutrophils. Shown is the distribution of marker molecules in discontinuous Percoll gradients. Postnuclear supernatants were fractionated on a three-step gradient (A) or a flotation gradient (B), and fractions of 1.5 ml were collected from the bottoms of the respective centrifuge tubes. The fractions from the three-step gradient were analyzed for myeloperoxidase (marker for azurophil granules; α; open circles), vitamin B12-binding protein (marker for the specific granules; β1; closed squares), gelatinase (marker for specific and gelatinase granules; β1 and β2; open squares), and alkaline phosphatase measured in the presence of detergent (marker for secretory vesicles and plasma membrane; closed triangles). The fractions from the flotation gradient were analyzed for myeloperoxidase, vitamin B12-binding protein, latent alkaline phosphatase (marker for the secretory vesicles; γ1; open diamonds), and HLA class I (marker for the plasma membrane; γ2; closed diamonds). Abscissa, fraction number; ordinate, amount of marker (arbitrary units [AU]).
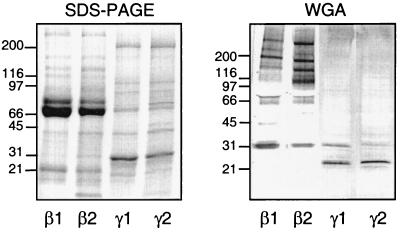
All subcellular fractions showed positive staining for WGA-binding proteins, but the pattern of binding varied significantly (Fig. 6). However, one protein, a 30-kDa WGA-binding protein, was common among the subcellular fractions, although the amount in the plasma membrane was very small compared to the other fractions. In addition, the specific (β1) and gelatinase (β2) granules contained a vast amount of WGA-binding proteins, the major ones being of 195, 140, 120, 65, 60, and 40 kDa. The specific granule (β1) fraction in addition contained a 160-kDa WGA-binding protein, while two proteins of 100 and 50 kDa were specific for the gelatinase granules (β2). The plasma membrane (γ2) and secretory vesicle (γ1) fractions contained a 20-kDa protein as the major WGA-binding protein in addition to the 30-kDa protein.
FIG. 6.
Analysis of neutrophil proteins that are recognized by WGA. Proteins from the neutrophil-specific granules (β1), gelatinase granules (β2), secretory vesicles (γ1), and plasma membrane (γ2), corresponding to the fractionated content of 5 × 105 cells, were separated on SDS-PAGE gels which were stained with Coomassie blue (left) or blotted onto a PVDF membrane (right). The blot was incubated with WGA-HRP (1 μg/ml) and developed with peroxidase substrate (VIP kit; Vector). Molecular sizes are given to the left in kilodaltons.
As stated above, the fact that the NADPH-oxidase responses to WGA gradually increase in parallel with the mobilization of the secretory vesicles and gelatinase or specific granules suggests that the receptor mediating the responses may be present in all mobilizable organelles. This makes the 30-kDa protein an attractive receptor candidate.
Inhibition of the WGA-induced NADPH activity.
The WGA-induced response was determined in the presence of free N-acetylglucosamine (GlcNAc; 10 mM). The GlcNAc totally inhibited both extra- and intracellular CL (data not shown), indicating that WGA indeed interacts with the neutrophils in a carbohydrate-specific manner.
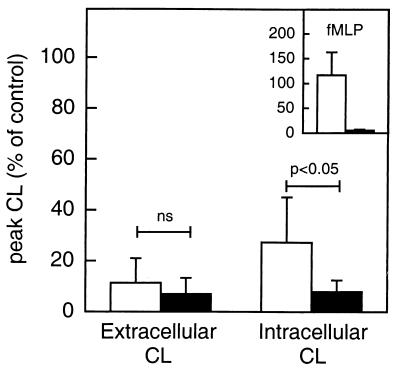
WGA binds not only GlcNAc but also sialic acid. To determine if sialic acid-containing glycoconjugates were involved in the WGA-induced activation of neutrophils, the cells were treated with neuraminidase. The cleavage of sialic acid residues from the cell surfaces of responding neutrophils (shown with fMLP 10′ cells; Fig. 7) significantly inhibited the release of reactive oxygen species (reduced by around 75%), while the intracellular response was not at all affected (Fig. 7). These results indicate that the WGA-induced activation of the oxidase leading to an extracellular release of oxygen metabolites involves a surface glycoconjugate receptor containing sialic acid. In addition, the receptors (or receptor epitopes) mediating the two NADPH-oxidase responses (extra- and intracellular, respectively) can be concluded to be different from each other.
FIG. 7.
Effect of neuraminidase on WGA-induced NADPH-oxidase activation. Responding (fMLP 10′) neutrophils were treated with 0.2 U of neuraminidase per ml for 5 min on ice and washed twice. The results are expressed as the peak values of the CL responses induced by WGA (2 μg/ml) in control (open bars) or neuraminidase-treated (closed bars) neutrophils (105 cells). The extracellular responses were measured in the presence of isoluminol (5 × 10−5 M) and HRP (4 U), while the intracellular responses were measured in the presence of luminol (5 × 10−5 M), SOD (50 U), and catalase (2,000 U). Shown are the means plus standard deviations (error bars) of four experiments. The CL is given in increments of 106 cpm (Mcpm).
Since the intracellular response to WGA was not inhibited by neuraminidase, the interaction causing this response may involve a neutrophil GlcNAc residue functioning as the binding epitope of the receptor. The WGA binding to this residue was mimicked by another lectin, GlcNAc-binding GS-II. However, GS-II (10 μg/ml) did not induce NADPH-oxidase activity in in vitro-primed cells, and neither could this lectin inhibit the activation by WGA (data not shown).
WGA-induced signal transduction events.
The signal transduction pathways engaged by the WGA-binding receptors were analyzed by using inhibitors of different signal-transducing molecules (Table 1).
To investigate whether either of the signal transduction pathways involved a heterotrimeric G-protein, responding cells (fMLP 10′) were preincubated in the presence or absence of pertussis toxin (0.5 μg/ml) for 120 min at 37°C. When the cells were challenged with WGA, the extracellular response was ablated independent of whether pertussis toxin was present or not (Fig. 8). The receptor inducing the activation of plasma membrane-bound NADPH-oxidase thus seems to be sensitive to the prolonged incubation, and consequently no conclusion can be drawn as to the involvement of G-proteins in this response. The intracellular response to WGA was also heat sensitive, although not to the same degree. Pertussis toxin significantly inhibited the remaining intracellular response (Fig. 8), indicating that a heterotrimeric G-protein may be involved in the signaling pathway launched by the WGA receptor to activate intracellular NADPH-oxidase.
FIG. 8.
Effect of pertussis toxin on WGA-induced NADPH-oxidase activation. Responding (fMLP 10′) neutrophils were incubated at 4°C or 37°C in the absence (open bars) or presence (closed bars) of pertussis toxin (0.5 μg/ml) for 120 min prior to activation. The results induced by WGA (2 μg/ml) or fMLP (10−7 M; extracellular response) (inset) in 105 neutrophils are expressed as a percentage of the CL response in control (4°C) cells stimulated correspondingly. The extracellular responses were measured in the presence of isoluminol (5 × 10−5 M) and HRP (4 U), while the intracellular responses were measured in the presence of luminol (5 × 10−5 M), SOD (50 U), and catalase (2,000 U). Shown are the means plus standard deviations (error bars) of six experiments. Student’s t test for paired samples was used for the statistical analysis of the data. ns, not significant.
Protein kinase C activity was required for the WGA-induced NADPH-oxidase activity both intra- and extracellularly, as shown by the sensitivity for staurosporine (Table 1). The inhibition of signaling through phospholipase D, either by the addition of ethanol or by the use of zLYCK (30, 51), inhibited both the extra- and intracellular oxidase activities. Okadaic acid as well as calyculin A was used to inhibit protein phosphatases 1 and 2A. In the presence of these inhibitors, the extracellular response was enhanced, while the intracellular response either was slightly higher than the control (okadaic acid) or decreased (calyculin A). Phosphatidylinositol 3-kinase was involved in both responses as indicated by the sensitivity to wortmannin.
DISCUSSION
This study shows that the binding of WGA to glycoconjugates on human neutrophils isolated after in vivo exudation results in an activation of the superoxide anion and hydrogen peroxide-generating NADPH-oxidase. During the process of extravasation, new receptors are exposed due to the mobilization of intracellular organelles to the cell surface. These organelles are mobilized to different degrees, the secretory vesicles being the most easily mobilized followed by the gelatinase granules and the specific granules, in that order, while the azurophil granules are essentially retained during the extravasation process (48). It has earlier been shown that exudate cells exhibit an enhanced responsiveness to the chemotactic factor fMLP as well as to complement-derived opsonins, and that these functional changes are due to the upregulation of fMLP receptors as well as complement receptors from the vesicle and granule pools (6). In addition, we have recently shown that the human lectin galectin-3 activates exudate neutrophils but not peripheral blood neutrophils due to an increased exposure of galectin-3 receptors on the cell surfaces of exudate cells (28). In light of this, the data presented here can be interpreted to mean that the receptors for WGA are also stored in mobilizable organelles. This is supported by earlier studies of the WGA-induced activation of neutrophil NADPH-oxidase, in which the presence of cytochalasin B was a prerequisite for activation (39). Cytochalasin B is a fungal metabolite known to block motile functions such as locomotion and phagocytosis by inhibiting the polymerization of contractile microfilaments. However, it also facilitates the secretion of granule constituents by removing actin filaments which normally block the access of the granules to the plasma membrane (24). The response to WGA in cytochalasin B-treated cells could thus be explained by a facilitated mobilization of granules associated with an upregulation of intracellular receptors.
To more specifically localize the WGA receptor(s) involved in oxidase activation, three different in vitro protocols were used to sequentially mobilize the vesicles or granules in peripheral blood cells. Both the intra- and extracellular NADPH-oxidase responses induced by WGA increased in parallel with the degree of granule and vesicle mobilization. Based on these results, the receptors mediating the oxidative responses are suggested to be localized in the secretory vesicles and/or the gelatinase granules, possibly with an additional pool in the specific granules.
A number of WGA-binding proteins could be detected in neutrophil subcellular organelle fractions. The literature reports a vast amount of neutrophil proteins isolated by WGA affinity chromatography or binding WGA on protein blots. Several receptors expose WGA-binding carbohydrates, e.g., the fMLP, C5a, interleukin 8, and granulocyte-macrophage colony-stimulating factor (GM-CSF) receptors, as well as Mac-1 (CR3) (8, 16, 21, 26, 40, 44), and some of these may correspond to proteins that are detected by WGA in this study. Whether any of these receptors can be activated not only by their natural ligand but also by a lectin binding to the carbohydrate residues on the receptor remains to be elucidated.
The activation of the NADPH-oxidase can be induced along several different signal transduction pathways. The stimulation of neutrophils with fMLP through the fMLP receptor is a G-protein-dependent event that results in phospholipase C activation leading to the release of intracellular free calcium and protein kinase C activation. The NADPH-oxidase activity launched by fMLP is exclusively extracellular (12). In contrast, the elevation of intracellular calcium by the Ca2+ ionophore ionomycin induces intracellular NADPH-oxidase activation only. Some stimuli such as opsonins from the complement system (25), the mannose-specific lectin concanavalin A (34), and influenza A viruses (23) give rise to an intracellular production of reactive oxygen species upon interaction with neutrophils. The signals generated by WGA mediate both the extracellular release and the intracellular production of superoxide anion, placing this lectin in the same category of stimulants as galectin-3 (28) and protein kinase C activators such as phorbol myristate acetate (37) and dioctanoylglycerol (17).
Based on neuraminidase sensitivity, the activation of the two pools of NADPH-oxidase (the plasma membrane- and specific granule-localized pools mediating the extracellular release and intracellular production of reactive oxygen species, respectively) can be concluded to be induced by different receptor structures, and the intracellular signals induced by these receptors differ, shown by the differences in their sensitivities to inhibitors of signal-transducing molecules. Different protein phosphatases may be involved in the intra- and extracellular responses. Okadaic acid greatly increased the extracellular response, while the intracellular response was much less affected. Calyculin A, on the other hand, induced changes in both the extra- and intracellular responses, although in different directions, enhancing the extracellular response and inhibiting the intracellular. Taken together, these data may indicate that the extracellular response involves the activation of protein phosphatase 2A, which is inhibited by both okadaic acid and calyculin A, while the intracellular response may involve protein phosphatase 1, which is inhibited by calyculin A but not as potently by okadaic acid (11).
The lack of sensitivity of the intracellular response to neuraminidase treatment does not necessarily mean that sialic acid is not involved in this activation. The sensitivity to neuraminidase has been shown to differ depending on the structure of the sialic acid-containing glycoconjugate as well as the surrounding membrane structures, and large fractions of sialic acid may remain on the cell surface after neuraminidase treatment (19, 43). In addition, agonists such as the sialic acid-specific lectin maackia amurensis agglutinin (MAA) as well as sialic acid-binding Actinomyces spp., can also activate cells after neuraminidase treatment (unpublished observation).
In conclusion, the data presented here point to the importance of taking into account the fact that neutrophils exhibit markedly different levels of responsiveness to various stimulating agents, physiological as well as nonphysiological, depending on their states of priming. By responding properly to priming agents, the neutrophils may limit their receptor exposure to include only those receptors needed at one precise moment, and the neutrophil activation and degranulation processes can thus be modulated to limit the inflammatory process.
ACKNOWLEDGMENTS
I thank Per Follin for kind help with the exudate chambers. The skillful technical assistance of Lisbeth Björck and Marie Samuelsson is gratefully acknowledged.
The work was supported by the Fredrik and Ingrid Thuring Foundation, the Swedish Society for Medicine, the Lars Hierta Foundation, the Magnus Bergvall Foundation, and the Anna-Greta Crafoord Foundation for Rheumatological Research.
REFERENCES
- 1.Andersson T, Dahlgren C, Lew P D, Stendahl O. Cell surface expression of fMet-Leu-Phe receptors on human neutrophils. Correlation to changes in the cytosolic free Ca2+ level and action of phorbol myristate acetate. J Clin Investig. 1987;79:1226–1233. doi: 10.1172/JCI112941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bjerrum O W, Borregaard N. Mixed enzyme-linked immunosorbent assay (MELISA) for HLA class I antigen: a plasma membrane marker. Scand J Immunol. 1990;31:305–313. doi: 10.1111/j.1365-3083.1990.tb02773.x. [DOI] [PubMed] [Google Scholar]
- 3.Borregaard N, Heiple J M, Simons E R, Clark R A. Subcellular localization of the b-cytochrome component of the human neutrophil microbicidal oxidase: translocation during activation. J Cell Biol. 1983;97:52–61. doi: 10.1083/jcb.97.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borregaard N, Lollike K, Kjeldsen L, Sengeløv H, Bastholm L, Nielsen M H, Bainton D F. Human neutrophil granules and secretory vesicles. Eur J Haematol. 1993;51:187–198. doi: 10.1111/j.1600-0609.1993.tb00629.x. [DOI] [PubMed] [Google Scholar]
- 5.Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Scand J Lab Investig. 1968;21:77–89. [PubMed] [Google Scholar]
- 6.Briheim G, Coble B, Stendahl O, Dahlgren C. Exudate polymorphonuclear leukocytes isolated from skin chambers are primed for enhanced response to subsequent stimulation with chemoattractant f-Met-Leu-Phe and C3-opsonized yeast particles. Inflammation. 1988;12:141–152. doi: 10.1007/BF00916397. [DOI] [PubMed] [Google Scholar]
- 7.Burnette W N. “Western blotting”: electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981;112:195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- 8.Christiansen N P, Skubitz K M. Identification of the major lectin-binding surface proteins of human neutrophils and alveolar macrophages. Blood. 1988;71:1624–1632. [PubMed] [Google Scholar]
- 9.Clark R A, Leidal K G, Pearson D W, Nauseef W M. NADPH oxidase of human neutrophils. Subcellular localization and characterization of an arachidonate-activatable superoxide-generating system. J Biol Chem. 1987;262:4065–4074. [PubMed] [Google Scholar]
- 10.Cohen M S, Metcalf J A, Root R K. Regulation of oxygen metabolism in human granulocytes: relationship between stimulus binding and oxidative response using plant lectins as probes. Blood. 1980;55:1003–1010. [PubMed] [Google Scholar]
- 11.Cohen P. The structure and regulation of protein phosphatases. Annu Rev Biochem. 1989;58:453–508. doi: 10.1146/annurev.bi.58.070189.002321. [DOI] [PubMed] [Google Scholar]
- 12.Dahlgren C. Difference in extracellular radical release after chemotactic factor and calcium ionophore activation of the oxygen radical-generating system in human neutrophils. Biochim Biophys Acta. 1987;930:33–38. doi: 10.1016/0167-4889(87)90152-2. [DOI] [PubMed] [Google Scholar]
- 13.Dahlgren C, Carlsson S R, Karlsson A, Lundqvist H, Sjölin C. The lysosomal membrane glycoproteins Lamp-1 and Lamp-2 are present in mobilizable organelles, but are absent from the azurophil granules of human neutrophils. Biochem J. 1995;311:667–674. doi: 10.1042/bj3110667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dahlgren C, Follin P, Johansson A, Lock R, Lundqvist H, Walan Å. Chemiluminescence as a means of following the function of phagocytic cells. Trends Photochem Photobiol. 1991;2:427–443. [Google Scholar]
- 15.DeChatelet L R, Cooper M R. A modified procedure for the determination of leukocyte alkaline phosphatase. Biochem Med. 1970;4:61–68. doi: 10.1016/0006-2944(70)90103-1. [DOI] [PubMed] [Google Scholar]
- 16.DiPersio J F, Golde D W, Gasson J D. GM-CSF: receptor structure and transmembrane signaling. Int J Cell Cloning. 1990;8:63–75. doi: 10.1002/stem.5530080707. [DOI] [PubMed] [Google Scholar]
- 17.Follin P, Johansson A, Dahlgren C. Intracellular production of reactive oxygen species in human neutrophils following activation by the soluble stimuli FMLP, dioctanoylglycerol and ionomycin. Cell Biochem Funct. 1991;9:29–37. doi: 10.1002/cbf.290090106. [DOI] [PubMed] [Google Scholar]
- 18.Follin P, Wymann M P, Dewald B, Ceska M, Dahlgren C. Human neutrophil migration into skin chambers is associated with production of NAP-1/IL8 and C5a. Eur J Haematol. 1991;47:71–76. doi: 10.1111/j.1600-0609.1991.tb00564.x. [DOI] [PubMed] [Google Scholar]
- 19.Ganguly P, Fossett N G. Role of surface sialic acid in the interaction of wheat germ agglutinin with human platelets. Biochem Biophys Res Commun. 1979;89:1154–1160. doi: 10.1016/0006-291x(79)92129-6. [DOI] [PubMed] [Google Scholar]
- 20.Gottlieb C, Lau K, Wasserman L R, Herbert V. Rapid charcoal assay for intrinsic factor (IF), gastric juice unsaturated B12 binding capacity, antibody to IF, and serum unsaturated B12 binding capacity. J Hematol. 1965;25:875–883. [PubMed] [Google Scholar]
- 21.Grob P M, David E, Warren T C, DeLeon R P, Farina P R, Homon C A. Characterization of a receptor for human monocyte-derived neutrophil chemitactic factor/interleukin-8. J Biol Chem. 1990;265:8311–8316. [PubMed] [Google Scholar]
- 22.Hartshorn K L, Daigneault D E, White M R, Tauber A I. Anomalous features of human neutrophil activation by influenza A virus are shared by related viruses and sialic acid-binding lectins. J Leukoc Biol. 1992;51:230–236. doi: 10.1002/jlb.51.3.230. [DOI] [PubMed] [Google Scholar]
- 23.Hartshorn K L, Daigneault D E, White M R, Tuvin M, Tauber J L, Tauber A I. Comparison of influenza A virus and formyl-methionyl-leucyl-phenylalanine activation of the human neutrophil. Blood. 1992;79:1049–1057. [PubMed] [Google Scholar]
- 24.Henson P M, Henson J E, Fittchen C, Bratton D L, Riches D W H. Inflammation: basic principles and clinical correlates. 2nd ed. New York, N.Y: Raven Press, Ltd.; 1992. Degranulation and secretion by phagocytic cells; pp. 511–539. [Google Scholar]
- 25.Johansson A, Jesaitis A J, Lundqvist H, Magnusson K E, Sjölin C, Karlsson A, Dahlgren C. Different subcellular localization of cytochrome b and the dormant NADPH-oxidase in neutrophils and macrophages: effect on the production of reactive oxygen species during phagocytosis. Cell Immunol. 1995;161:61–71. doi: 10.1006/cimm.1995.1009. [DOI] [PubMed] [Google Scholar]
- 26.Johnson R J, Simpson S, Van Epps D E, Chenoweth D E. Wheat germ agglutinin inhibits the C5a receptor interaction: implications for receptor microheterogeneity and ligand binding site. J Leukoc Biol. 1992;52:3–10. doi: 10.1002/jlb.52.1.3. [DOI] [PubMed] [Google Scholar]
- 27.Karlsson A, Carlsson S R, Dahlgren C. Identification of the lysosomal membrane glycoprotein Lamp-1 as a receptor for type-1-fimbriated (mannose-specific) Escherichia coli. Biochem Biophys Res Commun. 1996;219:168–172. doi: 10.1006/bbrc.1996.0200. [DOI] [PubMed] [Google Scholar]
- 28.Karlsson A, Follin P, Leffler H, Dahlgren C. Galectin-3 activates the NADPH-oxidase in exudated but not peripheral blood neutrophils. Blood. 1998;91:3430–3438. [PubMed] [Google Scholar]
- 29.Karlsson A, Markfjäll M, Lundqvist H, Strömberg N, Dahlgren C. Detection of glycoprotein receptors on blotting membranes by binding of live bacteria and amplification by growth. Anal Biochem. 1995;224:390–394. doi: 10.1006/abio.1995.1055. [DOI] [PubMed] [Google Scholar]
- 30.Kessels G C, Gervaix A, Lew P D, Verhoeven A J. The chymotrypsin inhibitor carbobenzyloxy-leucine-tyrosine-chloromethylketone interferes with phospholipase D activation induced by formyl-methionyl-leucyl-phenylalanine in human neutrophils. J Biol Chem. 1991;266:15870–15875. [PubMed] [Google Scholar]
- 31.Kjeldsen L, Bjerrum O W, Hovgaard D, Johnsen A H, Sehested M, Borregaard N. Human neutrophil gelatinase: a marker for circulating blood neutrophils. Purification and quantitation by enzyme linked immunosorbent assay. Eur J Haematol. 1992;49:180–191. doi: 10.1111/j.1600-0609.1992.tb00045.x. [DOI] [PubMed] [Google Scholar]
- 32.Kjeldsen L, Sengeløv H, Lollike K, Nielsen M H, Borregaard N. Isolation and characterization of gelatinase granules from human neutrophils. Blood. 1994;83:1640–1649. [PubMed] [Google Scholar]
- 33.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 34.Lock R, Johansson A, Orselius K, Dahlgren C. Analysis of horseradish peroxidase-amplified chemiluminescence produced by human neutrophils reveals a role for the superoxide anion in the light emitting reaction. Anal Biochem. 1988;173:450–455. doi: 10.1016/0003-2697(88)90213-8. [DOI] [PubMed] [Google Scholar]
- 35.Lundahl J, Dahlgren C, Eklund A, Hed J, Hernbrand R, Tornling G. Quarts selectively down-regulates CR1 on activated human granulocytes. J Leukoc Biol. 1993;53:99–103. doi: 10.1002/jlb.53.1.99. [DOI] [PubMed] [Google Scholar]
- 36.Lundqvist H, Dahlgren C. Isoluminol-enhanced chemiluminescence: a sensitive method to study the release of superoxide anion from human neutrophils. Free Radic Biol Med. 1996;20:785–792. doi: 10.1016/0891-5849(95)02189-2. [DOI] [PubMed] [Google Scholar]
- 37.Lundqvist H, Follin P, Khalfan L, Dahlgren C. Phorbol myristate acetate induced NADPH-oxidase activity in human neutrophils: only half the story has been told. J Leukoc Biol. 1995;59:270–279. doi: 10.1002/jlb.59.2.270. [DOI] [PubMed] [Google Scholar]
- 38.Lundqvist H, Gustafsson M, Johansson A, Särndahl E, Dahlgren C. Neutrophil control of formylmethionyl-leucyl-phenylalanine induced mobilization of secretory vesicles and NADPH-oxidase activation: effect of an association of the ligand-receptor complex to the cytoskeleton. Biochim Biophys Acta. 1994;1224:43–50. doi: 10.1016/0167-4889(94)90111-2. [DOI] [PubMed] [Google Scholar]
- 39.Magnusson K E, Dahlgren C, Sjölander A. Distinct patterns of granulocyte luminol-dependent chemiluminescence response to lectins WGA and RCA-I. Inflammation. 1988;12:17–24. doi: 10.1007/BF00915888. [DOI] [PubMed] [Google Scholar]
- 40.Malech H L, Gardner J P, Heiman D F, Rosenzweig S A. Asparagine-linked oligosaccharides on formyl peptide chemotactic receptors of human phagocytic cells. J Biol Chem. 1985;260:2509–2514. [PubMed] [Google Scholar]
- 41.Ofek I, Sharon N. Lectinophagocytosis: a molecular mechanism of recognition between cell surface sugars and lectins in the phagocytosis of bacteria. Infect Immun. 1988;56:539–547. doi: 10.1128/iai.56.3.539-547.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oppenheim J J, Rosenstreich D L. Signals regulating in vitro activation of lymphocytes. Prog Allergy. 1976;20:65–194. doi: 10.1159/000398282. [DOI] [PubMed] [Google Scholar]
- 43.Perez H D, Elfman F, Lobo E. Removal of human polymorphonuclear leukocyte sialic acid inhibits reexpression (or recycling) of formyl peptide receptors. J Immunol. 1987;139:1978. [PubMed] [Google Scholar]
- 44.Perez H D, Elfman F, Lobo E, Sklar L, Chenoweth D, Hooper C. A derivative of wheat germ agglutinin specifically inhibits formyl-peptide-induced polymorphonuclear leukocyte chemotaxis by blocking re-expression (or recycling) of receptors. J Immunol. 1986;136:1803–1812. [PubMed] [Google Scholar]
- 45.Pusztai A. Plant lectins. Cambridge, England: Cambridge University; 1991. [Google Scholar]
- 46.Rosen S D, Bertozzi C R. The selectins and their ligands. Curr Opin Cell Biol. 1994;6:663–673. doi: 10.1016/0955-0674(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 47.Sandberg A L, Mudrick L L, Cisar J O, Brennan M J, Mergenhagen S E, Vatter A E. Type 2 fimbrial lectin-mediated phagocytosis of oral Actinomyces spp. by polymorphonuclear leukocytes. Infect Immun. 1986;54:472–476. doi: 10.1128/iai.54.2.472-476.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sengeløv H, Follin P, Kjeldsen L, Lollike K, Dahlgren C, Borregaard N. Mobilization of granules and secretory vesicles during in vivo exudation of human neutrophils. J Immunol. 1995;154:4157–4165. [PubMed] [Google Scholar]
- 49.Sengeløv H, Kjeldsen L, Borregaard N. Control of exocytosis in early neutrophil activation. J Immunol. 1993;150:1535–1543. [PubMed] [Google Scholar]
- 50.Sengeløv H, Nielsen M H, Borregaard N. Separation of human neutrophil plasma membrane from intracellular vesicles containing alkaline phosphatase and NADPH oxidase activity by free flow electrophoresis. J Biol Chem. 1992;267:14912–14917. [PubMed] [Google Scholar]
- 51.Serrander L, Fällman M, Stendahl O. Activation of phospholipase D is an early event in integrin-mediated signalling leading to phagocytosis in human neutrophils. Inflammation. 1996;20:439–450. doi: 10.1007/BF01486745. [DOI] [PubMed] [Google Scholar]
- 52.Sharon N, Lis H. Lectins: cell-agglutinating and sugar-specific proteins. Science. 1972;177:949–959. doi: 10.1126/science.177.4053.949. [DOI] [PubMed] [Google Scholar]