Abstract
Background:
Changes in graft length according to knee flexion and the ideal knee flexion angle at the time of graft fixation for posterolateral corner (PLC) reconstruction have yet to be clearly defined.
Purposes:
To investigate graft length changes according to knee flexion and determine the optimal graft fixation angle for knee flexion in PLC reconstruction.
Study Design:
Descriptive laboratory study.
Methods:
Ten healthy male volunteers underwent computed tomography at varying knee flexion angles (0°, 30°, 45°, 60°, and 90°). The Larson, LaPrade, Arciero, and Kim techniques were performed on 3-dimensional knee models reconstructed from the computed tomography scans. The lengths of each theoretically reconstructed graft were recorded and compared according to knee flexion angle changes.
Results:
In the Larson technique, the lengths of both arms of the sling were the longest at 30° of knee flexion but were not significantly different between 45° and 60° of knee flexion. In the LaPrade, Arciero, and Kim techniques, the length of the lateral collateral ligament arm at 30° of knee flexion was significantly longer than that at other knee flexion angles (P < .05), except at 0° of knee flexion. The length of the popliteus tendon arm in the LaPrade and Kim techniques, and the length of the popliteofibular ligament arm in the Arciero technique, increased with knee flexion and became the longest at 60° of knee flexion (P < .05).
Conclusion:
In the LaPrade, Arciero, and Kim techniques, the lengths of the lateral collateral ligament and popliteus complex component arms were greatest at 30° and 60° of knee flexion, respectively. In the Larson technique, the lengths of the anterior and posterior arms were greatest at 30° of knee flexion. The authors recommend securing each arm of the graft at the point of its greatest length.
Clinical Relevance:
This study presents in vivo data regarding graft length changes according to knee flexion and offers an optimal graft fixation angle for PLC reconstructions through various techniques.
Keywords: lateral/posterolateral knee ligaments, biomechanics of ligament, posterolateral corner reconstruction, graft fixation, graft length, knee flexion angle
The incidence of high-grade posterolateral corner (PLC) injuries is 16% among acute knee ligament injuries. 21 Most PLC injuries (72%-92%) are not isolated and are often combined with other ligament injuries, including those to the anterior cruciate and/or posterior cruciate ligaments.7-9,19,21 Operative treatment for high-grade PLC injury is preferred because nonoperatively treated PLC injuries lead to chronic pain, residual laxity, and osteoarthritic changes to the injured knee.13,25,32 Reconstruction of PLC structures is more successful than repair in terms of failure rate (6%-9% vs 37%-40%).9,23,24,28
The principal structures of the PLC that provide stability for varus, external rotation, and posterior tibial translation of the knee are the lateral (fibular) collateral ligament (LCL), popliteofibular ligament (PFL), and popliteus tendon (PT). Reconstructive procedures for the PLC aim to reproduce at least 2 or all 3 of these structures. Larson 22 originally described fibula-based reconstruction with a single femoral tunnel to create the LCL and PFL arms owing to the isometry from the entire fibular head to the lateral femoral epicondyle. LaPrade et al 17 developed an anatomic tibiofibula-based reconstruction using 2 grafts in an effort to anatomically reconstruct all 3 primary contributors of the PLC. Arciero 1 modified the technique of Larson to reestablish the LCL and PFL, placed more anatomically with 2 femoral tunnels. Kim et al 16 reported tibiofibula-based reconstruction, which re-created the LCL and PT and was biomechanically comparable to the Arciero technique. 12
The main goal of ligament reconstruction is to use a graft to restore the preinjury location and tension of the native ligament, thereby allowing the ligament to ultimately regain its strength and function. Recent advances in the knowledge of the anatomy and biomechanics of the PLC have led to an effort to perform anatomic reconstruction that approximates the native structures.4,17,18,20 However, anatomically reconstructed grafts function as anisometric structures because the tensions of the native PLC structures change with knee flexion.10,26 To obtain the appropriate tension of the anisometric reconstructed graft that can mimic the native structure, considering the graft length (which varies according to the knee flexion angle at the time of graft fixation) is extremely important. An improperly undertensioned graft can lead to residual laxity, 3 whereas an overconstrained graft can lead to loss of knee motion, increased ligamentous laxity, graft attenuation over time, graft breakdown, and increased articular contact pressures. 2
Despite the importance of the knee flexion angle at graft fixation in PLC reconstruction, there has been no consensus on the optimal tensioning angles, as in vivo data regarding graft length changes according to knee flexion are lacking. Therefore, we aimed to investigate graft length changes according to knee flexion angle in a simulated PLC reconstruction using 3-dimensional (3D) reconstructed models of a healthy knee with different knee flexion angles and determine the optimal knee flexion angle at the time of graft fixation according to various reconstruction techniques. We hypothesized that the graft length would change according to knee flexion, that there would be an optimal knee flexion angle at which the graft length would be maximized, and that the graft should be fixed in each reconstruction technique.
Methods
Patients
After obtaining approval from the institutional review board of our hospital, we prospectively recruited 10 healthy male volunteers between May 2022 and July 2023. Written informed consent was obtained from each participant before screening and enrollment. Participants who were 20 years of age or older and had no knee-related symptoms; no ligament, meniscal, or cartilage injuries; no previous trauma or operative history of the knee; no osseous deformity, including varus/valgus malalignment deformity of the lower extremities; and osteoarthritis evaluated from standing knee radiography classified as Kellgren-Lawrence grade <2 were included. 14 The demographic data of the 10 participants at the time of the study were as follows: mean age, 35 years (range, 32-38 years); mean height, 180.2 cm (range, 171-192 cm); mean weight, 75.7 kg (range, 64-101 kg); and mean body mass index, 23.2 (range, 20.6-27.4).
Simulation of PLC Reconstruction
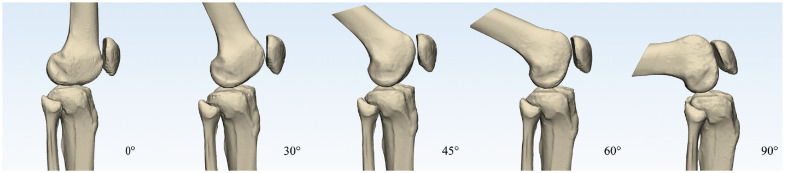
Computed tomography (CT) of the right knee was performed at 0°, 30°, 45°, 60°, and 90° of knee flexion for each participant. The degree of knee flexion was measured using a 12-inch goniometer by a fellowship-trained orthopaedic surgeon (S.-H. J.) at the time of scanning. The knee flexion angle was double-checked according to the CT images using anatomic axes of the femur and tibia after the scan at each knee flexion angle. The CT images were obtained using a high-resolution CT scanner (Sensation 64; Siemens Healthcare) with scan parameters as follows: tube voltage, 120 to 140 kVp; tube current, 86 to 140 mA; acquisition matrix, 512 × 512 pixels; field of view, 195 to 333 mm; and slice thickness, 0.6 to 1 mm. The Digital Imaging and Communications in Medicine data of the CT scan were imported into Mimics software (Version 21; Materialise), and ten 3D knee models with 5 different flexion angles for each patient were constructed (Figure 1).
Figure 1.
In vivo 3-dimensional knee models with 0°, 30°, 45°, 60°, and 90° of knee flexion.
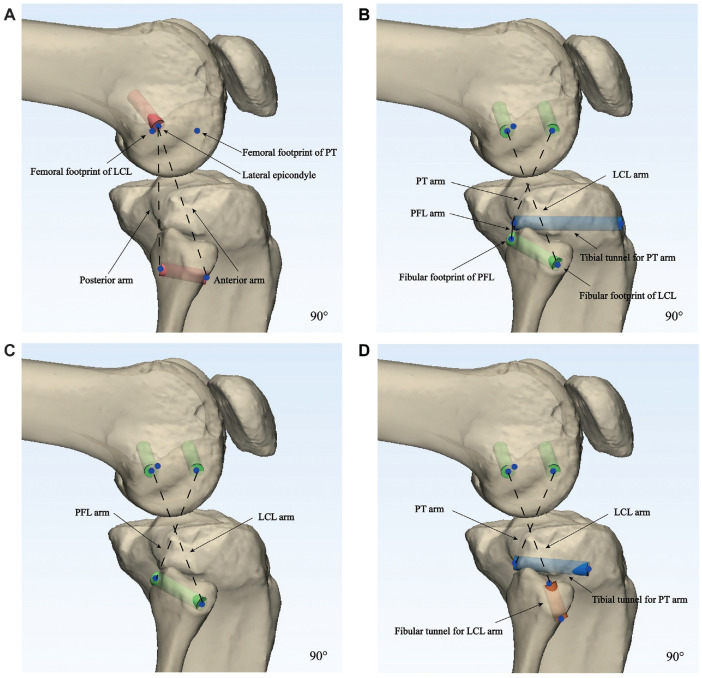
Simulated PLC reconstructions according to the 4 different reconstruction techniques, the Larson, LaPrade, Arciero, and Kim techniques, were performed on the 3D knee models at different knee flexion angles. The footprints of the lateral structures were made based on the bony landmarks and the quantitative locational relationship between the lateral epicondyle and the footprints of the LCL, PFL, and PT, as described in previous studies.5,18 The lateral epicondyle was defined as the most prominent point of the lateral femoral condyle. The femoral footprint of the LCL was set to be approximately 1.4 mm proximal and 3.1 mm posterior to the lateral epicondyle. 18 The fibular footprint of the LCL was defined at approximately 8.2 mm posterior to the anterior edge of the fibular head and 28.4 mm distal to the tip of the fibular styloid process. 18 The femoral origin of the PT was placed at the most anterior one-fifth of the popliteal sulcus, approximately 18.5 mm from the origin of the LCL. 18 For the Larson technique, the femoral tunnel with a diameter of 6 mm was placed at the lateral epicondyle, and the transfibular tunnel was placed at the center of the fibula in an anterior to posterior direction at the level of its maximal diameter (Figure 2A). 22
Figure 2.
Tunnel formation according to the 4 different techniques, the (A) Larson, (B) LaPrade, (C) Arciero, and (D) Kim techniques, for posterolateral corner reconstruction on each 3-dimensional knee model at 90° of knee flexion. The same methods were also applied at 0°, 30°, 45°, and 60° of knee flexion. The dotted lines show the virtual graft arms for each technique. LCL, lateral collateral ligament; PFL, popliteofibular ligament; PT, popliteus tendon.
For the LaPrade technique, the transfibular tunnel for the LCL and PFL arms was made anterolateral to posteromedial from the insertion of the LCL to the attachment site of the PFL on the posteromedial aspect of the fibular styloid adjacent to the proximal tibiofibular joint; the tibial tunnel for the PT and PFL arms was placed on the posterior popliteal tibial sulcus approximately 10 mm distal to the margin of the articular surface (Figure 2B). 17 The transfibular tunnel had a diameter of 6 mm and was adjusted so as not to invade the cortical rim of the fibula in the case of tunnel blowout. Two femoral tunnels with a diameter of 6 mm were created at the femoral footprints of the LCL and PT for the LCL and PT arms, respectively. For the Arciero technique, the transfibular and femoral tunnels were identical to those of the LaPrade technique for the LCL and PFL arms (Figure 2C).1,18 For the Kim technique, the transfibular tunnel for the LCL arm was placed from the anteroinferior portion of the fibula to a point just posteromedial to the LCL, and the tibial tunnel for the PT arm was identical to the tibia tunnel of the LaPrade technique. The femoral tunnels used for the Kim technique were identical to those for the LaPrade technique, at the center of the origin of the LCL and PT (Figure 2D).15,18
Measurement of the Reconstructed Graft Length
The shortest distance between the centers of the graft tunnels in the simulated PLC reconstruction was measured at 5 different knee flexion angles, considering the surface distance of the bony structures of the femur, tibia, and fibula (Figure 3).
Figure 3.
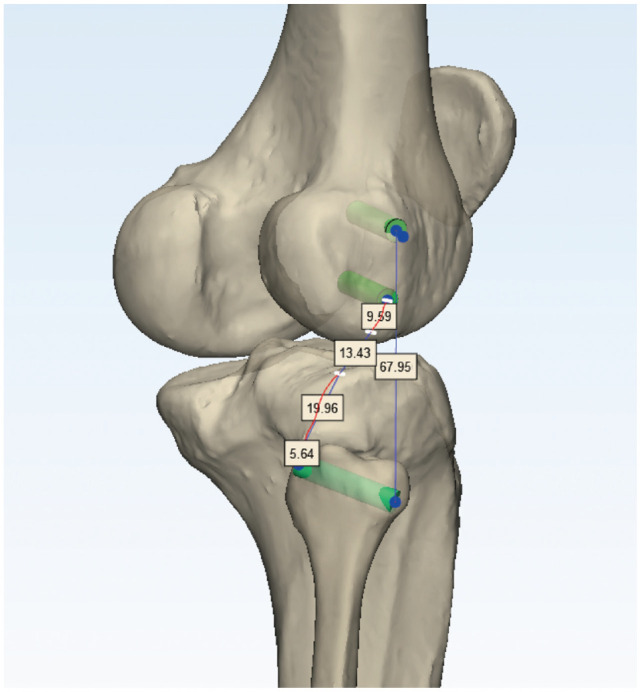
Measurement of the graft length in the simulated posterolateral corner (PLC) reconstruction of the Arciero technique. The shortest distance between the center of the graft tunnels in the simulated PLC reconstruction was measured at 5 different knee flexion angles in consideration of the surface distance of the bony structures of the femur, tibia, and fibula.
For the Larson technique, the lengths of the anterior and posterior arms were measured. For the LaPrade technique, the lengths of the LCL, PFL, and PT arms were measured. For the Arciero technique, the length of the PFL arm was measured. For the Kim technique, the length of the LCL arm was measured. However, the lengths of the LCL arm for the Arciero technique and the PT arm for the Kim technique were not measured because the femoral and tibial footprints were the same as those of the LaPrade technique, respectively. Two different researchers (K.C. and J.H.) measured these values to increase the reliability, and the mean of the 2 numerical values was used. The intraclass correlation coefficients for interobserver reliability of each arm length of the graft were 0.915 (95% CI, 0.799-0.927) for the anterior arm and 0.913 (95% CI, 0.822-0.955) for the posterior arm in the Larson technique; 0.924 (95% CI, 0.864-0.957) for the LCL arm, 0.940 (95% CI, 0.894-0.966) for the PT arm, and 0.944 (95% CI, 0.901-0.968) for the PFL arm in the LaPrade technique; 0.958 (95% CI, 0.0.912-0.979) for the PFL arm in the Arciero technique; and 0.892 (95% CI, 0.795-0.941) for the LCL arm in the Kim technique.
Statistical Analysis
A power analysis was performed using G*Power Version 3.1. 6 The statistical power was 95.9% for the LCL arm of the LaPrade technique. The Shapiro-Wilk test was used for normality. A repeated-measures analysis of variance was used to analyze the differences in graft length changes according to the knee flexion angle in each technique. The Bonferroni test was performed for post hoc analysis to compare each graft arm length at different flexion angles. The interobserver reliability of the measurement of variables was analyzed using an intraclass correlation coefficient set at a 95% confidence interval. Statistical significance was set at P < .05. IBM SPSS Statistics for Windows (Version 26.0; IBM Corp) was used for statistical analyses.
Results
The graft length changed with changes in the knee flexion angle, and each graft arm of the different PLC reconstruction techniques showed a distinct pattern of length changes (Appendix Table A1).
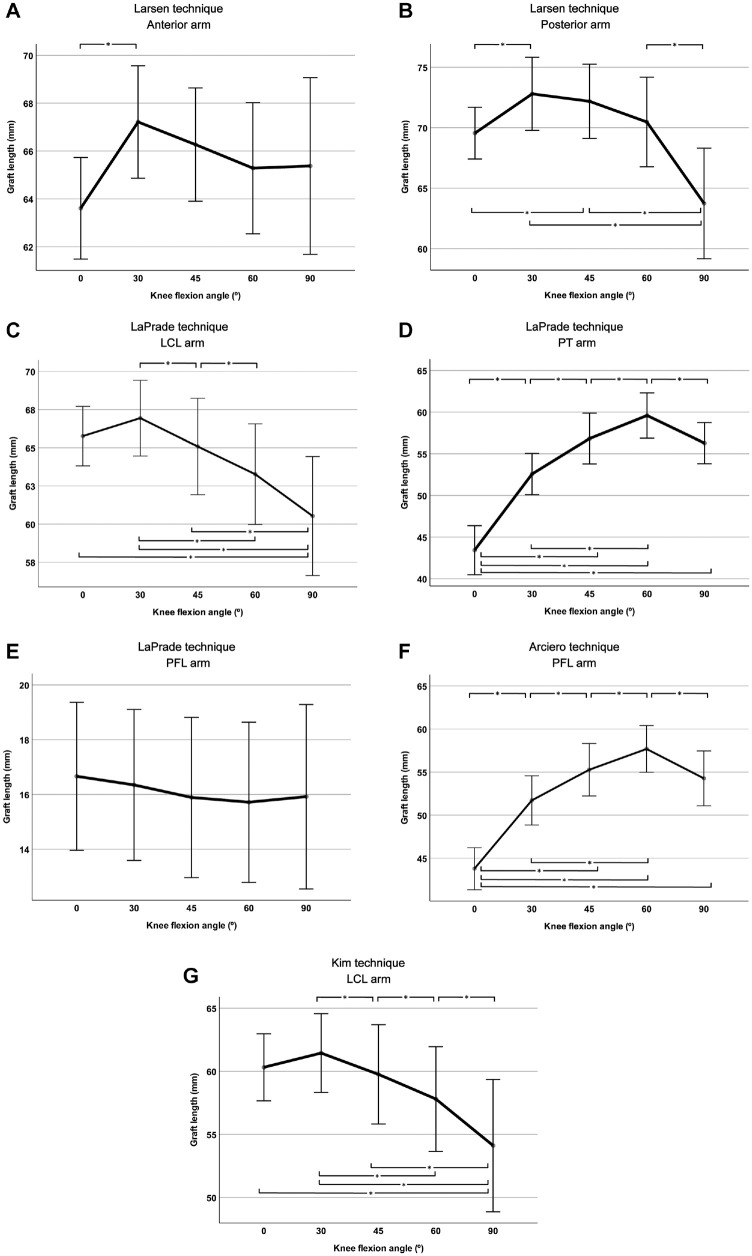
In the Larson technique, the length of the anterior arm of the sling was the longest at 30° of knee flexion and showed a significant difference from the length at 0° of knee flexion (P < .01) but did not significantly differ from the lengths at 45°, 60°, and 90° of knee flexion (P > .05) (Figure 4A).
Figure 4.
Length changes of each graft arm of the 4 different posterolateral corner reconstruction techniques, the (A and B) Larson, (C-E) LaPrade, (F) Arciero, and (G) Kim techniques, according to the knee flexion changes in simulated posterolateral corner reconstructions of an in vivo 3-dimensional knee model. *Statistically significant difference between the knee flexion angles (P < .05). LCL, lateral collateral ligament; PFL, popliteofibular ligament; PT, popliteus tendon.
The length of the posterior arm of the sling was the longest at 30° of knee flexion and was significantly different from that at 0° (P = .031) and 90° (P = .005) (Figure 4B). However, the lengths of the posterior arm of the sling were not significantly different between 30°, 45°, and 60° of knee flexion (P > .05). In the LaPrade and Kim techniques, the lengths of the LCL arm decreased as the knee flexion angle increased (Figure 4, C and G). The lengths of the LCL arm for the LaPrade and Kim techniques at 30° of knee flexion were the longest and were significantly different from the lengths at 45° (P = .045 and P = .047, respectively), 60° (P = .001 and P = .002, respectively), and 90° (P = .003 and P = .003, respectively) of knee flexion but were not different from the length at 0° of knee flexion in both reconstruction techniques. As the knee flexion angle increased from 0° to 60° of knee flexion, the PT arm for the LaPrade technique and the PFL arm for the Arciero technique increased. The lengths of the PT arm for the LaPrade technique and the PFL arm for Arciero technique at 60° of knee flexion were the longest, which differed significantly from the lengths at 0° (P < .001 and P < .001, respectively), 30° (P < .001 and P < .001, respectively), 45° (P = .002 and P = .002, respectively), and 90° (P = .041 and P = .047, respectively) of knee flexion (Figure 4, D and F). For the LaPrade technique, there was no significant difference between the PFL arm lengths at any knee flexion angle (P > .05) (Figure 4E).
Discussion
The most important finding of this study was that the graft should be fixed at different flexion angles for each arm of the anatomic PLC reconstruction techniques, such as the LaPrade, Arciero, and Kim techniques, because the graft length changes with changes in knee flexion (Table 1). To achieve proper tension of the graft that best reproduces the anisometric native ligament, the graft should be fixed at a knee flexion angle at which the tension and length of the native ligament are maximized throughout knee flexion. The length of the LCL arm in the LaPrade, Arciero and Kim techniques was greatest at 30° of knee flexion and decreased with further knee flexion. The length of the PT arms in the LaPrade and Kim techniques and the PFL arm in the Arciero technique increased as the knee flexion angle increased, with the longest length at 60° of knee flexion. Accordingly, the LCL arm should be secured at 30° of knee flexion; the PT arm in the LaPrade and Kim techniques and the PFL arm in the Arciero technique should be fixed at 60° of knee flexion. In contrast, in the Larson technique, we recommend that both arms be fixed at 30° of flexion as they were longest at this flexion angle, although not significantly different from the lengths at 45° or 60°.
Table 1.
Summary of Recommended Graft Fixation Angles a
| Variable | Recommended Knee Flexion Angle of Graft Fixation |
|---|---|
| Larson technique | |
| Anterior and posterior arm of the sling | 30° |
| LaPrade, Arciero, and Kim techniques | |
| LCL component | 30° |
| Popliteus complex components | 60° |
Popliteus complex components include the popliteus tendon arm of the LaPrade and Kim techniques and the popliteofibular ligament (PFL) arm of the Arciero technique. The PFL arm of the LaPrade technique can be fixed at any knee flexion angle. LCL, lateral collateral ligament.
Few studies have investigated the length changes in the PLC structures with respect to changes in knee flexion angle. Wang and Walker 31 demonstrated in their cadaveric study that the length of the LCL constantly diminished by 20% from 0° to 120° with little effect of tibial rotation. Another cadaveric study by Sugita and Amis 29 noted that the LCL became significantly loose as the knee bent. Sigward et al 27 demonstrated that the relative length of all anatomically reconstructed PLC structures, including the LCL, PFL, and PT, increased as the knee flexion angle increased from 0° to 30°, 45°, 70°, and 90° in their cadaveric PLC reconstruction study of optimum isometric femoral fixation sites. The length change patterns in the PFL or PT arm of the graft and the LCL arm in our study were not in agreement with these studies. Although the PFL or PT arm length increased as the knee flexion angle increased, it peaked at 60° of knee flexion and decreased as it reached 90°. The graft length of the LCL arm decreased as the knee flexion angle increased, with the peak length at 30° of knee flexion. The different results among the previous cadaveric studies may be because of the nature of those studies, and the discordance with our results may be because of an inherent distinction between a cadaveric study and an in vivo experiment. The results of the cadaveric studies were subject to the conditions of the specimens and testing environments. The effects of postmortem changes on cadaveric specimens and the use of elderly joints with weak structures are potential disadvantages thereof. Furthermore, the factitious movement of the knee joint using a machine in a cadaveric study may not reflect actual knee biomechanics, and the absence of active tension in dynamic stabilizers around the knee joint, such as the biceps femoris muscles and iliotibial tract, could limit the interpretation of the results.
The force distribution on the PLC structures according to the knee flexion angle was closely related to the graft length during reconstruction. Direct force measurements of individual PLC structures in cadaveric specimens during functional loading provide explicit information about the function of each PLC structure. 20 The loading responses of varus and external rotation forces on the LCL peak at 30° of knee flexion diminish with higher knee flexion from 30° to 90°. The loading responses of external rotation on the PT and PFL increase with knee flexion and peak at 60° of knee flexion. These patterns of force measurement for each PLC structure according to knee flexion angle were very similar to the results of length changes with changes in knee flexion in our study.
In the study by Larson 22 initially describing the prescribed technique, the anterior and posterior arms were fixed at 30° of knee flexion. This angle was based on cadaveric biomechanical studies, which showed that posterolateral structures contribute the most to varus and external rotatory stability at 30° of knee flexion.10,11 This result is in agreement with ours, that the lengths of the anterior and posterior arms were maximized at 30° of knee flexion, although knee flexion angles from 30° to 60° only created a mean length difference of approximately 2 mm. The Larson technique is a nonanatomic reconstruction and is less preferred compared with the anatomic reconstruction techniques among expert groups. 4
LaPrade et al 17 developed the surgical technique for PLC reconstruction, which reconstructed the 3 major stabilizing structures. They placed each arm of the graft at knee flexion angles where the load response of each ligament reached its peak: 30° for the LCL arm and 60° for the PFL and PT arms. 20 Our study findings support the recommendations of LaPrade et al for the knee flexion angle for graft fixation of those 3 components. The LCL and PT arms in the simulated LaPrade technique were greatest at 30° and 60° of knee flexion, respectively, implying that the graft should be fixed at 30° for the LCL arm and 60° for the PT arm, which is concordant with our results. Of note is that the LaPrade technique was designed to fix the PFL and PT arms simultaneously. 17 Because the length of the PFL arm was not significantly different throughout the arc of knee flexion, the fixation of the PFL arm at 60°, as LaPrade et al 17 recommended, seems appropriate.
Arciero 1 recommended concomitantly securing the LCL and PFL arms with approximately 30° of knee flexion. However, cadaveric studies have shown that individual posterolateral structures are tensioned differently throughout the arc of knee flexion. Cadaveric biomechanical studies showed that the LCL became tight when the knee was at or near full extension, acting as a primary varus restraint, and became lax as the knee flexed, while the PFL complex played an important role in tibial external rotation in deep flexion.11,29 Our results also suggest that separate tensioning of the LCL and PFL arms is reasonable because the length of the LCL arm decreases as the knee flexion angle increases and the length of the PFL arm increases as the knee flexion angle decreases. However, it may be beneficial to fix the LCL arm of the graft at 30° of knee flexion and the PFL arm at 60° of knee flexion because the peak length of each arm is reached at different knee flexion angles, although the LCL arm length at 30° was not significantly different from that at 0° of knee flexion.
Kim et al 16 advocated for their technique, re-creating the LCL and PT and fixing the LCL arm at 0° of knee flexion; however, they did not specify the knee flexion angle of graft fixation for the PT arm. Our study showed that the overall results representing the graft length of the LCL and PT arms according to the knee flexion angle in the Kim technique were similar to those of the LCL and PT arms of the LaPrade technique, respectively, which changed the knee flexion angle at the time of graft fixation from 0° to 30° for the LCL arm and 60° for the PT arm.
Limitations
Several limitations of this study should be considered when interpreting its results. First, the sample size was relatively small. However, the statistical power was high (>80%) for the comparison of graft lengths between various knee flexion angles. Second, bony landmarks and quantitative relationships were used to define the footprints of the PLC structures. Although this apporoch was based on solid anatomic evidence, individual variations may exist. Patients of different sizes may have different quantitative relationships among lateral femoral structures. Moreover, magnetic resonance imaging could be better than CT to directly determine the footprints of the PLC structures; however, CT scans have strengths in the determination of the bony landmarks. 30 Clinically, surgeons rely on bony landmarks and their quantitative relationships to identify the footprints and create the aperture for the reconstructed graft. Third, the simulated PLC reconstruction on a 3D intact knee model may not exactly reproduce clinical PLC reconstruction. For instance, the simulated reconstruction presumed that the graft was located in the center of the bone tunnel; however, the graft of the clinical PLC reconstruction could be in an eccentric location in the tunnel, especially when using an interference screw for fixation. Moreover, the positions of the femur and tibia in actual reconstructions on the injured knee, particularly the tibial rotation in combined ligament injuries, could be different from those in the simulation on the intact knee. 10 Despite the shortcomings, using a 3D knee model enabled the measurement of graft length on a healthy knee instead of a cadaveric knee. Fourth, 3D knee models were constructed with fragmentary knee flexion angles rather than continuous angles. Although this is difficult to perform because of various practical restrictions, a study of more subdivided knee flexion angles would be helpful to draw a more straightforward conclusion. Fifth, the study included only men; variations of knee morphology according to sex may exist. Last, the CT scans for the 3D knee models were taken in the supine position, without weightbearing or external stress. Therefore, the scans may not reflect the length or tension of the graft in daily life. However, clinical PLC reconstruction is performed in the supine position, and knee flexion during a CT scan is performed with a healthy knee that reflects actual knee biomechanics; thus, our results could be applied to clinical PLC reconstructions.
Conclusion
In the LaPrade, Arciero, and Kim techniques, the lengths of the LCL and popliteus complex component arms were greatest at 30° and 60° of knee flexion, respectively. In the Larson technique, the lengths of the anterior and posterior arms were greatest at 30° of knee flexion. We recommend securing each arm of the graft at the point of its greatest length.
Appendix Table A1.
Graft Length at Different Flexion Angles a
| Variable | Knee Flexion Angle | P | ||||
|---|---|---|---|---|---|---|
| 0° | 30° | 45° | 60° | 90° | ||
| Larson technique, mm | ||||||
| Anterior arm length | 63.6 ± 3.0 | 67.2 ± 3.3 | 66.3 ± 3.3 | 65.3 ± 3.61 | 65.4 ± 5.2 | .029 |
| Posterior arm length | 69.6 ± 3.0 | 72.8 ± 4.2 | 72.2 ± 4.3 | 70.5 ± 5.2 | 63.7 ± 6.4 | <.001 |
| LaPrade technique, mm | ||||||
| LCL arm length | 65.8 ± 2.7 | 66.9 ± 3.5 | 65.1 ± 4.4 | 63.3 ± 4.6 | 60.5 ± 5.5 | <.001 |
| PT arm length | 43.4 ± 4.1 | 52.6 ± 3.5 | 56.8 ± 4.2 | 59.6 ± 3.8 | 56.3 ± 3.4 | <.001 |
| PFL arm length | 16.7 ± 3.8 | 16.3 ± 3.9 | 15.9 ± 4.1 | 15.7 ± 4.1 | 15.9 ± 4.7 | .086 |
| Arciero technique, mm | ||||||
| PFL arm length | 43.8 ± 3.4 | 51.7 ± 4.0 | 55.3 ± 4.3 | 57.7 ± 3.8 | 54.3 ± 4.4 | <.001 |
| Kim technique, mm | ||||||
| LCL arm length | 60.3 ± 3.7 | 61.4 ± 4.4 | 59.8 ± 5.5 | 57.8 ± 5.8 | 54.1 ± 7.3 | <.001 |
Data are presented as mean ± SD. Bold P values indicate statistical significance (P < .05). The lengths of the lateral collateral ligament (LCL) arm for the Arciero technique and the popliteus tendon (PT) arm for the Kim technique were identical to those of the LCL and PT arms for the LaPrade technique, respectively. PFL, popliteofibular ligament.
Footnotes
Final revision submitted May 21, 2024; accepted May 23, 2024.
The authors declared that they have no conflicts of interest in the authorship and publication of this contribution. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from Yonsei University, Gangnam Severance Hospital (3-2022-0140).
ORCID iD: Sung-Hwan Kim  https://orcid.org/0000-0001-5743-6241
https://orcid.org/0000-0001-5743-6241
References
- 1. Arciero RA. Anatomic posterolateral corner knee reconstruction. Arthroscopy. 2005;21(9):1147. [DOI] [PubMed] [Google Scholar]
- 2. Austin JC, Phornphutkul C, Wojtys EM. Loss of knee extension after anterior cruciate ligament reconstruction: effects of knee position and graft tensioning. J Bone Joint Surg Am. 2007;89(7):1565-1574. [DOI] [PubMed] [Google Scholar]
- 3. Bae BS, Yoo S, Lee SH. Ramp lesion in anterior cruciate ligament injury: a review of the anatomy, biomechanics, epidemiology, and diagnosis. Knee Surg Relat Res. 2023;35(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chahla J, Murray IR, Robinson J, et al. Posterolateral corner of the knee: an expert consensus statement on diagnosis, classification, treatment, and rehabilitation. Knee Surg Sports Traumatol Arthrosc. 2019;27(8):2520-2529. [DOI] [PubMed] [Google Scholar]
- 5. Chung K, Kim SJ, Choi CH, Kim SH, Choi Y, Jung M. Does knee flexion influence the relationship between the femoral tunnel and the lateral anatomic structures during ACL reconstruction? Clin Orthop Relat Res. 2019;477(10):2228-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009;41(4):1149-1160. [DOI] [PubMed] [Google Scholar]
- 7. Geeslin AG, LaPrade RF. Location of bone bruises and other osseous injuries associated with acute grade III isolated and combined posterolateral knee injuries. Am J Sports Med. 2010;38(12):2502-2508. [DOI] [PubMed] [Google Scholar]
- 8. Geeslin AG, LaPrade RF. Outcomes of treatment of acute grade-III isolated and combined posterolateral knee injuries: a prospective case series and surgical technique. J Bone Joint Surg Am. 2011;93(18):1672-1683. [DOI] [PubMed] [Google Scholar]
- 9. Geeslin AG, Moulton SG, LaPrade RF. A systematic review of the outcomes of posterolateral corner knee injuries, part 1: surgical treatment of acute injuries. Am J Sports Med. 2016;44(5):1336-1342. [DOI] [PubMed] [Google Scholar]
- 10. Gollehon DL, Torzilli PA, Warren RF. The role of the posterolateral and cruciate ligaments in the stability of the human knee. A biomechanical study. J Bone Joint Surg Am. 1987;69(2):233-242. [PubMed] [Google Scholar]
- 11. Grood ES, Stowers SF, Noyes FR. Limits of movement in the human knee. Effect of sectioning the posterior cruciate ligament and posterolateral structures. J Bone Joint Surg Am. 1988;70(1):88-97. [PubMed] [Google Scholar]
- 12. Kang KT, Koh YG, Son J, et al. Finite element analysis of the biomechanical effects of 3 posterolateral corner reconstruction techniques for the knee joint. Arthroscopy. 2017;33(8):1537-1550. [DOI] [PubMed] [Google Scholar]
- 13. Kannus P. Nonoperative treatment of grade II and III sprains of the lateral ligament compartment of the knee. Am J Sports Med. 1989;17(1):83-88. [DOI] [PubMed] [Google Scholar]
- 14. Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim SJ, Lee SK, Kim SH, Kim SH, Jung M. Clinical outcomes for reconstruction of the posterolateral corner and posterior cruciate ligament in injuries with mild grade 2 or less posterior translation: comparison with isolated posterolateral corner reconstruction. Am J Sports Med. 2013;41(7):1613-1620. [DOI] [PubMed] [Google Scholar]
- 16. Kim SJ, Park IS, Cheon YM, Ryu SW. New technique for chronic posterolateral instability of the knee: posterolateral reconstruction using the tibialis posterior tendon allograft. Arthroscopy. 2004;20(suppl 2):195-200. [DOI] [PubMed] [Google Scholar]
- 17. LaPrade RF, Johansen S, Wentorf FA, Engebretsen L, Esterberg JL, Tso A. An analysis of an anatomical posterolateral knee reconstruction: an in vitro biomechanical study and development of a surgical technique. Am J Sports Med. 2004;32(6):1405-1414. [DOI] [PubMed] [Google Scholar]
- 18. LaPrade RF, Ly TV, Wentorf FA, Engebretsen L. The posterolateral attachments of the knee: a qualitative and quantitative morphologic analysis of the fibular collateral ligament, popliteus tendon, popliteofibular ligament, and lateral gastrocnemius tendon. Am J Sports Med. 2003;31(6):854-860. [DOI] [PubMed] [Google Scholar]
- 19. LaPrade RF, Terry GC. Injuries to the posterolateral aspect of the knee. Association of anatomic injury patterns with clinical instability. Am J Sports Med. 1997;25(4):433-438. [DOI] [PubMed] [Google Scholar]
- 20. LaPrade RF, Tso A, Wentorf FA. Force measurements on the fibular collateral ligament, popliteofibular ligament, and popliteus tendon to applied loads. Am J Sports Med. 2004;32(7):1695-1701. [DOI] [PubMed] [Google Scholar]
- 21. LaPrade RF, Wentorf FA, Fritts H, Gundry C, Hightower CD. A prospective magnetic resonance imaging study of the incidence of posterolateral and multiple ligament injuries in acute knee injuries presenting with a hemarthrosis. Arthroscopy. 2007;23(12):1341-1347. [DOI] [PubMed] [Google Scholar]
- 22. Larson RV. Isometry of the lateral collateral and popliteofibular ligaments and techniques for reconstruction using a free semitendinosus tendon graft. Oper Tech Sports Med. 2001;9(2):84-90. [Google Scholar]
- 23. Levy BA, Dajani KA, Morgan JA, Shah JP, Dahm DL, Stuart MJ. Repair versus reconstruction of the fibular collateral ligament and posterolateral corner in the multiligament-injured knee. Am J Sports Med. 2010;38(4):804-809. [DOI] [PubMed] [Google Scholar]
- 24. Moulton SG, Geeslin AG, LaPrade RF. A systematic review of the outcomes of posterolateral corner knee injuries, part 2: surgical treatment of chronic injuries. Am J Sports Med. 2016;44(6):1616-1623. [DOI] [PubMed] [Google Scholar]
- 25. Shon OJ, Park JW, Kim BJ. Current concepts of posterolateral corner injuries of the knee. Knee Surg Relat Res. 2017;29(4):256-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sidles JA, Larson RV, Garbini JL, Downey DJ, Matsen FA, III. Ligament length relationships in the moving knee. J Orthop Res. 1988;6(4):593-610. [DOI] [PubMed] [Google Scholar]
- 27. Sigward SM, Markolf KL, Graves BR, Chacko JM, Jackson SR, McAllister DR. Femoral fixation sites for optimum isometry of posterolateral reconstruction. J Bone Joint Surg Am. 2007;89(11):2359-2368. [DOI] [PubMed] [Google Scholar]
- 28. Stannard JP, Brown SL, Farris RC, McGwin G, Jr, Volgas DA. The posterolateral corner of the knee: repair versus reconstruction. Am J Sports Med. 2005;33(6):881-888. [DOI] [PubMed] [Google Scholar]
- 29. Sugita T, Amis AA. Anatomic and biomechanical study of the lateral collateral and popliteofibular ligaments. Am J Sports Med. 2001;29(4):466-472. [DOI] [PubMed] [Google Scholar]
- 30. Victor J, Van Doninck D, Labey L, Innocenti B, Parizel PM, Bellemans J. How precise can bony landmarks be determined on a CT scan of the knee? Knee. 2009;16(5):358-365. [DOI] [PubMed] [Google Scholar]
- 31. Wang CJ, Walker PS. The effects of flexion and rotation on the length patterns of the ligaments of the knee. J Biomech. 1973;6(6):587-596. [DOI] [PubMed] [Google Scholar]
- 32. Yoon HK, Park SH, Oh HC, Ha JW, Choi H. Combined PCL and PLC reconstruction improves residual laxity in PCL injury patients with posterolateral knee laxity less than grade III. Yonsei Med J. 2023;64(5):313-319. [DOI] [PMC free article] [PubMed] [Google Scholar]