Abstract
Background:
Joint effusion at 3 months after anterior cruciate ligament (ACL) reconstruction is a risk factor for ACL reinjury. However, factors associated with joint effusion at 3 months postoperatively and the effect of joint effusion on subsequent quadriceps muscle strength and graft remodeling remain unknown.
Purposes:
To identify factors associated with joint effusion and investigate the association between joint effusion and quadriceps muscle strength and graft remodeling in the postoperative period.
Study Design:
Case-control study; Level of evidence, 3.
Methods:
In this retrospective multicenter study, the medical records of patients who underwent single-bundle ACL reconstruction between 2015 and 2021 were reviewed. The study included the data of 174 patients (mean age, 23.5 ± 10.6 years). Demographic data, including sex, age at surgery, time from injury to surgery in months, body mass index, preinjury Tegner activity score, presence of meniscus, and chondral injuries, were collected. Magnetic resonance imaging was performed 3 months postoperatively. Joint effusion was defined as grade 3 (range of grades, 0-3) according to the ACL Osteoarthritis Score. Isokinetic strength testing was performed at 60 deg/s, while the limb symmetry index (LSI) of quadriceps strength was evaluated at 6 months postoperatively. Moreover, graft remodeling was evaluated using magnetic resonance imaging-derived signal intensity ratio (SIR) measures at 1 year postoperatively. The authors used multivariate logistic and linear regression analyses to identify the factors influencing joint effusion at 3 months and those associated with postoperative quadriceps strength LSI and SIR values, respectively.
Results:
Greater preinjury Tegner activity scores (odds ratio, 1.59; 95% CI, 1.08 to 2.34; P = .02) increased the odds of joint effusion at 3 months postoperatively. Multivariable linear regression analysis revealed that joint effusion (β = −23.8; 95% CI, −36.0 to −11.7; P < .001) was an independent factor associated with LSI of the quadriceps. Furthermore, joint effusion (β = 1.33; 95% CI, 0.53 to 2.14; P = .001) was associated with a higher SIR value of the reconstructed graft.
Conclusion:
The preinjury Tegner activity score was a factor associated with joint effusion at 3 months postoperatively, and joint effusion was associated with subsequent muscle weakness and delayed graft remodeling.
Keywords: knee ligaments, ACL, magnetic resonance imaging, physical therapy/rehabilitation, muscle physiology, graft remodeling, joint effusion, quadriceps strength, signal intensity ratio
While good clinical outcomes have been reported for anterior cruciate ligament (ACL) reconstruction (ACLR) in many cases, there are certain cases of reinjury and poor clinical outcomes.16,19 Although the amount of synovial fluid in the knee is greater at 3 months after ACLR surgery compared with that after nonoperative treatment, the degree of synovial fluid varies. 7 Joint effusion with a larger fluid volume at 3 months postoperatively is one of the risk factors for ACL reinjury independent of confounders. In a multivariate logistic regression analysis, the presence of joint effusion (odds ratio [OR], 34.5; 95% CI, 6.63-179.7) increased the odds of ACL reinjury. 12 As it takes approximately 1 year to return to sports after ACLR, 8 joint effusion evaluation at 3 months postoperatively may be beneficial because of the possibility of modifying subsequent rehabilitation and even intervention.
Joint effusion can lead to quadriceps weakness with impaired neural activation of the quadriceps muscle, known as arthrogenic muscle inhibition (AMI). 33 Poor quadriceps muscle strength recovery after ACLR is associated with poor postoperative performance, 5 poor patient-reported outcome, 6 and increased risk of ACL reinjury. 10
Additionally, synovial fluid after ACLR has been reported to contain high levels of inflammatory cytokines, such as tumor necrosis factor (TNF), interleukin (IL)-6, and IL-8. 15 These inflammatory cytokines are expressed in the graft of patients with biological failure at 1 year after ACLR, 32 which may delay graft remodeling. As reported in a multivariate analysis, delayed graft remodeling in the first postoperative year—that is, enhanced signal intensity ratio (SIR) on magnetic resonance imaging (MRI)—was associated with reinjury of the reconstructed graft (OR, 1.428; 95% CI, 1.030-1.978) 24 and may be associated with a delayed return to sports. 38
Thus, joint effusion after ACLR may negatively affect quadriceps muscle strength and graft remodeling related to clinical outcomes. Although knee joint effusion is generally associated with synovial inflammation and is thought to be caused by excessive mechanical loading and cartilage degeneration, 18 the risk factors for joint effusion at 3 months after ACLR remain unclear. Furthermore, the effect of joint effusion at 3 months after ACLR on postoperative quadriceps muscle strength and graft remodeling has not yet been investigated.
This study retrospectively evaluated the data of patients who underwent ACLR performed by the same surgeon (A.K.) at 2 sites. The objectives were to identify factors associated with joint effusion at 3 months after ACLR at the 2 sites and to examine the association between the joint effusion effect at 3 months after ACLR and quadriceps muscle strength at 6 months postoperatively, as well as that between the joint effusion effect at 3 months after ACLR and graft remodeling at 1 year postoperatively. We hypothesized that concomitant cartilage injury would be associated with joint effusion at 3 months postoperatively and that joint effusion at 3 months postoperatively would impair quadriceps muscle strength recovery and delay graft remodeling.
Methods
This retrospective study was approved by the medical ethical committee of our institute and was conducted in accordance with the Declaration of Helsinki. The need for informed consent was waived by the committee because of the retrospective nature of the study.
This multisite study investigated the factors associated with joint effusion after ACLR at 3 months postoperatively. Furthermore, we examined the association between the joint effusion effect at 3 months after ACLR and quadriceps muscle strength at 6 months postoperatively at site 1 as well as the association between the joint effusion effect at 3 months after ACLR and graft remodeling at 1 year postoperatively at site 2.
All patients who underwent a primary single-bundle ACLR for ACL injury between 2015 and 2021 at site 1 and between 2016 and 2021 at site 2 by the same knee surgeon (A.K.) were considered for inclusion. Patients were excluded from this analysis if they were operated with a bone-tendon-bone autograft, underwent all-epiphyseal ACLR with concomitant reconstruction of multiple ligaments, or experienced ACL reinjury within 1 year of primary ACLR. Additionally, patients who did not undergo MRI at 3 months postoperatively at both sites, those who had incomplete data regarding quadriceps strength at site 1, and those who did not undergo MRI at 1 year postoperatively at site 2 were excluded. Demographic data were documented from medical records, including sex, age at surgery, time from injury to surgery (months), body mass index, and preinjury Tegner activity score. 36 The Tegner Activity Scale score is a self-reported measure that patients use to describe their ability to participate in work-related and sports activities. This scale is a single-question survey rated on an 11-point scale (ranging between 0 and 10) in which the patient's activity or work level is self-assessed. A score of 0 indicates the highest level of disability, whereas a score of 10 signifies the capability of engaging in activities at an elite sports athletic level. Surgical records were reviewed for meniscal and chondral injuries.
Surgical Procedure
Arthroscopic ACLR was performed with a single-bundle technique using an autologous hamstring tendon graft (semitendinosus ± gracilis tendon) using the same procedure at both sites. The tibial tunnel was positioned at the center of the ACL footprint, while the femoral tunnel was located behind the resident's ridge and at the center of the ACL footprint, achieved through an outside-in technique. An ACL TightRope (Arthrex Inc) was used to anchor the graft to the femur. At the tibial site, the graft was fixed using a Double Spiked Plate (Smith & Nephew) along with a screw combined with sutures, with the knee positioned at 20° of flexion. During tibial fixation, manual tension equivalent to 30 N was applied to the graft.
Postoperative Rehabilitation
Postoperative rehabilitation was performed according to the same protocol at both sites. The knee joint was kept immobilized for 1 week. Subsequently, exercises to enhance the joint's range of motion were introduced, gradually expanding the range on a weekly basis. After 6 weeks, no limitations were imposed. Patients were allowed to partially bear weight after 4 days and to fully bear weight after 3 weeks. In instances where simultaneous meniscal repair was undertaken, weightbearing was postponed by a week, and the commencement of range of motion exercises was deferred by 2 weeks. Cycling was allowed at 6 weeks postoperatively, whereas jogging in a straight line was initiated at 3 months postoperatively. Double-leg jumping activities were commenced at 4 months postoperatively. If rehabilitation objectives were achieved, a complete return to sports was suggested within 8 to 10 months. Aspiration was performed if effusion caused pain or limited the range of motion, preventing the patient from undergoing rehabilitation. However, in this series, there were no cases in which aspiration was performed until 3 months postoperatively.
Joint Effusion Evaluation
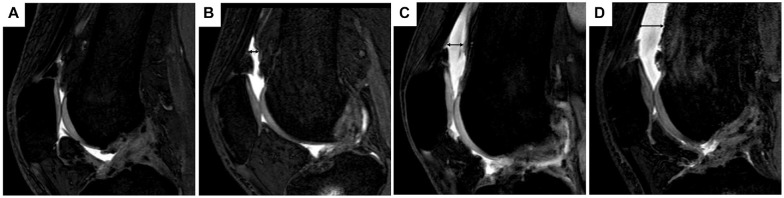
MRI assessments were conducted at 3 months postoperatively, before the patient's progression to jogging during rehabilitation. A 3-T MRI system (Philips Ingenia; Philips GmbH) with a circular polarized surface coil was employed for MRI scans. The evaluation of joint effusion was carried out using the ACL Osteoarthritis Score (ACLOAS), performed by 2 orthopaedic surgeons (N.K. and N.A.). 27 The evaluation was conducted using sagittal T2-weighted fast field echo with water-selective excitation imaging. All MRI data were imported into a workstation (OsiriX; Pixmeo SARL). Joint effusion grading was determined based on the fluid-equivalent signal length in the suprapatellar recess, observable on the sagittal images of a central slice. The grading scale was as follows: grade 0, <2 mm; grade 1, 2 to 5 mm; grade 2, 5 to 10 mm; and grade 3, ≥10 mm (Figure 1). Grade 3 was defined as a joint effusion. Kappa values were calculated to assess the interobserver reliability in grading synovial fluid extent. The interobserver reliability for the grading was 0.86, which signifies excellent agreement.
Figure 1.
Evaluation of joint effusion by magnetic resonance imaging. Assessment of the extent of joint effusion involves assigning scores ranging between 0 and 3, determined by the length of the fluid-equivalent signal observed in the suprapatellar recess on sagittal images of a central slice. (A) In cases where there was little joint effusion, the black double-headed arrow was not depicted. (B-D) The black double arrow signifies the measurement of the suprapatellar recess length. (A) Grade 0, <2 mm; (B) grade 1, 2 to <5 mm; (C) grade 2, 5 to <10 mm; (D) grade 3, ≥10 mm.
Isokinetic Quadriceps Strength Testing
Isokinetic assessments were performed using the Biodex System III (Biodex Medical Systems) preoperatively and at 6 months postoperatively at site 1. During the assessment, the patients were seated with their hips flexed at 90°, and their trunks and thighs were secured using straps. The dynamometer arm was secured just above the ankle joint. Patients were instructed to perform knee extensions and flexions with maximal force and speed. Quadriceps torque was measured isokinetically within a range of approximately 0° to 90° of knee motion at 60 deg/s. The highest recorded peak torque measurement in newton meters was collected. To quantify limb symmetry, the limb symmetry index (LSI), which is the ratio of the strength on the involved side to that on the uninvolved side, expressed as a percentage (injured/noninjured × 100), was determined.
Graft Remodeling Evaluation
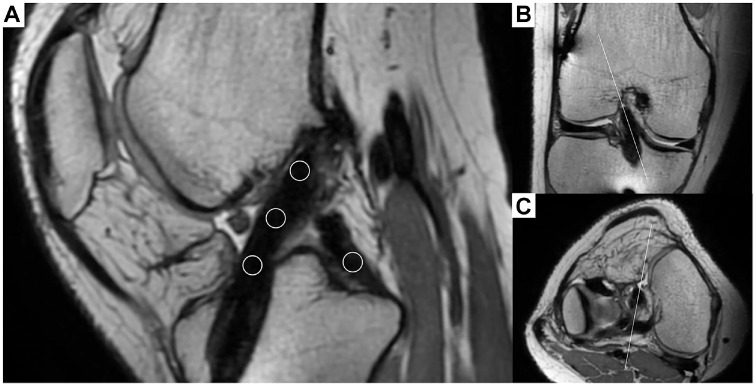
MRI was performed using a 3-T system (Philips Ingenia) with a circular polarized surface coil at 1 year postoperatively at site 2. The evaluation of graft remodeling was performed by 2 orthopaedic surgeons (N.K, N.A) using 3-dimensional proton density–weighted volumetric isotropic turbo spin-echo acquisition. All MRI data were imported into a workstation (OsiriX). Based on previous reports,21,24 the signal intensity was measured by taking measurements at 4 regions of interest: (1) proximal, (2) midsubstance, (3) distal portion of the graft, and (4) distal tibial attachment of the posterior cruciate ligament (PCL) using a circular region of interest with the sagittal oblique reconstructed image parallel to the graft (Figure 2). The SIR was calculated for each graft region using the following formula: SIR = (SIGNALacl/SIGNALpcl). The mean of these 3 regions (proximal, midsubstance, and distal portions of the graft) was calculated and used as the SIR value of the graft. Intraclass correlation coefficients were used to assess the interobserver reliability of the SIR evaluation. The interobserver reliability for the SIR evaluation was 0.81, indicating excellent agreement.
Figure 2.
Evaluation of graft remodeling using magnetic resonance imaging. (A) Images were reconstructed to be parallel to the center of the graft to evaluate graft remodeling. Circular regions of interest are located at 3 points measured along the graft (proximal, midsubstance, distal portion of the graft) and at the base of the posterior cruciate ligament at the center of the tibial attachment to calculate the signal intensity ratio. The reconstructed image is created to be parallel to the graft along the white line in the (B) coronal and (C) axial images.
Statistical Analysis
Statistical analyses were performed using SPSS software (Version 26.0; IBM). Continuous variables are presented as mean ± SD. The normality of the distribution was assessed using the Shapiro-Wilk test. The Pearson chi-square test, Student t test, and Mann-Whitney U test were used to compare qualitative, normally distributed, and nonnormally distributed continuous variables, respectively, between the joint effusion and no joint effusion groups. To determine which factors were associated with joint effusion at 3 months postoperatively, a univariate logistic regression analysis was performed. Subsequently, factors with P values of <.10 in the univariate analysis were included in the multivariate logistic regression analysis. One-way analysis of variance with the Tukey post hoc test was used to compare the mean differences in the postoperative LSI and SIR values across ACLOAS grades. To determine the factors associated with postoperative LSI of quadriceps strength and SIR value, univariate linear regression analysis was performed. Factors with P values of <.10 in the univariate analysis were included in the multivariate linear regression analysis. A P value of <.05 was considered significant.
The sample size was based on convenience sampling, collecting all available cases. Sample calculations were not performed before this study. The study conducted a post hoc test on multivariable regression analyses to ensure that the sample size was sufficient for statistical power. For all 3 analyses, with an effect size of 0.35 and an alpha value of .05, the post hoc analysis indicated that the sample size for this study had adequate statistical power of ≥0.97.
Results
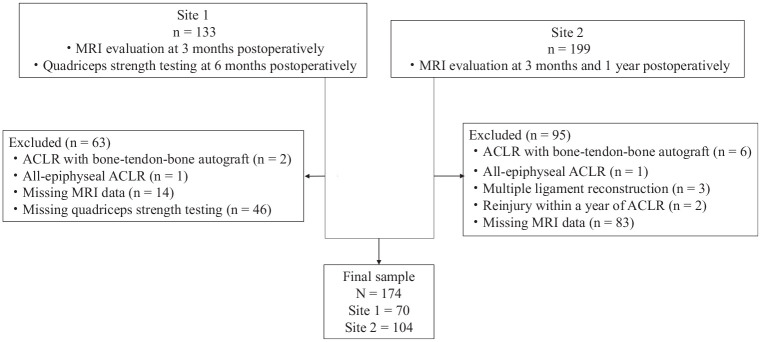
A total of 174 patients were included in our analysis. Of these, 70 patients were at site 1 and 104 were at site 2 (Figure 3). The total patient demographics and surgical characteristics of each site are summarized in Table 1.
Figure 3.
Flowchart of the study participants. ACLR, anterior cruciate ligament reconstruction; MRI, magnetic resonance imaging.
Table 1.
Patient Demographics and Surgical Characteristics a
| Parameter | Total (N = 174) | Site 1 (n = 70) | Site 2 (n = 104) |
|---|---|---|---|
| Sex, male/female | 90/84 | 42/28 | 48/56 |
| Age, y | 23.5 ± 10.6 | 20.5 ± 5.1 | 25.5 ± 12.7 |
| Time to surgery, mo | 7.4 ± 15.4 | 5.4 ± 6.9 | 8.7 ± 19.0 |
| BMI, kg/m2 | 23.7 ± 3.5 | 23.9 ± 3.7 | 23.5 ± 3.3 |
| Preinjury Tegner activity score | 6.7 ± 1.4 | 7.6 ± 1.5 | 6.2 ± 1.1 |
| Meniscal injury | 98 | 47 | 51 |
| Chondral injury | 24 | 8 | 16 |
| ACLOAS grade, 0/1/2/3, n (%) | 56/48/54/16 (32.2/27.6/31.0/9.2) |
15/22/26/7 (21.4/31.4/37.1/10.0) |
41/26/28/9 (39.4/25.0/26.9/8.7) |
Data presented as mean ± SD or n unless otherwise indicated. ACLOAS, Anterior Cruciate Ligament Osteoarthritis Score; BMI, body mass index.
For joint effusion scoring observed on MRI at 3 months postoperatively, 16 patients (9.2%) were evaluated as grade 3 and defined as having joint effusion. There was a statistically significant difference between the joint effusion and no joint effusion groups only in the preinjury Tegner activity scores (Table 2). Univariate logistic regression analysis revealed that a higher preinjury Tegner activity score (OR, 1.66; 95% CI, 1.13-2.43; P = .01) was associated with increased odds of joint effusion presence at 3 months postoperatively. Multivariate logistic regression analysis revealed that a higher preinjury Tegner activity score (OR, 1.59; 95% CI, 1.08-2.34; P = .02) was associated with increased odds of joint effusion presence at 3 months postoperatively (Table 3).
Table 2.
Comparison Between Joint and No joint Effusion Groups a
| Parameter | Joint Effusion (n = 16) | No joint Effusion (n = 158) | P |
|---|---|---|---|
| Sex, male/female | 10/6 | 74/68 | .26 |
| Age, y | 24.4 ± 10.9 | 23.4 ± 10.6 | .28 |
| Time to surgery, mo | 4.8 ± 5.4 | 7.6 ± 16.1 | .34 |
| BMI, kg/m2 | 24.3 ± 2.4 | 23.6 ± 3.6 | .13 |
| Preinjury Tegner activity score | 7.6 ± 1.4 | 6.7 ± 1.4 | .008 |
| Meniscal injury | 12 | 86 | .09 |
| Chondral injury | 3 | 21 | .38 |
Data presented as mean ± SD or n. BMI, body mass index.
Table 3.
Logistic Regression Analysis for Risk Factors Associated With Joint Effusion at 3 Months Postoperatively a
| Variable | Univariate Model | Multivariate Model | ||||||
|---|---|---|---|---|---|---|---|---|
| B | OR | 95% CI | P | B | OR | 95% CI | P | |
| Sex, female | −0.49 | 0.62 | 0.21-1.78 | .37 | ||||
| Age | 0.008 | 1.01 | 0.96-1.06 | .72 | ||||
| Time to surgery, mo | −0.02 | 0.98 | 0.91-1.05 | .51 | ||||
| BMI | 0.05 | 1.05 | 0.91-1.21 | .46 | ||||
| Tegner Activity Scale | 0.51 | 1.66 | 1.13-2.43 | .01 | 0.47 | 1.59 | 1.08-2.34 | .02 |
| Meniscal injury | 0.92 | 2.51 | 0.78-8.12 | .09 | 0.72 | 2.06 | 0.62-6.85 | .24 |
| Chondral injury | 0.41 | 1.51 | 0.40-5.73 | .55 | ||||
BMI, body mass index; OR, odds ratio.
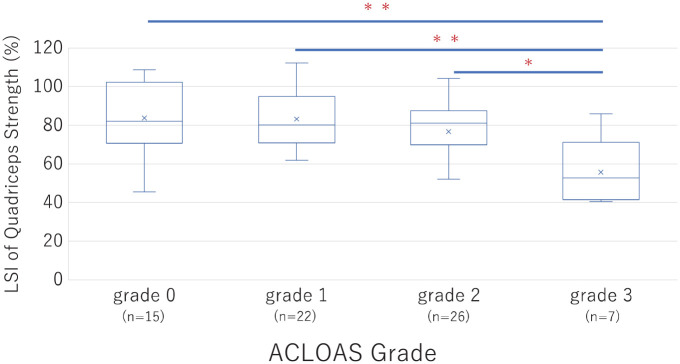
The postoperative LSI of quadriceps strength significantly decreased according to ACLOAS grade progression (Figure 4). A simple linear regression analysis revealed that sex, age, body mass index, preoperative LSI of the quadriceps, and presence of joint effusion were significantly associated with postoperative LSI of the quadriceps strength (Table 4). Multivariable linear regression analysis revealed that age (β = −1.70; 95% CI −2.61 to −0.80; P < .001), preoperative LSI of the quadriceps (β = 0.33; 95% CI, 0.12 to 0.55; P = .003), and joint effusion presence (β = −23.8; 95% CI, −36.0 to −11.7; P < .001) were also independently associated factors (Table 5).
Figure 4.
The limb symmetry index (LSI) of quadriceps strength was compared among the Anterior Cruciate Ligament Osteoarthritis Score (ACLOAS) grades. *P < .05; **P < .001.
Table 4.
Univariate Linear Regression Analysis Factors Affecting LSI of Quadriceps Strength at 6 Months Postoperatively a
| β± SE | 95% CI | P | |
|---|---|---|---|
| Sex, female | 7.54 ± 4.28 | −1.01 to 16.1 | .08 |
| Age | −1.53 ± 0.38 | −2.28 to −0.78 | <.001 |
| Time to surgery, mo | −0.007 ± 0.010 | −0.028 to 0.014 | .50 |
| BMI | −1.27 ± 0.57 | −2.40 to −0.14 | .03 |
| Preinjury Tegner activity score | 0.33 ± 1.48 | −2.60 to 3.28 | .82 |
| Meniscal injury | −1.99 ± 4.56 | −11.1 to 7.12 | .67 |
| Chondral injury | −3.93 ± 6.73 | −17.4 to 9.50 | .56 |
| Preoperative LSI of quadriceps | 0.34 ± 0.12 | 0.10 to 0.58 | .006 |
| Joint effusion | −24.9 ± 6.48 | −37.9 to −12.0 | <.001 |
BMI, body mass index; LSI, limb symmetry index.
Table 5.
Multivariate Linear Regression Analysis of Factors Affecting LSI of Quadriceps Strength at 6 Months Postoperatively a
| β± SE | 95% CI | P | |
|---|---|---|---|
| Intercept | 108.6 ± 17.9 | 72.8 to 144.5 | <.001 |
| Sex, female | −5.71 ± 4.38 | −14.5 to 13.06 | .20 |
| Age | −1.70 ± 0.45 | −2.61 to −0.80 | <.001 |
| BMI | −0.69 ± 0.52 | −1.72 to 0.35 | .19 |
| Preoperative LSI of quadriceps | 0.33 ± 0.11 | 0.12 to 0.55 | .003 |
| Joint effusion | −23.8 ± 6.06 | −36.0 to −11.7 | <.001 |
BMI, body mass index; LSI, limb symmetry index.
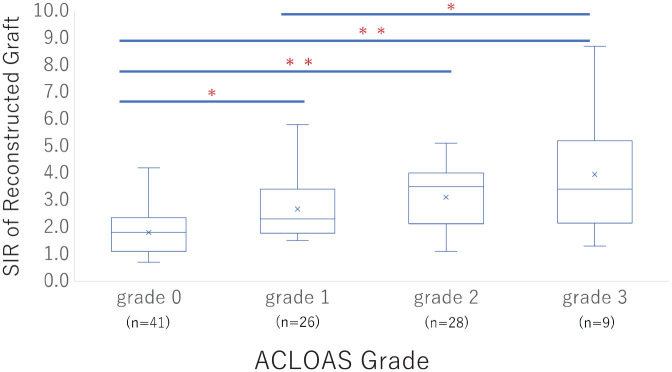
The SIR of the reconstructed graft significantly increased with the ACLOAS grade progression (Figure 5). A simple linear regression analysis revealed that sex, presence of meniscal injury, presence of chondral injury, and presence of joint effusion were significantly associated with the SIR of the reconstructed graft (Table 6). Multivariable linear regression analysis revealed that female sex (β = −0.53; 95% CI, −0.98 to −0.075; P = .02), chondral injury (β = 1.28; 95% CI, 0.64 to 1.92; P < .001), and joint effusion (β = 1.33; 95% CI, 0.53 to 2.14; P = .001) were also independently associated factors (Table 7).
Figure 5.
The signal intensity ratio (SIR) of the reconstructed graft was compared among the Anterior Cruciate Ligament Osteoarthritis Score (ACLOAS) grades. *P < .05; **P < .001.
Table 6.
Univariate Linear Regression Analysis of Factors Affecting the Signal Intensity Ratio of Reconstructed Graft at 1 Year Postoperatively a
| β± SE | 95% CI | P | |
|---|---|---|---|
| Sex, female | −0.67 ± 0.25 | −1.18 to −0.17 | .01 |
| Age | 0.016 ± 0.0.10 | −0.004 to 0.036 | .12 |
| Time to surgery, mo | 0.002 ± 0.007 | −0.012 to 0.016 | .76 |
| BMI | 0.059 ± 0.039 | −0.019 to 0.14 | .14 |
| Preinjury Tegner activity score | 0.089 ± 0.12 | −0.15 to 0.33 | .46 |
| Meniscal injury | 0.59 ± 0.26 | 0.086 to 1.10 | .02 |
| Chondral injury | 1.45 ± 0.33 | 0.79 to 2.11 | <.001 |
| Joint effusion | 1.52 ± 0.44 | 0.64 to 2.40 | <.001 |
BMI, body mass index.
Table 7.
Multivariate Linear Regression Analysis of Factors Affecting Signal Intensity Ratio of Reconstructed Graft at 1 Year Postoperatively
| β± SE | 95% CI | P | |
|---|---|---|---|
| Intercept | 2.47 ± 0.21 | 2.05 to 2.90 | <.001 |
| Sex, female | −0.53 ± 0.23 | −0.98 to −0.075 | .02 |
| Meniscal injury | 0.12 ± 0.24 | −0.36 to 0.59 | .63 |
| Chondral injury | 1.28 ± 0.32 | 0.64 to 1.92 | <.001 |
| Joint effusion | 1.33 ± 0.41 | 0.53 to 2.14 | .001 |
Discussion
In this study, we found that the preinjury Tegner activity score was a factor associated with joint effusion at 3 months postoperatively, and joint effusion at 3 months postoperatively was a factor associated with low quadriceps strength at 6 months postoperatively and delayed graft remodeling at 1 year postoperatively. Previous studies have reported that nonmodifiable patient factors, such as age, 28 bone morphology,9,19 and surgical technique–related parameters, including graft type and graft diameter,25,31 were related to the clinical outcome after ACLR. Notably, this study showed that postoperative factors are associated with clinical outcomes after ACLR and that joint effusion at 3 months after ACLR may indicate the requirement for close monitoring.
The present study found that synovial fluid volume varied between grade 0 and grade 3 and affected subsequent knee joint function. A previous study showed that joint effusion amount was significantly higher at 3 and 6 months postoperatively than that when nonoperative treatment was administered and was comparable at 1 year postoperatively. 7 Furthermore, synovial fluid volume at 2 years after ACLR, evaluated with the same technique as in this study, was of grade 0 (69.8%), grade 1 (22.4%), grade 2 (4.3%), and grade 3 (3.4%), and there was no correlation between the synovial fluid volume and patient-reported outcomes at 2 and 5 years postoperatively. 35 Therefore, examination of the joint effusion at 3 months postoperatively could provide valuable information.
Contrary to the hypothesis of the present study, concomitant cartilage injury was not a factor associated with joint effusion at 3 months postoperatively. In general, knee joint effusion is associated with synovial inflammation, which is thought to be caused by excessive mechanical loading and cartilage degeneration. 18 Cartilage damage alters joint contact pressure and induces further cartilage damage, leading to a negative spiral. A possible reason for this contradiction in the present study is that the patients were evaluated at 3 months postoperatively, which is not long enough for this effect to occur, and the patients had not returned to sports, and thus the load on the joint was insignificant.
In a study examining risk factors for joint effusion on computed tomography 3 months after ACLR compared to the healthy side, the risk factors were higher preinjury Tegner score and meniscal injury. 17 Here, although the joint effusion was evaluated differently, the high preinjury Tegner activity score was an associated factor. It is unclear why a high preinjury Tegner activity score is a risk factor for joint effusion, as this may indicate a high level of athleticism, high motivation to return to competition, and excessive self-training. To confirm this hypothesis, strict rehabilitation management, including self-training interventions, is needed.
The LSI of quadriceps muscle strength in patients with joint effusion (ACLOAS grade 3) at 3 months postoperatively was 55.7% ± 17.0% in this study. Quadriceps muscle strength has been reported to be associated with knee function and patient satisfaction after ACLR.5,6 Although there is no consensus on the appropriate timing of return to sports after ACLR, patients typically return to sports at 9 to 12 months postoperatively, 8 with sports-specific training beginning at 6 months postoperatively. Not achieving sufficient muscle strength at 6 months postoperatively is associated with subsequent reinjury.14,30 In a previous study, at a mean follow-up period of 41 months, the group with no reinjury had an LSI of 81.8% ± 32.0% at 6 months postoperatively, compared to 53.4% ± 20.6% in the group with reinjury. 30 The quadriceps muscle strength of patients with joint effusion in this study was low as in previous studies.
Consistent with previous studies, older age and lower preoperative muscle strength were associated with muscle weakness at 6 months postoperatively.13,37 Particularly, in a previous study, older age was also found to be a protective factor against reinjury. 23 This may be attributed to lower levels and less frequent participation in sports and activities in older patients. Participants should be compared by age group in future studies to test this hypothesis. Regarding preoperative muscle strength, Ueda et al 37 reported that in ACLR using hamstrings, the cut-off value for an LSI of quadriceps strength of at least 85% at 6 months postoperatively was a preoperative LSI of quadriceps strength of 70.2%, indicating that muscle strength recovery through preoperative rehabilitation was important.
Obvious weakness and atrophy of the quadriceps muscles are often observed after ACLR. The concept of AMI has been employed to explain the central inhibition of some muscle groups after injuries or surgical procedures, 41 and joint effusion is one major factor. 34 Injection of glucose saline into the knee joint in healthy individuals reduces the quadriceps peak torque and muscle fiber conduction velocity, and AMI disappears when joint fluid is aspirated.11,26,34 Thus, joint effusion itself may cause neural inhibition and prolong muscle strength recovery. Furthermore, the components of synovial fluid after ACLR may contain inflammatory cytokines. 16 Muscle weakness after ACLR reportedly reflects changes in signaling pathways that control muscle protein synthesis and degradation; in particular, transforming growth factor-β induces muscle atrophy by activating the ubiquitin-proteasome pathway. 29
The SIR of ACLOAS grade 3 was 3.9 ± 2.3, which was significantly higher than those of grade 0 (1.8 ± 0.83) and grade 1 (2.7 ± 1.1) in this study. There was a negative correlation between SIR and ligament tensile strength and load to failure in an animal study. 40 There was an association between SIR at 1 year postoperatively and the subsequent graft reinjury rates, with a 40% increased risk of retear with a 1-point increase in SIR. 24 Furthermore, a lower SIR was found to be a factor that may allow an earlier return to sports. 38
No other studies have shown an association between sex and SIR, but in this study, male individuals had a higher SIR at 1 year postoperatively. A systematic review of clinical outcomes after ACLR based on sex reported that men had a higher return to competition rate than women but a higher reinjury rate. 20 Furthermore, a higher SIR value has been reported to be associated with reinjury. 24 These studies seem to support the results of the present study, although we did not examine subsequent injury.
In this study, the presence of chondral injury was associated with a higher SIR at 1 year postoperatively. ACLR for ACL injury with concomitant chondral injury has been reported to have inferior clinical outcomes at 5 to 10 years 2 and noninferior clinical outcomes at 10 to 15 years. 39 These studies primarily assessed knee stability and function and did not evaluate reinjury. No correlation was found between SIR and clinical and functional outcomes at 1 year postoperatively, 4 but high SIR and reinjury rates have been associated. 24 Therefore, future studies should investigate the long-term reinjury rate of ACLR with concomitant cartilage injury.
The gene and protein expression of inflammatory cytokines, such as TNF-α, IL-6, and IL-8, increased in grafts with graft biological failure, including reinjury, compared to nonfailure cases, suggesting that inflammation may be associated with biological remodeling. 32 Synovial fluid at 10 days postoperatively had significantly higher concentrations of IL-6, matrix metalloproteinase-3, macrophage inflammatory protein beta, and vascular endothelial growth factor than before ACLR. 1 Additionally, at 4 months postoperatively, the synovial fluid had significantly higher levels of IL-6, IL-8, IL-10, and TNF than those after nonoperative therapy, 15 suggesting that proinflammatory cytokines in the synovial fluid in the early postoperative period may have delayed graft remodeling. A previous work stated that postoperative SIR was low in patients who had platelet-rich fibrin added to the graft during ACLR. 3 Based on our findings, whether the injection of a bioadjuvant agent for patients with joint effusion postoperatively can accelerate the remodeling process needs to be investigated.
Limitations
This study had several limitations. First, it had a retrospective design and, therefore, had a selection bias. Moreover, many patients were excluded because of missing data. A prospective study is required to demonstrate the validity of our findings. Second, only quadriceps muscle strength and SIR graft assessments were performed, and other clinical assessments, including those of the Lysholm, International Knee Documentation Committee scores, return to sports rate, and reinjury rate, were not conducted. Third, graft type should be considered when assessing quadriceps muscle recovery. The present results may not apply to other graft types. To explore this issue, we should delve into this point after identifying the type of graft and technique. Fourth, this study exclusively examined graft remodeling within the intra-articular portion of the graft. It did not delve into the assessment of bone-tendon healing or the tunnel position and postoperative tunnel widening. Furthermore, we did not provide any analysis or information regarding the intratunnel graft signals. The process of graft healing and integration is influenced by a multitude of factors, making it a complex phenomenon. These factors can include patient-related variables, such as the rehabilitation type, 24 and surgical factors, such as the graft type and source. 22 This study included only patients who underwent hamstring tendon autografts; therefore, its findings may not necessarily apply to cases involving different graft types or sources.
Conclusion
The preinjury Tegner activity score was a factor associated with joint effusion at 3 months postoperatively. Furthermore, joint effusion with a larger fluid volume at 3 months postoperatively was associated with subsequent muscle weakness and delayed graft remodeling.
Acknowledgments
The authors thank Editage for English-language editing.
Footnotes
Final revision submitted April 29, 2024; accepted May 7, 2024.
The authors declared that there are no conflicts of interest in the authorship and publication of this contribution. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from University of Tsukuba Hospital (R05-140).
References
- 1. Anil U, Jejurikar N, Kenny L, Strauss EJ. Changes in synovial fluid biomarker concentration before and after ACL reconstruction. Bull Hosp Jt Dis (2013). 2019;77(3):189-193. [PubMed] [Google Scholar]
- 2. Balasingam S, Sernert N, Magnusson H, Kartus J. Patients with concomitant intra-articular lesions at index surgery deteriorate in their knee injury and osteoarthritis outcome score in the long term more than patients with isolated anterior cruciate ligament rupture: a study from the Swedish National Anterior Cruciate Ligament Register. Arthroscopy. 2018;34(5):1520-1529. [DOI] [PubMed] [Google Scholar]
- 3. Beyzadeoglu T, Pehlivanoglu T, Yildirim K, Buldu H, Tandogan R, Tuzun U. Does the application of platelet-rich fibrin in anterior cruciate ligament reconstruction enhance graft healing and maturation? A comparative MRI study of 44 cases. Orthop J Sports Med. 2020;8(2):2325967120902013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bouguennec N, Robinson J, Douiri A, Graveleau N, Colombet PD. Two-year postoperative MRI appearances of anterior cruciate ligament hamstrings autografts are not correlated with functional outcomes, anterior laxity, or patient age. Bone Joint Open. 2021;2(8):569-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Crotty NMN, Daniels KAJ, McFadden C, Cafferkey N, King E. Relationship between isokinetic knee strength and single-leg drop jump performance 9 months after ACL reconstruction. Orthop J Sports Med. 2022;10(1):23259671211063800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ebert JR, Edwards P, Joss B, Annear P, Radic R, D'Alessandro P. Isokinetic torque analysis demonstrates deficits in knee flexor and extensor torque in patients at 9-12 months after anterior cruciate ligament reconstruction, despite peak torque symmetry. Knee. 2021;32:9-18. [DOI] [PubMed] [Google Scholar]
- 7. Frobell RB, Le Graverand MP, Buck R, et al. The acutely ACL injured knee assessed by MRI: changes in joint fluid, bone marrow lesions, and cartilage during the first year. Osteoarthritis Cartilage. 2009;17(2):161-167. [DOI] [PubMed] [Google Scholar]
- 8. Glattke KE, Tummala SV, Chhabra A. Anterior cruciate ligament reconstruction recovery and rehabilitation: a systematic review. J Bone Joint Surg Am. 2022;104(8):739-754. [DOI] [PubMed] [Google Scholar]
- 9. Grassi A, Macchiarola L, Urrizola Barrientos F, et al. Steep posterior tibial slope, anterior tibial subluxation, deep posterior lateral femoral condyle, and meniscal deficiency are common findings in multiple anterior cruciate ligament failures: an MRI case-control study. Am J Sports Med. 2019;47(2):285-295. [DOI] [PubMed] [Google Scholar]
- 10. Grindem H, Snyder-Mackler L, Moksnes H, Engebretsen L, Risberg MA. Simple decision rules can reduce reinjury risk by 84% after ACL reconstruction: the Delaware-Oslo ACL cohort study. Br J Sports Med. 2016;50(13):804-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hopkins JT. Knee joint effusion and cryotherapy alter lower chain kinetics and muscle activity. J Athl Train. 2006;41(2):177-184. [PMC free article] [PubMed] [Google Scholar]
- 12. Kikuchi N, Kanamori A, Okuno K, et al. Joint effusion at 3 months after anterior cruciate ligament reconstruction is associated with reinjury. Knee Surg Sports Traumatol Arthrosc. 2023;31(5):1798-1804. [DOI] [PubMed] [Google Scholar]
- 13. Kuenze C, Weaver A, Grindstaff TL, et al. Age-, sex-, and graft-specific reference values from 783 adolescent patients at 5 to 7 months after ACL reconstruction: IKDC, Pedi-IKDC, KOOS, ACL-RSI, single-leg hop, and thigh strength. J Orthop Sports Phys Ther. 2023;53(4):1-8. [DOI] [PubMed] [Google Scholar]
- 14. Kyritsis P, Bahr R, Landreau P, Miladi R, Witvrouw E. Likelihood of ACL graft rupture: not meeting six clinical discharge criteria before return to sport is associated with a four times greater risk of rupture. Br J Sports Med. 2016;50(15):946-951. [DOI] [PubMed] [Google Scholar]
- 15. Larsson S, Struglics A, Lohmander LS, Frobell R. Surgical reconstruction of ruptured anterior cruciate ligament prolongs trauma-induced increase of inflammatory cytokines in synovial fluid: an exploratory analysis in the KANON trial. Osteoarthritis Cartilage. 2017;25(9):1443-1451. [DOI] [PubMed] [Google Scholar]
- 16. Lindanger L, Strand T, Mølster AO, Solheim E, Inderhaug E. Return to play and long-term participation in pivoting sports after anterior cruciate ligament reconstruction. Am J Sports Med. 2019;47(14):3339-3346. [DOI] [PubMed] [Google Scholar]
- 17. Lindström M, Wredmark T, Wretling ML, Henriksson M, Felländer-Tsai L. Post-operative bracing after ACL reconstruction has no effect on knee joint effusion. A prospective, randomized study. Knee. 2015;22(6):559-564. [DOI] [PubMed] [Google Scholar]
- 18. Mathiessen A, Conaghan PG. Synovitis in osteoarthritis: current understanding with therapeutic implications. Arthritis Res Ther. 2017;19(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mohan R, Webster KE, Johnson NR, Stuart MJ, Hewett TE, Krych AJ. Clinical outcomes in revision anterior cruciate ligament reconstruction: a meta-analysis. Arthroscopy. 2018;34(1):289-300. [DOI] [PubMed] [Google Scholar]
- 20. Mok AC, Fancher AJ, Vopat ML, et al. Sex-specific outcomes after anterior cruciate ligament reconstruction: a systematic review and meta-analysis. Orthop J Sports Med. 2022;10(2):232596712210768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Okutan AE, Kalkışım M, Gürün E, Ayas MS, Aynacı O. Tibial slope, remnant preservation, and graft size are the most important factors affecting graft healing after ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2022;30(5):1584-1593. [DOI] [PubMed] [Google Scholar]
- 22. Panos JA, Webster KE, Hewett TE. Anterior cruciate ligament grafts display differential maturation patterns on magnetic resonance imaging following reconstruction: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2020;28(7):2124-2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pullen WM, Bryant B, Gaskill T, Sicignano N, Evans AM, DeMaio M. Predictors of revision surgery after anterior cruciate ligament reconstruction. Am J Sports Med. 2016;44(12):3140-3145. [DOI] [PubMed] [Google Scholar]
- 24. Putnis SE, Oshima T, Klasan A, et al. Magnetic resonance imaging 1 year after hamstring autograft anterior cruciate ligament reconstruction can identify those at higher risk of graft failure: an analysis of 250 cases. Am J Sports Med. 2021;49(5):1270-1278. [DOI] [PubMed] [Google Scholar]
- 25. Rahardja R, Zhu M, Love H, Clatworthy MG, Monk AP, Young SW. Effect of graft choice on revision and contralateral anterior cruciate ligament reconstruction: results from the New Zealand ACL Registry. Am J Sports Med. 2020;48(1):63-69. [DOI] [PubMed] [Google Scholar]
- 26. Rice D, McNair PJ, Dalbeth N. Effects of cryotherapy on arthrogenic muscle inhibition using an experimental model of knee swelling. Arthritis Rheum. 2009;61(1):78-83. [DOI] [PubMed] [Google Scholar]
- 27. Roemer FW, Frobell R, Lohmander LS, Niu J, Guermazi A. Anterior Cruciate Ligament OsteoArthritis Score (ACLOAS): longitudinal MRI-based whole joint assessment of anterior cruciate ligament injury. Osteoarthritis Cartilage. 2014;22(5):668-682. [DOI] [PubMed] [Google Scholar]
- 28. Runer A, Csapo R, Hepperger C, Herbort M, Hoser C, Fink C. Anterior cruciate ligament reconstructions with quadriceps tendon autograft result in lower graft rupture rates but similar patient-reported outcomes as compared with hamstring tendon autograft: a comparison of 875 patients. Am J Sports Med. 2020;48(9):2195-2204. [DOI] [PubMed] [Google Scholar]
- 29. Sandri M. Signaling in muscle atrophy and hypertrophy. Physiology (Bethesda). 2008;23:160-170. [DOI] [PubMed] [Google Scholar]
- 30. Severyns M, Plawecki S, Odri GA, et al. Correlation of isokinetic testing and ACL failure with the short graft tape suspension technique at six months. Arthrosc Sports Med Rehabil. 2022;4(2):e585-e590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Snaebjörnsson T, Hamrin-Senorski E, Svantesson E, et al. Graft diameter and graft type as predictors of anterior cruciate ligament revision: a cohort study including 18,425 patients from the Swedish and Norwegian National Knee Ligament Registries. J Bone Joint Surg Am. 2019;101(20):1812-1820. [DOI] [PubMed] [Google Scholar]
- 32. Song B, Jiang C, Luo H, et al. Macrophage M1 plays a positive role in aseptic inflammation-related graft loosening after anterior cruciate ligament reconstruction surgery. Inflammation. 2017;40(6):1815-1824. [DOI] [PubMed] [Google Scholar]
- 33. Sonnery-Cottet B, Saithna A, Quelard B, et al. Arthrogenic muscle inhibition after ACL reconstruction: a scoping review of the efficacy of interventions. Br J Sports Med. 2019;53(5):289-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Spencer JD, Hayes KC, Alexander IJ. Knee joint effusion and quadriceps reflex inhibition in man. Arch Phys Med Rehabil. 1984;65(4):171-177. [PubMed] [Google Scholar]
- 35. Struglics A, Turkiewicz A, Larsson S, et al. Molecular and imaging biomarkers of local inflammation at 2 years after anterior cruciate ligament injury do not associate with patient reported outcomes at 5 years. Osteoarthritis Cartilage. 2020;28(3):356-362. [DOI] [PubMed] [Google Scholar]
- 36. Tegner Y, Lysholm J. Rating systems in the evaluation of knee ligaments injuries. Clin Orthop Relat Res. 1985;198:43-49. [PubMed] [Google Scholar]
- 37. Ueda Y, Matsushita T, Araki D, et al. Factors affecting quadriceps strength recovery after anterior cruciate ligament reconstruction with hamstring autografts in athletes. Knee Surg Sports Traumatol Arthrosc. 2017;25(10):3213-3219. [DOI] [PubMed] [Google Scholar]
- 38. Vari N, Marot V, Ripoll T, et al. Preserving the semitendinosus distal attachment is associated with improved graft remodeling after ACL reconstruction. Am J Sports Med. 2023;51(8):2064-2072. [DOI] [PubMed] [Google Scholar]
- 39. Wang K, Eftang CN, Ulstein S, Årøen A, Jakobsen RB. Concomitant full-thickness cartilage lesions do not affect patient-reported outcomes at minimum 10-year follow-up after ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2022;30(5):1836-1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Weiler A, Peters G, Mäurer J, Unterhauser FN, Südkamp NP. Biomechanical properties and vascularity of an anterior cruciate ligament graft can be predicted by contrast-enhanced magnetic resonance imaging: a two-year study in sheep. Am J Sports Med. 2001;29(6):751-761. [DOI] [PubMed] [Google Scholar]
- 41. Young A. Current issues in arthrogenous inhibition. Ann Rheum Dis. 1993;52(11):829-834. [DOI] [PMC free article] [PubMed] [Google Scholar]