Abstract
Glycated haemoglobin (HbA1c) is considered the gold standard for predicting glycaemia-associated risks for the microvascular and macrovascular complications of diabetes mellitus over 5–10 years. The value of HbA1c in the care of patients with type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM) is unassailable, yet HbA1c targets remain contentious. Guidelines from diabetes care organizations recommend conflicting HbA1c targets — generally between 6.5% and 8%. However, all such organizations advocate for individualization of HbA1c targets, leaving both health-care providers and their patients confused about what HbA1c target is appropriate in an individual patient. In this Review, we outline the landmark T1DM and T2DM trials that informed the current guidelines, we discuss the evidence that drives individualized HbA1c targets, we examine the limitations of HbA1c, and we consider alternatives for monitoring glycaemic control. Ultimately, in synthesizing this literature, we argue for an HbA1c target of <7% for most individuals, but emphasize the importance of helping patients determine their own personal goals and determinants of quality of life that are independent of a particular glycaemic target. We also recognize that as newer technologies and anti-hyperglycaemic therapies emerge, glycaemic targets will continue to evolve.
Diabetes mellitus affects upwards of 30 million people (~10% of the population) in the USA and 422 million adults worldwide, a number that is estimated to rise to 640 million by 2040 (REFS1–4). This epidemic, which has mostly been mediated by the increase in type 2 diabetes mellitus (T2DM) over the last 30 years, has considerable consequences both individually and globally. Patients with diabetes mellitus have a twofold to tenfold higher risk of cardiovascular disease-related death than age-matched normoglycaemic individuals and are at substantially higher risk of all-cause mortality, cardiovascular complications (such as coronary heart disease, heart failure, stroke, and peripheral arterial disease) and microvascular complications (including retinopathy, neuropathy and nephropathy)5–8. Furthermore, the estimated health-care expenditure on diabetes mellitus was US$1.2 trillion globally in 2015 and US$404 billion in the USA alone in 2017 (REFS9,10). This expenditure includes the direct costs of treatment of diabetes mellitus and the indirect costs attributed to the complications of poor glycaemic control11. As such, much of the research effort in patient care has centred around attaining glycaemic control, with an aim of improving quality and length of life.
The history and popularization of glycated haemoglobin (HbA1c) are discussed in BOX 1. In the past decade, HbA1c has become the universally accepted standard for the diagnosis and monitoring of diabetes mellitus. Yet, identifying the appropriate HbA1c targets for different patient groups remains difficult due to ongoing debate about the ‘optimal’ HbA1c. Guidelines provided by diabetes care organizations recommend conflicting targets (discussed in detail later) but share a common thread: choosing an HbA1c target that fits an individual patient. In this Review, we summarize the clinical trials that have led to the discussion of HbA1c targets, we outline the current guidelines for HbA1c targets, and we discuss the future of monitoring glycaemic control.
Box 1 |. The history of HbA1c — a tool to measure glycaemic control.
The devastating cardiovascular and microvascular sequelae of diabetes mellitus became more apparent in the 1920s, after the diagnosis and prevalence of the disease increased, and the development of insulin extended survival115,116. At the time, whether these outcomes were a direct consequence of hyperglycaemia was difficult to determine due to the inability to track the glycaemic control of individuals over time. The discovery of HbA1c changed the landscape of diabetes mellitus management by providing a tool to monitor overall glycaemia.
In the 1960s, fascinated by haemoglobin variants, Samuel Rahbar observed an unusual haemoglobin in patients with diabetes mellitus that made up 7.5–10.6% of total haemoglobin117,118. Normoglycaemic individuals also had this haemoglobin variant, but at consistently lower concentrations (4–6%)117. It was quickly established that this haemoglobin was haemoglobin A, the most common haemoglobin tetramer in red blood cells, with the addition of a hexose molecule. This haemoglobin variant was termed HbA1c. At the time, it was postulated that HbA1c could reflect blood sugar concentrations. Subsequent studies in diabetic mouse models revealed that increases in the percentage of HbA1c occurred 3–4 weeks after the onset of hyperglycaemia in diabetic animals and declined with improved glycaemic control119. With these data in hand, Cerami and colleagues demonstrated that the HbA1c reflected urine glucose levels in humans120. Careful regulation of blood glucose concentrations in patients with diabetes mellitus normalized HbA1c over 6 weeks120,121. It was thus acknowledged that a haemoglobin <6.0% was associated with normal glycaemia and, in 2010, HbA1c ≥6.5% was added to the diagnostic criteria for diabetes mellitus122.
With these studies, a new tool for monitoring glycaemic control was born. Commercial assays for measurement of HbA1c were developed quickly. In 1985, the World Health Organization formally acknowledged the potential of HbA1c and eventually updated their guidelines to include HbA1c >6.5% as a diagnostic criterion for diabetes mellitus123,124. Defining optimal glycaemic and HbA1c targets thus became an important focus of large-scale clinical trials.
Guideline-defining clinical trials
Five large randomized clinical trials that we review, in addition to multiple smaller studies, have fuelled the HbA1c target debate. These trials were designed to examine whether treatment intensification aiming for an HbA1c in the normal range reduces complications in diabetes mellitus. These studies compared intensive treatment (achieving HbA1c 6.3–7.4%) with standard treatment (achieving HbA1c 7.3–9%) and evaluated microvascular and macrovascular outcomes. The results of these trials are summarized below (TABLE 1; Supplementary Table 1). Long-term follow-up is summarized in BOX 2. Though at face value these trials seem similar, considerable heterogeneity regarding patient populations, glycaemic targets, baseline HbA1c, intervention, the method of therapy escalation, length of follow-up and outcomes make it challenging to synthesize the trials into a recommendation for a single HbA1c target. These differences, coupled with strong and varied opinions on essential elements of the trials, contribute to the ongoing debate.
Table 1 |.
Summary of the major findings in guideline-defining trials
| Study (year) | Diabetes mellitus type | Intensive glycaemic control target | HbA1c achieved (%)a | Microvascular outcomes | Macrovascular events | Mortality | Adverse events | Ref. |
|---|---|---|---|---|---|---|---|---|
| DCCT (1993) | T1DM | HbA1c <6.05% | Mean 7.4 vs 9.1 | Sustained retinopathy: RRR 76% (95% CI 62–85%) Microalbuminuria >40 mg/24 h: RRR 34% (95% CI 2–56%) Neuropathy: RRR 69% (95% CI 24–87%) |
No significant difference | No significant difference | Hypoglycaemia: 62 per 100 patient-years (intensive) vs 19 per 100 patient-years (conventional) Weight gain: 33% (intensive) vs 9.3% (conventional) |
13 |
| UKPDS (1998) (SU, basal insuLin)b | T2DM | FPG <110 mg/dl in the intensive arm vs FPG <270 mg/dlin the control arm | Median 7.0 vs 7.9 | Combined microvascular: RRR 25% (95% CI 7–40%) | No significant difference | No significant difference | Weight gain: 3.1 kg (95% CI −0.9 to 7 kg) | 22 |
| UKPDS (1998) (metformin)b | T2DM | FPG <110 mg/dl in the intensive arm vs FPG <270 mg/dlin the control arm | Median 7.4 vs. 8.0 | AnyT2DM-re1ated end point: RRR 32% (95% CI 13–47%) Retinopathy: minimal slowing of progression, but not sustained |
Myocardial infarction: RRR 39% (P = 0.01) Composite macrovascular diseases: RRR 30% (95% CI 5–48%) |
Any T2DM-related death: RRR 42% (95% CI 9–63%) All-cause mortality: RRR 36% (95% CI 9–55%) |
96% increased risk of T2DM-re1ated death; 60% increased risk of mortality with early addition of metformin to sulfonylurea therapy | 23 |
| ACCORD (2008) | T2DM | HbA1c <6.0% | Median 6.4 vs 7.5 | No significant difference | MACE: HR 0.90 (95% CI 0.78–1.04) Non-fatal MI: HR 0.76 (95% CI 0.62–0.92) Death from CV causes: HR 1.35 (95% CI 1.04–1.76) |
All-cause mortality: HR 1.22 (95% CI 1.01–1.46) | Increased risk of hypoglycaemia requiring any assistance (16.2% vs. 5.1%) Weight gain: 3.5 kg vs 0.4 kg |
25 |
| ADVANCE (2008) | T2DM | HbA1c <6.5% | Mean 6.5 vs 7.3 | Major microvascular events: HR 0.86 (95% CI 0.77–0.97) Renal events: HR 0.79 (95% CI 0.66–0.93) No significant difference in neuropathy or retinopathy |
No significant difference | No significant difference | Hypoglycaemia: HR 1.86 (95% CI 1.42–2.4), although hypoglycaemia events were uncommon at 2.7% (intensive) and 1.5% (control) | 27 |
| VADT (2009) | T2DM | Absolute HbA1c reduction of 1.5% | Median 6.9 vs. 8.4 | Any increase in albuminuria: 9.1% (intensive) vs. 13.8% (control) (P=0.01) | No significant difference | No significant difference | Any serious AE: 24.1% (intensive) vs 17.6% (control) (P=0.05) Hypoglycaemia significantly increased in the intensive group BMI 33.8 kg/m2 vs 32.3 kg/m2 (P = 0.01) |
31 |
This is a revised summary table; the full table is available as Supplementary Table 1. ACCORD, Action to Control Cardiovascular Risk in Diabetes; ADVANCE, Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation; AE, adverse event; BMI, body mass index; CV, cardiovascular; DCCT, Diabetes Control and Complications Trial; FPG, fasting plasma glucose; HbA1c, glycated haemoglobin; HR, hazard ratio; MACE, major adverse cardiac event (defined as time to first major cardiovascular event: non-fatal myocardial infarction, non-fatal stroke or death from cardiovascular disease); MI, myocardial infarction; RRR, relative risk reduction; SU, sulfonylurea; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; UKPDS, UK Prospective Diabetes Study; VADT, Veterans Affairs Diabetes Trial.
Intensive arm versus control arm.
UKPDS trial results are stratified according to whether the intensive intervention arm used sulfonylurea or basal insulin, or metformin.
Box 2 |. Long-term follow-up of major trials of glycaemic targets.
This box summarizes outcomes after long-term follow-up of the major trials of glycaemic targets and discusses the evidence for metabolic memory, or a legacy effect.
DCCT (1993)13
Incidence of any CVD at 17 years and 30 years, respectively: RR 42% (95% CI 9–63%), RR 30% (95% CI 7–48%)
Incidence of non-fatal myocardial infarction, stroke or death from CVD at 17 years and 30 years respectively: RR 57% (95% CI 12–79%), RR 32% (95% CI −3 to 56%)
UKPDS (1998; SU, basal insulin)22
10-year follow up of UKPDS24
Any T2DM-related end point: RR 0.91 (95% CI 0.83–0.99)
Microvascular disease: RR 0.76 (95% CI 0.64–0.89)
T2DM-related mortality: HR 0.83 (95% CI 0.73–0.96)
Myocardial infarction: RR 0.85 (95% CI 0.74–0.97)
UKPDS (1998; metformin)23
10-year follow up of UKPDS24
Any T2DM-related end point: RR 0.79 (95% CI 0.66–0.95)
Microvascular disease: RR 0.84 (95% CI 0.6–1.17)
T2DM-related mortality: HR 0.70 (95% CI 0.53–0.92)
Myocardial infarction: RR 0.67 (95% CI 0.51–0.89)
ACCORD (2008)25
9-year follow-up of ACCORD26
All-cause mortality: no difference
CV mortality: HR 1.2 (95% CI 1.03–1.39)
ADVANCE (2008)27
10-year follow-up of ADVANCE28
Risk of ESKD: HR 0.54 (95% CI 0.34–0.85)
No difference in mortality outcomes
VADT (2009)31
Major CV event at 10 years and 20 years, respectively: HR 0.83 (95% CI 0.70–0.99), HR 0.91 (95% CI 0.78–1.06)
Any CV death: no difference
Death from any cause: no difference
ACCORD, Action to Control Cardiovascular Risk in Diabetes; ADVANCE, Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation; CV, cardiovascular; CVD, cardiovascular disease; DCCT, Diabetes Control and Complications Trial; EDIC, Epidemiology of Diabetes Interventions and Complications; ESKD, end-stage kidney disease; SU, sulfonylurea; T2DM, type 2 diabetes mellitus; UKPDS, UK Prospective Diabetes Study; VADT, Veterans Affairs Diabetes Trial.
The Diabetes Control and Complications Trial.
The Diabetes Control and Complications Trial (DCCT) evaluated whether intensive treatment aimed at maintaining near normal glucose levels with insulin delivered by multiple daily injections or continuous infusion pump and informed by frequent glucose monitoring would prevent or delay the onset of microvascular consequences in patients with type 1 diabetes mellitus (T1DM) of duration 1–15 years, compared with conventional treatment with one or two daily injections of insulin12,13. No HbA1c target was set for the conventional treatment group. By contrast, the aim of intensive treatment was normoglycaemia and an HbA1c of <6%. However, attaining and maintaining this HbA1c target was difficult. Nearly half of the participants in the intensive treatment group achieved an HbA1c of <6.05% once during the study, but <5% of this group maintained this target over the 6.5 years of the trial. Average HbA1c in the intensive treatment group was approximately 7% versus 9% in the conventional treatment group.
The onset and progression of retinopathy were significantly reduced in the intensive treatment group compared with the conventional treatment group. Furthermore, the incidence of nephropathy and neuropathy was also lower in the intensive treatment group14. Adverse events included weight gain and statistically significant increases in the incidence of severe hypoglycaemia in the intensive treatment group (TABLE 1; Supplementary Table 1).
Conclusions about macrovascular disease were less convincing, although a non-significant reduction in the rate of macrovascular disease was observed. Of note, no difference in mortality between the groups was observed. The authors attributed the lack of significant difference to the relative youth of the cohort and short follow-up time, leading to the development of the Epidemiology of Diabetes Interventions and Complications (EDIC) study15,16.
EDIC: long-term follow-up of DCCT.
Regardless of their previous treatment arm in DCCT, all DCCT–EDIC participants were advised to follow the intensive treatment regimen. HbA1c levels in the conventional and intensive treatment groups thus converged during the follow-up years (8.2% and 8.0% respectively). However, the time-weighted mean HbA1c remained statistically significantly higher in the conventional treatment group due to the difference in HbA1c observed during the years of the DCCT17. Despite the convergence in HbA1c levels, intensive treatment for 6.5 years during the randomized DCCT period statistically significantly reduced the risk of cardiovascular events at 17 and 30 years of follow-up15,18 (BOX 2). Intensive control was associated with less atherosclerosis, as measured in terms of the coronary artery calcification score19,20. These data demonstrate that, compared with conventional treatment, intensive glycaemic control for 6.5 years in the first 7 to 20 years following the diagnosis of T1DM reduces the risk of developing cardiovascular disease later in life, in addition to the micro vascular benefits demonstrated in the DCCT. The mechanism behind this effect has been attributed to ‘metabolic memory’, also termed the ‘legacy effect’ in the UK Prospective Diabetes Study (UKPDS) described below.
Taken together, even though an HbA1c of 6% was rarely achieved in the study itself, the DCCT–EDIC provided evidence of a benefit of aiming for an HbA1c of near 6%, with caution for hypoglycaemia. The DCCT demonstrated beneficial effects on microvascular outcomes from achieving an average HbA1c of 7% for 6.5 years in patients with a duration of T1DM of 1–15 years and minimal or no complications at study start. Whether this finding is generalizable to more diverse populations — such as those with long-standing T1DM, T2DM, substantial comorbidities or complications — or whether such intensive treatment could be managed in routine practice is less certain and was addressed in subsequent trials as discussed in the following sections.
The UK Prospective Diabetes Study.
The aim of the UKPDS was to determine whether intensive blood glucose control reduces the risk of microvascular and macrovascular complications in patients with newly diagnosed T2DM21. Approximately 4,000 participants were randomized to conventional treatment with continued diet and weight control or ‘intensive treatment’ aiming for fasting plasma glucose (FPG) levels of <6 mmol/l (108 mg/dl). Intensive treatment consisted of the addition of sulfonylurea or once-daily insulin. In both arms, insulin was used as a rescue therapy for symptomatic hyperglycaemia or fasting glucose levels of >15 mmol/l (270 mg/dl)22. The intensive treatment group had an initial decrease in HbA1c to ~6%, which subsequently increased over the course of the study. As in the DCCT, a statistically significant difference in microvascular end points was found between the intensive and conventional treatment groups. A non-significant 16% reduction in the risk of myocardial infarction was also found. However, T2DM-related all-cause mortality was not different between the treatment groups (TABLE 1; Supplementary Table 1).
In an integral stratification at the UKPDS sites, individuals with overweight (>120% ideal body weight) were randomized to treatment with diet alone (conventional treatment), sulfonylurea or insulin (as in the overall study intensive treatment group) or metformin (n = 342)23. Treatment with metformin resulted in a 32% lower risk of developing any T2DM-related end point, including microvascular and macrovascular complications. Metformin treatment also resulted in statistically significant reductions in all-cause mortality, and in the rates of myocardial infarction and composite macrovascular diseases. Metformin treatment minimally lowered the rate of progression of retinopathy.
The findings of the UKPDS studies supported the conclusion of the DCCT that tighter glycaemic control improves diabetes mellitus-related outcomes, but left many questions. The absolute reduction in the risk of microvascular outcomes with the use of sulfonylurea was modest and was coupled with statistically significant hypoglycaemia and weight gain. Treatment modality seems to be important, as sulfonylureas and insulin improved microvascular outcomes, whereas metformin improved macrovascular outcomes despite achieving similar HbA1c levels. The persistent reduction in microvascular outcomes and small but statistically significant differences in the occurrence of myocardial infarction and death from any cause were seen in a 10-year observational extension of the UKPDS24. As such, the UKPDS findings support the DCCT–EDIC findings that early control could have lasting effects on diabetic complications, but the UKPDS findings also suggest that the medications utilized dictate outcomes.
Action to Control Cardiovascular Risk of Diabetes.
The aim of the Action to Control Cardiovascular Risk of Diabetes (ACCORD) trial was to examine the effects of intensive treatment in patients with long-standing T2DM and high cardiovascular risk25. Patients were randomized to intensive treatment that aimed for an HbA1c of <6%, or to standard treatment that aimed for an HbA1c of 7–7.9%. These targets were pursued with differential application of oral anti-hyperglycaemic agents, insulin, glucose monitoring and follow-up. The primary outcome of the ACCORD trial was the first occurrence of non-fatal myocardial infarction, non-fatal stroke or death from cardiovascular causes. At 1 year after randomization, HbA1c had stabilized at 6.4% and 7.5% in the intensive treatment and standard treatment groups, respectively.
Intensive treatment resulted in statistically significantly higher rates of hypoglycaemia, weight gain and fluid retention. Moreover, the intensive treatment group had a statistically significantly higher mortality than the standard treatment group (TABLE 1; Supplementary Table 1). Thus, the study was terminated early. Long-term follow-up did not identify improved outcomes for intensive control in participants, although trends towards a lower risk of non-fatal myocardial infarction persisted26. Many mechanisms have been proposed for this higher mortality without strong evidence of causation, including the rate or the magnitude of the reduction in HbA1c, the occurrence of hypoglycaemia and interaction between drug classes. Regardless, the ACCORD trial was the first study to identify harms, specifically death, as a consequence of intensive glucose lowering with an HbA1c target of <6%.
The ADVANCE trial.
Similar to the ACCORD trial, the aim of the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) trial was to determine whether intensive glucose control to a target of ≤6.5% influenced the risk of complications in T2DM. The ADVANCE trial recruited geographically more broadly than the ACCORD trial and enrolled patients at high cardiovascular risk with long-standing T2DM27. At the end of the follow-up period, mean HbA1c values were statistically significantly different between the two arms, with HbA1c of 6.5% and 7.3% for the intensive and standard treatment groups, respectively. A small but statistically significant difference in the composite end point of the incidence of major microvascular and macrovascular events between groups was observed: 18.1% in the intensive treatment group versus 20% in the standard treatment group. Microvascular events, particularly new-onset or progression of nephropathy, contributed disproportionately to this difference, as no differences were observed in the incidence of macrovascular events or death between the groups. Moreover, no differences in retinopathy or mortality were observed during long-term follow-up28 (TABLE 1; Supplementary Table 1). Unlike the ACCORD trial, this trial did not identify an increased risk of death as a potential harm of intensive treatment. However, the ADVANCE trial also did not confirm major benefits in pursuing the intensive treatment goals, at least in comparison with moderate control (that is, HbA1c 7.3%).
The Veterans Affairs Diabetes Trial.
The Veterans Affairs Diabetes Trial (VADT) was designed to evaluate the effect of intensive control on macrovascular complications in older patients with long-standing T2DM and complications29,30. The VADT randomized military veterans with poorly controlled T2DM (HbA1c >7.5%) to either intensive treatment (HbA1c target of <6% achieved with two oral agents and insulin) or standard treatment (HbA1c target of <9%)31. The primary outcome was the time to the first occurrence of a major cardiovascular event31. VADT had a goal of 1.5% difference in HbA1c levels between the treatment groups, which was achieved, with the intensive group approaching an HbA1c of ~7%. Both treatment groups had fewer than predicted cardiovascular events, but no difference was observed in the time to the first occurrence of a major cardiovascular event. Of note, intensively treated individuals had statistically significantly more episodes of hypoglycaemia and more serious adverse events than the standard treatment group. The only statistically significant differences observed in microvascular events was a lower risk of progression of albuminuria in the intensive treatment group (TABLE 1; Supplementary Table 1).
At 10 years, intensive treatment was found to have resulted in a small but statistically significant 17% relative reduction in the time to first major cardiovascular event, suggesting a potential legacy effect32. This reduction was not maintained at 15 years, when the separation of HbA1c levels between the intensive and standard treatment groups had vanished33. These data indicate that long-term glycaemic control might result in a small reduction in the risk of cardiovascular events, but not mortality; however, the reduction in cardiovascular risk wanes as glycaemia worsens.
Interpreting the trials as a whole.
The marked differences in the design and results of these trials make synthesizing their outcomes into a single, cohesive HbA1c target challenging. Overall, these data create a consistent picture that microvascular risk reduction is an expected benefit of more intensive treatment aiming for HbA1c levels of <6–7%, with the greatest absolute risk and risk reduction occurring in patients with high HbA1c levels at baseline (>9%). As HbA1c approaches 7%, further HbA1c lowering provides diminishing returns, in part because the absolute risk of complications is low at this level of HbA1c. Whether these microvascular benefits are clinically important varies based on baseline HbA1c, the change in HbA1c, and the duration of intervention.
The case for aiming for a near-normal HbA1c to achieve cardiovascular benefit is much less convincing34. The cause of the excess mortality in the ACCORD trial remains unclear; however, this excess mortality was probably unrelated to the HbA1c level achieved but could have been associated with the method used to lower glucose levels35. The findings of the UKPDS lend further support to the notion that outcomes might be related to the specific methods or medications employed to lower blood glucose concentrations. Finally, observational extensions of several of these studies support the existence of a legacy effect or metabolic memory, suggesting that good glycaemic control achieved in the first decades of disease could provide sustained benefits over time. As such, despite these well-designed, well-executed trials, HbA1c targets remain controversial and interpretation of these trials has led to conflicting guidelines.
Current guidelines
Three US-based societies offer updated guidelines for HbA1c targets: the American Diabetes Association (ADA), the American Association of Clinical Endocrinologists (AACE)–American College of Endocrinology (ACE), and the American College of Physicians (ACP)36–38. Additional guidelines are available from other US-based and international health organizations39–42. There are consistencies in the recommendations of these guidelines. For example, they all recommend that individualized HbA1c targets be established based on the patient’s age, life expectancy, comorbidities and risk of hypoglycaemia. In general, an HbA1c of <8% should be achieved in all individuals, unless they have substantially reduced life expectancy or the method employed will result in unacceptable consequences such as cost, complexity or hypoglycaemia. The guidelines (summarized in TABLE 2) diverge on the HbA1c target that should be aimed for in healthy individuals.
Table 2 |.
Guidelines for HbA1c targets in non-pregnant adults
| Organization | HbA1c target (%) | Qualifications | Ref. | |
|---|---|---|---|---|
| For most patients | For patients with shortened life expectancy or severe hypoglycaemia | |||
| ADA and EASD | <7 | <8 | GLP1 receptor agonist and/or SGLT2 inhibitor should be considered independently of HbA1c target in certain patient populations (C/AD, HF, CKD) | 36 |
| AACE–ACE | <6.5 | 7–8 | – | 37 |
| ACP | 7–8 | Avoid setting target | Consider de-escalation of therapy if HbA1c <6.5% | 38 |
| NICE | <6.5, <7 on hypoglycaemic drugs | Relax target | – | 39 |
| VA/DoD | 6–7 | 8–9 | Recommend taking patient characteristics (e.g. race, ethnicity, CKD and laboratory issues) into account when interpreting HbA1c | 40 |
| ICSI | <7 to <8 | <8 | – | 41 |
| SIGN | <7 | – | Target of <6.5% at diagnosis may be appropriate | 42 |
AACE, American Association of Clinical Endocrinologists; ACE, American College of Endocrinology; ACP, American College of Physicians; ADA, American Diabetes Association; CAD, coronary artery disease; CKD, chronic kidney disease; EASD, European Association for the Study of Diabetes; HbA1c, glycated haemoglobin A1c; HF, heart failure; ICSI, Institute for Clinical Systems Improvement; NICE, National Institute for Health and Care Excellence; SIGN, Scottish Intercollegiate Guidelines Network; VA/DoD, US Department of Veterans Affairs and Department of Defense.
The ADA posits that an HbA1c of <7% is a reasonable target for most non-pregnant adults, citing lower microvascular complications in patients who achieve this target36. However, they note a much smaller absolute reduction in risk in patients with microvascular disease with an HbA1c of <6.5% and suggest that this level is an acceptable target only if it can be achieved without resulting in clinically significant hypoglycaemia or other adverse effects. The ADA cautions against aggressively reducing HbA1c in patients with high cardiovascular risk, reflecting the increased mortality observed in the ACCORD trial. In addition, the ADA offers different targets for older patients, with HbA1c <8% being advised for healthy patients over 65 years of age and <8.5% for older patients with notable comorbidities. Of note, the recommendations of the European Association for the Study of Diabetes (EASD) align with those of the ADA43. Most importantly, however, both the ADA and EASD qualify the HbA1c targets in the setting of atherosclerotic cardiovascular disease, chronic kidney disease and heart failure in stating that “the decision to treat with a GLP1 receptor agonist or SGLT2 inhibitor to reduce major adverse cardiovascular events, heart failure hospitalization, cardiovascular death, or chronic kidney disease progression should be considered independently of baseline HbA1c or individualized HbA1c target”43.
Although the target is slightly lower, the AACE–ACE and ADA guidelines are generally similar. For example, the AACE–ACE guidelines recommend individual targets but suggest that most patients should aim for an HbA1c of <6.5% if safe and affordable37. This target is set to reduce the lifetime risk of microvascular and macrovascular events in individuals with recent onset diabetes mellitus and no cardiovascular risk. Less stringent targets are set for older individuals and patients with hypoglycaemia.
Conversely, the ACP guidelines strongly recommend an HbA1c target between 7% and 8%38. The ACP approached the guidelines as a systematic review, collecting and rating the available guidelines36,37,39–42 to form a guidance statement38. All guidelines included in the ACP review recommend an HbA1c of 7% or less for most individuals. However, the ACP suggests a target between 7% and 8% for most individuals, citing evidence that targets of less than 7% do not reduce death or macrovascular outcomes. Hypoglycaemia and increased risk of death were named as concerns for targets less than 7%. No HbA1c target is suggested for patients who are 80 years of age or older, reside in a nursing home, or have chronic conditions (for example, dementia, cancer, end-stage kidney disease, severe chronic obstructive pulmonary disease or heart failure) that considerably limit life expectancy. They suggest that more aggressive targets might be acceptable for patients with more than 15 years life expectancy and who understand the risk of harms, “including but not limited to hypoglycaemia, patient burden, and pharmacologic costs”.38 Although the AACE–ACE guidelines recommend a target of <6.5%, the ACP’s third guidance statement suggests that if an HbA1c of <6.5% is achieved, physicians should consider de-intensification of pharmacological therapy to reduce cost, patient burden and risk of hypoglycaemia.
Globally, hyperglycaemia management guidelines with regard to targets substantially overlap. The differences are due to varying opinions on how best to personalize targets; however, this has not been examined in trials and is informed by observational data. Regardless, the discordant nature of the guidelines is confusing for clinicians and patients44. The question thus remains: is there an ‘ideal’ target and if not, how do we individualize HbA1c targets?
Epidemiology of HbA1c
At what HbA1c levels are outcomes affected — how low should we go?
Large meta-analyses and observational studies have provided insight into the HbA1c levels at which the rate of macrovascular outcomes is affected. For example, meta-analyses of randomized controlled trials have suggested that intensive treatment decreases macrovascular events in patients with T2DM45,46. Consistently, in a cohort of >200,000 patients with T2DM registered in the Swedish National Diabetes Register, HbA1c above 7% was strongly associated with risk in all outcomes, including death from any cause, stroke, myocardial infarction and heart failure47. However, studies have also demonstrated a U-shaped relationship between HbA1c and all-cause mortality and cardiac events48,49. One retrospective study of >900,000 patients with diabetes mellitus in the US Veterans Affairs Healthcare System demonstrated increasing cardiovascular disease-related death as HbA1c exceeded 7%50. Yet, an HbA1c of <6% was associated with higher all-cause mortality compared with an HbA1c between 6% and 6.9%, suggesting that a narrow range of HbA1c provides the most clinical benefit in patients with T2DM.
To complicate matters further, it has long been appreciated that timing of glycaemic control might affect outcomes. As mentioned above, studies from the DCCT–EDIC and UKPDS suggest that early, intensive control could have lasting benefits, an outcome termed ‘metabolic memory’ or the ‘legacy effect’15,24. The findings of observational studies support this hypothesis. For example, among patients with newly diagnosed T2DM, an HbA1c of >6.5% in the first year after diagnosis was associated with a higher risk of diabetic complications than an HbA1c of <6.5%51. Furthermore, an HbA1c of >7% in the first year was associated with an increased risk of future mortality51. These data suggest that early, tight control is critical in diabetes mellitus management. However, it is not possible to determine whether these differences were due to differences in disease severity at the time of diagnosis, patient or provider characteristics, or genetic factors influencing HbA1c.
Multifactorial intervention trials have been designed to determine whether the predictive value of HbA1c is improved by other factors. These trials are also affected by variable findings. For example, in the Steno-2 trial, 160 patients with T2DM were randomized to conventional treatment based on local guidelines (HbA1c <7.5% for the majority of the trial) or intensive treatment aiming for an HbA1c of <6.5% and tight blood pressure and cholesterol control52. Very few patients had achieved the target HbA1c by the end of the trial, with patients achieving HbA1c of 9.0% and 7.8% in the conventional and intensive treatment groups, respectively52. Patients in the intensive intervention group exercised more as recommended and achieved lower blood pressure, total cholesterol and LDL cholesterol than those in the conventional treatment group. Importantly, the intensive intervention reduced the risk of cardiovascular and microvascular events substantially, despite these patients achieving HbA1c only modestly below 8%, suggesting that HbA1c alone might not be the biggest predictor of outcomes52. These differences were maintained at 13 years follow-up, despite convergence of HbA1c, blood pressure and cholesterol levels53.
Less impressive effects were seen in the Japan Diabetes Optimal Treatment study for three major risk factors of cardiovascular disease (J-D OIT3) trial. In the J-DOIT3 trial in patients with T2DM the target HbA1c was <6.9% with similar blood pressure and lipid targets as in the Steno-2 trial; however, J-D OIT3 demonstrated minimal long-term benefit54. The HbA1c achieved in J-DOIT3 was 6.8% in the intensive treatment group as compared with 7.2% in the conventional treatment group. Moreover, blood pressure and LDL were statistically significantly lower in the intensive treatment group. No difference was seen in the primary outcome of major cardiovascular events, although post-hoc analysis revealed a reduction in cerebrovascular events in the intensive treatment group54. Of note, the conventional treatment group in the J-DOIT3 trial achieved good glycaemic control, and the limited contrast in HbA1c between the treatment groups constrains conclusions; however, the study does suggest that a modest reduction in HbA1c near 7% is associated with minimal benefit.
Perhaps more importantly, these studies recognize that HbA1c is not the only predictor of macrovascular outcomes and acknowledge other modifiable risk factors, such as blood pressure and cholesterol levels52,54. The findings of these observational studies support and extend suggestions from the randomized controlled trials. Namely, it seems that attaining an average HbA1c of ≤7.5% over decades is associated with generally good outcomes. Furthermore, early efforts at glycaemic control are associated with improvements in long-term prognosis. In addition, even when excellent glycaemic control is not achieved, good cardiovascular risk management is associated with improved outcomes for both cardiovascular and microvascular disease. However, an HbA1c of <7% in epidemiological studies is not consistently associated with better outcomes in patients with T2DM.
Similarly, large cohort studies in patients with T1DM have suggested that HbA1c is a strong predictor of death, myocardial infarction and stroke, together with albuminuria, duration of T1DM, blood pressure and LDL cholesterol55. In observational studies with up to 25 years follow-up in Swedish patients with T1DM, no patients with a long-term weighted mean HbA1c of <7.6% developed proliferative retinopathy or persistent macroalbuminuria56. As the development of kidney disease is strongly correlated with mortality in patients with diabetes mellitus, these data indicate that maintenance of a mean HbA1c of <7.6% over time could reduce mortality but provide no indication that normalizing HbA1c improves outcomes56,57.
What HbA1c level is achieved?
The National Health and Nutrition Examination Survey (NHANES) has examined the prevalence and control of diabetes mellitus over the last three decades. These nationally representative cross-sectional studies suggest that the results of the DCCT reported in 1993 and the subsequent associated guidance to aim for an HbA1c of <7% impacted national diabetes mellitus care. For example, the proportion of individuals with diabetes mellitus who achieved an HbA1c of <7% improved from 43.2% in 1989–1994 to 57.0% in 2003–2006 (REF.2). However, the publication of the ACCORD, ADVANCE and VADT trials in 2008 and subsequent modifications in guidance towards greater individualization was temporally associated with a decline in the proportion of patients with diabetes mellitus achieving an HbA1c of <7% to 50.8% in 2011–2014 (REFS2,58). A similar trend was seen for the prevalence of individuals with an HbA1c of <8%, with a peak of well above 75% of people with diabetes mellitus achieving this target in 2003–2006, followed by a decline to 70% in 2011–2014 (REF.2). These observations were confirmed and extended by the US Healthcare Effectiveness Data and Information Set. This tool includes data from over 1,000 health plans covering more than 171 million people, and demonstrated in 2014 that only 40% of commercially insured health-care maintenance organization patients and 30% of government-insured patients achieved an HbA1c of <7%59. More disturbingly, younger patients (aged 20–49 years) were significantly less likely to achieve an HbA1c of <7% than older adults (aged >75 years), which amplifies the long-term risk of poor outcomes in the young and could amplify the risk of adverse events in older people2.
At least in the clinical trial setting, an HbA1c of <7% can be achieved with minimal pharmacotherapy by most patients with recent onset T2DM. For example, more than 70% of patients with an HbA1c between 7% and 10% on no baseline pharmacotherapy who were randomized to subcutaneous semaglutide (0.5 or 1.0 mg weekly) had achieved an HbA1c of <7% after 30 weeks60. Furthermore, in trials, even patients with T2DM treated with basal insulin at baseline can achieve an HbA1c of <7% with the addition of non-insulin pharmacotherapy. For example, 79% of Japanese patients with T2DM and an HbA1c between 7% and 10% treated with stable basal insulin with or without metformin at baseline achieved an HbA1c of <7%, with the addition of subcutaneous semaglutide 1.0 mg weekly61. These examples suggest that HbA1c targets are attainable. However, confusion about appropriate glycaemic targets, cost, access to health care, or other factors, are barriers that dominate the landscape of diabetes care.
Limitations of HbA1c
The utility of HbA1c as a marker for glycaemic control and diabetes mellitus outcomes is unassailable, but HbA1c does have limitations. Years ago, variability within and among testing methods limited the clinical utility of HbA1c (REF.62). Considerable efforts to standardize the measurement of HbA1c have been essential to its rise as the gold standard for measuring glycaemic control63. Some variability remains across laboratories and different methods of measuring glycated haemoglobin; however, HbA1c is certainly more consistently and reproducibly measured than glucose. Moreover, it is perhaps under-appreciated that a wide range of mean glucose values can be associated with a given HbA1c level64–66.
Variability between HbA1c and glucose concentration.
Continuous glucose monitors (CGM; discussed in more detail later) demonstrate variability in the correlation between HbA1c and average CGM-measured glucose concentrations67,68. This variability could reflect imprecision in CGM measurements. One analysis compiled data from three randomized trials using CGM to determine the relationship between HbA1c and mean glucose concentration in patients with T1DM67. The analysis showed that the 95% confidence interval for mean glucose concentration for an HbA1c level of 8.0% substantially overlapped that for HbA1c levels of 7.0% and 9.0%67 (FIG. 1). Thus, an HbA1c of 8% could represent good, moderate or poor glycaemic control as determined by CGM. This finding is not surprising, as any mean is susceptible to outliers and can be associated with a wide distribution of values to achieve the same mean; however, glycaemic variability and imprecision in CGM data does not tell the whole story.
Fig. 1 |. CGM-measured mean glucose concentration versus HbA1c.
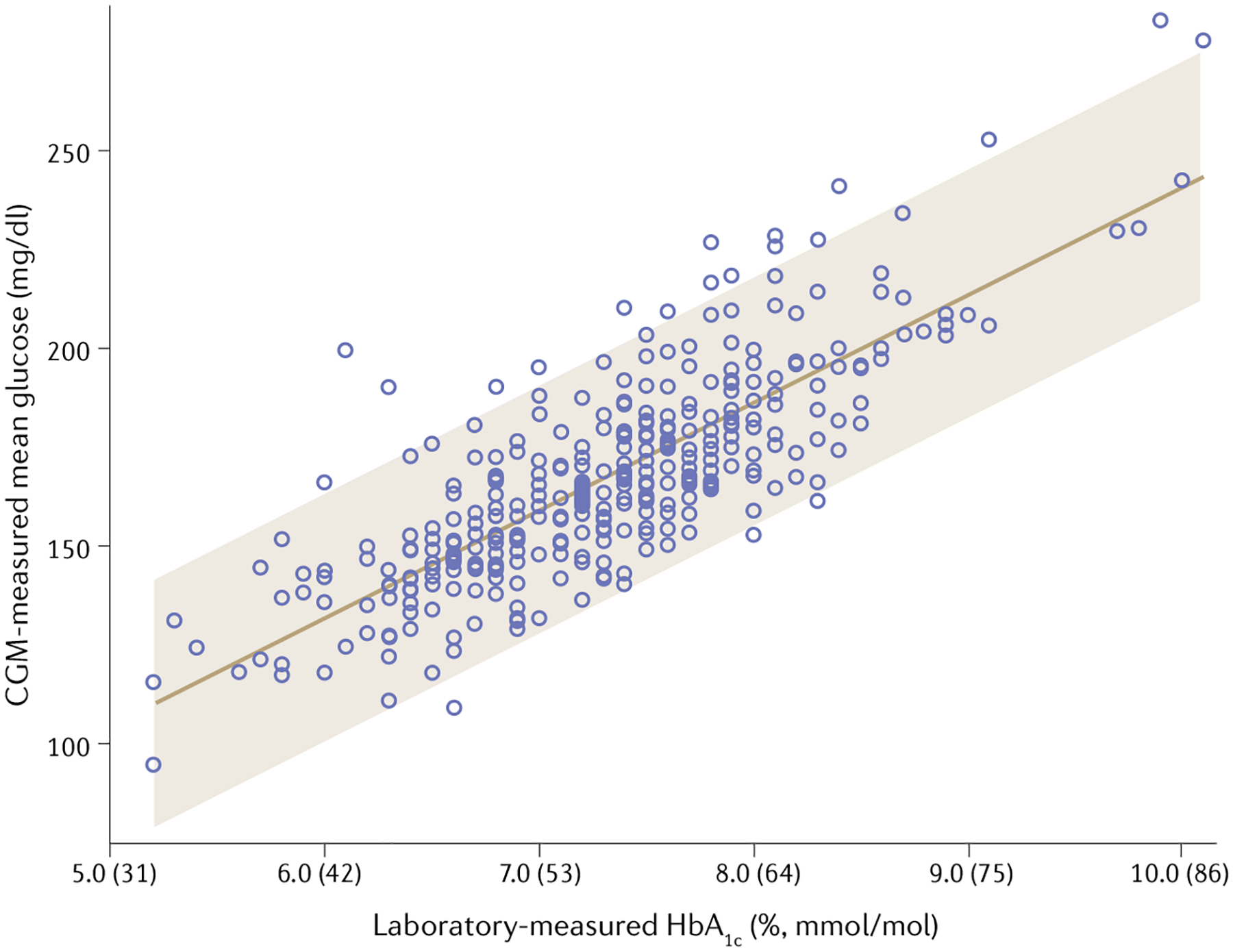
Continuous glucose monitoring (CGM) measurements from three randomized controlled trials were pooled and plotted against laboratory measured glycated haemoglobin A1c (HbA1c). The 95% confidence interval for a patient’s mean glucose concentration predicted from a laboratory measurement of HbA1c is shown by the shaded area. This figure highlights the wide range of mean glucose concentrations obtained by cCGM that a single HbA1c can represent. The figure has been reproduced with permission from REF.67, American Diabetes Association, Beck, R. W., Connor, C. G., Mullen, D. M., Wesley, D. M. & Bergenstal, R. M. The fallacy of average: how using HbA1c alone to assess glycemic control can be misleading. Diabetes Care 40, 994–999 (2017). Copyright and all rights reserved. Material from this publication has been used with the permission of American Diabetes Association.
Genetic and biological factors.
In addition to mean glucose over time, genetic and biological factors affect haemoglobin glycation69. Studies in twins have suggested that population variance in HbA1c levels is largely genetically determined70. Differences in red blood cell lifespan, erythrocyte membrane permeability and glucose variability affect haemoglobin glycation65,71,72. Additionally, conditions that affect haemoglobin will also affect its glycation, such as haemolysis, anaemia, transfusions and haemoglobinopathies. HbA1c can therefore be an inaccurate surrogate for average blood glucose concentrations in certain individuals and populations. Patients with kidney disease have reduced red blood cell survival, which artificially lowers HbA1c (REF.73). Conversely, iron deficiency has been associated with increased HbA1c (REF.64). Moreover, liver disease, which affects protein synthesis and causes anaemia, makes glycated haemoglobin an unreliable marker of glycaemia74. In addition, conditions such as haemolytic anaemia, thalassaemia and pregnancy will also alter erythrocyte lifespan and affect HbA1c interpretation75,76.
Even in the absence of these conditions, HbA1c can be misleading as an index of average glycaemia. For example, in cystic fibrosis-related diabetes, HbA1c underestimates the degree of hyperglycaemia77. Additionally, older age is associated with higher HbA1c for unclear reasons, although this effect is thought to be independent of glycaemia78. A study in middle-aged and older Chinese individuals without a prior diagnosis of diabetes mellitus suggests that age-related increases in HbA1c are associated with decreased erythrocyte count79. When data were adjusted for erythrocyte count, the negative association between age and HbA1c disappeared.
Although contentious, evidence suggests that race and ethnicity can affect HbA1c. For example, several studies have demonstrated that HbA1c overestimates mean blood glucose concentrations in Black American individuals compared with white American individuals80,81. Similar trends have been demonstrated in other racial–ethnic groups even after adjustment for factors that affect glycaemia81. Whether these differences are clinically meaningful remains controversial82,83. Studies directly comparing the prognostic value of clinical categories of HbA1c across ethnicities have found no differences in the association of HbA1c with retinopathy84. Others have indicated that retinopathy begins at lower HbA1c levels in Black American individuals than in white American individuals85. These studies provide evidence against setting a higher threshold for treatment of Black American people or other ethnic groups. Moreover, considerable and appropriate concern exists that interpretation of HbA1c differently for racial–ethnic minorities will increase health disparities86. Prior to use in clinical decision-making, more basic and clinical research is necessary to identify and understand specific genetic and socially-mediated risk that collectively result in racial–ethnic differences in HbA1c.
Many investigators have attempted to understand how to assess variations in HbA1c as compared to mean glucose concentrations between individuals. One proposed method is the haemoglobin glycation index (HGI), an index of the difference between observed HbA1c and the HbA1c predicted from mean plasma glucose or FPG concentrations, which might potentially be used to quantify interindividual differences in HbA1c that are independent of glucose concentration87,88. A patient with a high HGI would have a higher HbA1c than that predicted from glucose measurements. Numerous observational studies have demonstrated associations between high HGI and vascular complications, suggesting that the biological determinants of haemoglobin glycation affect outcomes independent of glycaemia88–90. Therefore, we suggest that lowering blood glucose concentrations, particularly with insulin, in patients with high HGI might result in unexpected hypoglycaemia. Evidence for this hypothesis has been provided by an analysis of intensive versus standard treatment in the ACCORD trial by HGI tertile, which demonstrated increasing frequency of adverse events and mortality with higher HGI91. Furthermore, machine learning analysis of the ACCORD trial data suggested that HGI could help individualize treatment and identify patients who might benefit from more intensive HbA1c targets and patients in whom an intensive glycaemic control strategy could cause notable harm92. However, analysis of the ADVANCE trial data demonstrated that HGI predicted the risk for complications, but was not better than HbA1c (REF.93). These issues and others have stalled the clinical use of HGI; however, further investigation is warranted to tailor HbA1c targets for individuals as suggested by the guidelines.
Short-term variations in glucose.
Another limitation of HbA1c is that it cannot assess short-term variations in glucose (that is, variations over 2–4 weeks). As observed in many studies (for example, ACCORD and VADT) in which insulin and sulfonylureas were used in glycaemic management, dose titration to achieve a prespecified lower HbA1c target results in significantly more hypoglycaemic episodes in intensively treated individuals25,31. These data demonstrate that adjusting anti-hyperglycaemic medications, particularly insulin, requires careful attention to daily blood glucose levels to mitigate hypoglycaemia.
Glycaemic variability.
Finally, some literature suggests that beyond HbA1c, glycaemic variability drives the risk of complications. This variability can be within-day (for example, postprandial hyperglycaemia), between days (for example, variability in fasting glucose), or even over years (including variability over time in HbA1c levels within a patient). HbA1c variability has been associated with both microvascular and macrovascular complications and mortality independently of average HbA1c level94. Studies to date have been limited with respect to adjustment for confounding but suggest that this is another area for debate and investigation.
What does this mean for HbA1c targets?
How should we incorporate these limitations with regard to the topic at hand — HbA1c targets? First, arguably the precision, accuracy and reproducibility of the HbA1c test is excellent and exceeds that of all other measures of glycaemia, including plasma glucose95. Second, in some patients, the HbA1c measure is known to not accurately reflect mean glycaemia. At extremes, the HbA1c test would be inappropriate, such as after a blood transfusion. In particular, when using insulin as a treatment, the HbA1c test alone is clearly inadequate for decision-making with regard to short-term medication adjustment, and routine glucose monitoring is recommended to prevent hypoglycaemia. Arguably, when using medications not associated with risk of hypoglycaemia, adjusting medications solely on the basis of HbA1c level is reasonable. The rationale being that in the trials and their subsequent epidemiologic analyses that demonstrated microvascular benefits of glucose lowering (TABLE 1; Supplementary Table 1), it was the HbA1c level that was aimed for and it was the HbA1c level achieved that was associated with benefits. Of note, we argue that the variance in average glycaemia demonstrated by CGM between individuals with the same HbA1c level is not a fatal flaw of the HbA1c test67. This variance is in part due to limitations of the HbA1c test and in part due to limitations of CGM. In most patients, the variance in mean glucose concentrations from the population mean is modest. And most importantly, in trials, HbA1c has been well validated both as a predictor of clinical outcomes and as a target for glycaemic interventions (TABLE 1; Supplementary Table 1) and is certainly easier and cheaper to obtain than frequent glucose measures64. Additional information is certainly obtainable from other measures. However, the HbA1c test for now is the one test with prospective randomized clinical trial data that validate its effectiveness as a measure to monitor glycaemic control.
Alternatives to HbA1c
Self-monitored blood glucose (SMBG) continues to be a commonly used tool in the assessment of glycaemia. Its utility in the management of insulin-treated patients with diabetes mellitus is incontrovertible. SMBG provides a single snapshot of blood glucose concentration as the levels fluctuate over time. By contrast, the HbA1c test provides an index of the overall effect of glucose on a tissue (that is, red blood cells) over months. These tests are complementary and are not substitutes for one another.
Glycated serum proteins have been proposed as an alternative to HbA1c. Serum proteins are not affected by erythrocyte turnover and as such are not influenced by conditions such as haemolysis and anaemia, or by blood transfusions. These proteins turn over more rapidly than red blood cells, and thus they can represent a shorter period of mean glucose levels (2–3 weeks)64. Fructosamine and glycated albumin are both ketoamines, which form when glucose binds serum proteins in a non-enzymatic process96. Fructosamine assays measure all glycated serum, which in human serum largely reflects the levels of glycated albumin, as glycated albumin makes up about 80% of all glycated proteins96. Glycated albumin can also be reported as the proportion of total albumin. Fructosamine and glycated albumin are strongly associated with HbA1c and fasting blood glucose96. These tools can be useful in practice in patients for whom HbA1c might not be accurate or where detection of rapid changes in glycaemia are necessary (for example, when adjusting medication dosage). However, widespread use has been hampered by lack of trial-driven treatment targets and assay standardization64.
1,5-Anhydroglucitol (1,5-AG), a six-carbon monosaccharide that competes with glucose for renal reabsorption, has also been studied as an adjunctive measure to assess glycaemic excursions in combination with HbA1c (REFS97,98). During periods of hyperglycaemia that are in excess of the renal threshold for glucose reabsorption, tubular glucose competes with 1,5-AG for reabsorption, leading to a reduction in 1,5-AG in serum. Despite similar HbA1c and FPG values, patients treated with multiple daily insulin injections demonstrated fewer glycaemic excursions as measured by SMBG and significantly higher serum 1,5-AG concentrations compared with patients on basal insulin alone, thereby suggesting a role for the molecule as an index of postprandial glycaemic control throughout the day99. Further studies have confirmed that 1,5-AG reflects glycaemic excursions more robustly than HbA1c or fructosamine, making it a potentially valuable complementary measure in the assessment of glycaemic control100. 1,5-AG was approved for use as a short-term marker of glycaemic control in diabetes mellitus in the USA in 2003, but has not been widely adopted97.
Continuous glucose monitoring.
The increasing availability and affordability of CGM has offered new insights into individual glycaemic patterns. If SMBG is a snapshot of glucose concentration at one specific moment, CGM is comparable to a movie of glucose fluctuations over time. CGM provides opportunities to evaluate glycaemic excursions, hypoglycaemia and hyperglycaemia, and daily patterns, thereby enabling subtle changes in treatment regimens in a way that was not previously possible. One particularly useful measure that CGM has identified is the time spent in the target glucose range (usually 70–180 mg/dl), referred to as time in range (TIR); another measure is time in ranges (TIRs), which includes TIR, time above range and time below range101. TIRs provides information about the frequency and duration of hypoglycaemia or hyperglycaemia and gives an overall assessment of glycaemic control.
Importantly, TIR has also been shown to be strongly associated with outcome measures. For example, an elegant analysis of data from patients with T1DM in the DCCT was able to validate TIR as an outcome measure for clinical trials102. Every quarter during the DCCT, seven blood fingerstick samples were collected (before meals, after meals, and at bedtime) and glucose concentrations were measured at a central laboratory. These data were analysed to calculate TIR (70–180 mg/dl) and regression models were used to assess the effects of TIR on the primary outcomes of DCCT, that is, retinopathy and microalbuminuria102. In patients with evidence of developing retinopathy, TIR was 32% compared with 44% in those with no evidence of retinopathy102. The results were strikingly similar for microalbuminuria (TIR 32% versus 42% in patients with and without microalbuminuria, respectively). Of note, 10% lower TIR was associated with 64% and 40% increases in the adjusted hazard ratios for retinopathy and microalbuminuria, respectively. Studies have also demonstrated a similar association between TIR and diabetic retinopathy in T2DM103.
CGM can be used to assess the correlation between the mean interstitial glucose concentration and HbA1c. Formulas have been validated that determine the glucose management index (formerly known as estimated HbA1c), which provides an expected HbA1c level from CGM-derived mean glucose concentration67,104. A glucose management index that does not match measured HbA1c might suggest the need for alternative HbA1c targets than those expected based on guidelines.
The measurement of TIRs has also been shown to empower individuals and help them manage their diabetes mellitus on a day-to-day basis105. For example, high and low glucose alerts can help patients adjust their insulin regimen, avoid hypoglycaemia, and increase awareness of nocturnal hypoglycaemia and hyperglycaemia. In these ways, CGM has been shown to increase hypoglycaemic confidence and decrease distress associated with diabetes mellitus, thus improving diabetes-related quality of life106. However, CGM, too, has limitations. CGM measures glucose concentrations in interstitial fluid, which can vary from plasma glucose levels. Interpretation of CGM takes experience and is often difficult for health-care providers, and its clinical impact can therefore be variable107. Many patients can be overwhelmed by the data, rather than empowered. Most importantly, even in countries where HbA1c is considered inexpensive, CGM remains unaffordable for most108. In the USA, CGM is not generally funded by insurers for T2DM, except in a patient using multiple daily injections of insulin and frequent blood glucose monitoring109.
Summary.
These alternative biomarkers and technologies are useful in particular contexts, but also have substantial limitations, which are largely outside the scope of this review. However, it remains clear that no single alternative is positioned to replace HbA1c for assessing glycaemic control in most patients with diabetes mellitus. Both SMBG and CGM have a clear role in the management of diabetes mellitus, particularly in intensively treated patients treated with insulin in the context of team-based care. Glycated serum proteins are highly useful in certain patients and situations but have not been widely validated either as markers for outcomes or as targets in prospective studies96. As a measure of overall glycaemia and target for treatment, the HbA1c test has myriad advantages over CGM including cost, patient burden, provider burden, precision, accuracy, reproducibility and a much larger evidence base supporting its use. That said, CGM provides much greater granularity regarding moment-to-moment glycaemic control. As technology improves and becomes more affordable, with the appropriate outcome trials, CGM could displace HbA1c as the optimal instrument, not only for patient self-management but also for guideline-driven advice from providers to target glycaemic control, particularly in patients with T1DM. For today, for most people with diabetes mellitus, the HbA1c test remains the gold standard.
The elusive optimal HbA1c target
Despite its shortcomings, HbA1c is the best validated biomarker of glycaemic control for use as a prognostic factor of the complications of diabetes mellitus across the spectrum of disease. The remaining question is at what target should we aim?
Based on nearly three decades of accumulating data, we suggest that for people with diabetes mellitus and moderate life expectancies (10–25 years), achieving an average HbA1c of ≤7.5% should minimize the risk of disabling microvascular complications. For patients with long life expectancies (for example, >30 years), one should aim to achieve near normal HbA1c as long as it can be practically and easily achieved without undue patient burden (such as cost or complexity) or notable adverse events. In most patients with T2DM early in the disease course, an HbA1c of <7% is achievable and will be easier to achieve than later in the course of the disease (barring new developments in diabetes mellitus care). The overarching goal should be to maintain the average HbA1c over the life-course at ≤7.5%. We would stipulate further that the effort to control glycaemia must be integrated into an overall programme of preventive care behaviours.
Finally, it is clear that the tools, including technology, pharmacotherapy and bedside manner, employed in managing diabetes mellitus are as important as the target in the effort to achieve the aim of lifelong optimal quality of life. In patients with T1DM, insulin is life-saving. In selected patients with T2DM, it is an acceptable option when used expertly, as using complex insulin regimens to pursue more stringent HbA1c targets in patients with T2DM might be associated with more harm than benefit. In patients with diabetes mellitus and diabetic kidney disease or heart failure, or who are at very high risk of cardiovascular events, an SGLT2 inhibitor and/or a GLP1 receptor agonist should be used in all patients, independently of HbA1c target or level achieved. We do not favour the routine withdrawal of medications based on an HbA1c level achieved, as suggested by the ACP38, although it is certainly appropriate in patients with any drug-related symptomatology, particularly weight gain or hypoglycaemia.
One reason to advocate with patients for an HbA1c target of <7% is to avoid therapeutic inertia. Data shows that in patients with T2DM with an HbA1c of >7.0%, >7.5% or >8.0% on one oral anti-hyperglycaemic drug, the median times to drug intensification are 2.9, 1.9 or 1.6 years, respectively110. The time increases to >7 years in patients on two oral anti-hyperglycaemic drugs110. Initiation of insulin took >6 years in individuals on oral anti-hyperglycaemic agents110. Aiming for a more ambitious HbA1c of <7% makes it more likely that the level achieved by a patient will be ~7.5% over time, and therefore more beneficial, than aiming for an HbA1c of <8%. It is important to appropriately frame the result in each context. A target of <7% is a goal, aspiration and hope, but not a requirement. However, we suggest that an HbA1c target of <8% should be considered a requirement in patients with a life expectancy of >10 years, as there are clear medium term measurable outcome differences in patients with persistent HbA1c levels above and below 8%.
In patients with T1DM, CGM has quickly become the standard of care. TIR and glucose management index are being used in conjunction with HbA1c to help optimize insulin regimens. In patients with T1DM, we advocate for the same targets (HbA1c <7% as an aspiration, HbA1c <8% as a requirement) and arguably the evidence base for these targets is stronger13–15,17,18,20,55,56. Elimination of severe and asymptomatic hypoglycaemia is as important a goal as trying to achieve a lower HbA1c or a higher TIR. CGM has also enabled major advances in insulin pump technology — hybrid closed loop systems — which are providing more TIR, whilst simultaneously reducing hypoglycaemia111. As these technologies become more advanced, there is the potential to nearly eliminate hypoglycaemia whilst achieving near normoglycaemia. Data from a 2019 study in individuals prior to the availability of this technology still suggest that aiming for an HbA1c of <6.5% in patients with T1DM does not result in significant improvement in microvascular outcomes and might result in worse outcomes related to hypoglycaemia112.
Conclusions
Unfortunately, disagreements between organizations and bluster among their advocates regarding specific HbA1c targets has done little to inform clinical decision-making. Ultimately, as stated in the existing guidelines, glycaemic targets must be individualized. Shared decision-making between health-care providers and patients that is based on mutual respect and an adequate understanding of the issues is essential113. A mutual understanding between patients and health-care providers of what a target means (aspirational target versus a requirement) is something that should be established. Plans should be in place for what occurs when a glycaemic target is reached. Frequent reviews of medications and potential adverse events of therapy are important.
Although an HbA1c target of <7% is appropriate for most individuals, this target is set as an aspiration with the intention of having most individuals achieve a lifelong HbA1c of ≤7.5%. We propose that targets should be more stringent in younger patients with long life expectancies with diabetes mellitus, due to the potential legacy effect (BOX 2) and the generally low risk of severe hypoglycaemia51. There is something to be said for aiming for ‘better’ (that is, any improvement as opposed to explicitly aiming for an HbA1c of <7%) in patients who have chronically inadequately controlled hyperglycaemia. Although we reflect that the ADA target of <7% is still appropriate in patients with poor control (HbA1c >9%), aiming for modest improvements (0.5–1%) and incremental gains is optimal. Specifically, creating a flexible, iterative patient-specific plan where a goal is achieved is better than dogmatically sticking to guidelines. Much less-aggressive treatment is appropriate for patients with a life expectancy of <10 years, as complications take a long time to progress to consequences and the near-term risk of hypoglycaemia is arguably graver.
Hypoglycaemia avoidance should be as important a goal as hyperglycaemia avoidance at virtually every stage of life, but particularly in patients with a limited lifespan. This issue is most critical in the setting of T1DM, where hypoglycaemia is more common and more frequently severe114. However, HbA1c levels substantially more than 8% are associated with progressive symptoms — polyuria, weight loss, fatigue, blurred vision, infections and thromboses. In frail older people and those with decreased life expectancy, carefully assessing symptoms and trying to find optimal care plans is just as challenging as the stringent efforts in young adults. With the availability of at least nine classes of medications that are not associated with hypoglycaemia approved for T2DM management, achieving targets without substantial hypoglycaemia risk is achievable in most patients with T2DM.
HbA1c is the gold standard for evaluating the medium term risk of complications related to hyperglycaemia. Adjunctive care with SMBG is essential in the setting of T1DM and CGM has emerged as an increasingly affordable and useful tool. No tool or technology will replace the art of helping patients determine their own personal goals and assess the determinants of their quality of life. Although HbA1c remains the general index of choice for monitoring glycaemia for most, health-care providers and their patients must work together to determine the appropriate HbA1c target and optimal approach to its attainment.
Supplementary Material
Key points.
Glycated haemoglobin (HbA1c) targets are controversial due to conflicting results from large-scale clinical trials in patients with type 1 and type 2 diabetes mellitus.
Observational studies in patients with type 1 diabetes have shown that achieving an average HbA1c of ≤7.5% over 25 years is associated with a low risk of disabling microvascular complications.
Data from large-scale outcome trials in patients with type 1 and type 2 diabetes mellitus have demonstrated that achieving an HbA1c of ~7% is associated with microvascular benefit as compared with higher levels of HbA1c, but less clear evidence exists for macrovascular outcomes.
Although it is the gold standard for monitoring glycaemic control, HbA1c has limitations that are not widely appreciated.
The advent of novel technology (especially continuous glucose monitors) and therapeutic agents (GLP1 receptor agonists and SGLT2 inhibitors) have created additional reasons for a more flexible approach to selecting HbA1c treatment targets. No tool, technology or pharmacotherapy will replace the importance of shared decision-making based on mutual respect and understanding between patients and health-care providers to individualize HbA1c targets.
Acknowledgements
K.R.K. acknowledges the support of the University of North Carolina Department of Medicine Physician Scientist Training Program. J.B.B. acknowledges the support of grants from the National Institutes of Health (UL1TR002489, P30DK124723). The reviewers were extremely helpful in suggesting numerous important revisions.
Competing interests
J.B.B.’s contracted consulting fees and travel support for contracted activities are paid to the University of North Carolina by Adocia, AstraZeneca, Dance Biopharm, Dexcom, Eli Lilly, Fortress Biotech, Fractyl, GI Dynamics, Intarcia Therapeutics, Lexicon, MannKind, Metavention, NovaTarg, Novo Nordisk, Orexigen, PhaseBio, Sanofi, Senseonics, vTv Therapeutics, and Zafgen; he reports grant support from AstraZeneca, Eli Lilly, Intarcia Therapeutics, Johnson & Johnson, Lexicon, Medtronic, NovaTarg, Novo Nordisk, Sanofi, Theracos, Tolerion, and vTv Therapeutics; he is a consultant to Cirius Therapeutics Inc., CSL Behring, Mellitus Health, Neurimmune AG, Pendulum Therapeutics, and Stability Health; he holds stock/options in Mellitus Health, Pendulum Therapeutics, PhaseBio, and Stability Health; and he is supported by grants from the National Institutes of Health, PCORI and ADA.
Footnotes
Supplementary information
Supplementary information is available for this paper at https://doi.org/10.1038/s41574-020-00425-6.
References
- 1.Centers for Disease Control and Prevention. National diabetes statistics report, 2020 (CDC, 2020). [Google Scholar]
- 2.Cowie CC Diabetes diagnosis and control: missed opportunities to improve health: The 2018 Kelly West Award Lecture. Diabetes Care 42, 994–1004 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Global report on diabetes (WHO, 2016). [Google Scholar]
- 4.International Diabetes Federation. IDF Diabetes Atlas 9th edn (IDF, 2019). [Google Scholar]
- 5.Almdal T, Scharling H, Jensen JS & Vestergaard H The independent effect of type 2 diabetes mellitus on ischemic heart disease, stroke, and death: a population-based study of 13,000 men and women with 20 years of follow-up. Arch. Intern. Med 164, 1422–1426 (2004). [DOI] [PubMed] [Google Scholar]
- 6.Sarwar N et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 375, 2215–2222 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y, Hu G, Yuan Z & Chen L Glycosylated hemoglobin in relationship to cardiovascular outcomes and death in patients with type 2 diabetes: a systematic review and meta-analysis. PLoS ONE 7, e42551 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Ferranti SD et al. Type 1 diabetes mellitus and cardiovascular disease: a scientific statement from the American Heart Association and American Diabetes Association. Diabetes Care 37, 2843–2863 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bommer C et al. Global economic burden of diabetes in adults: projections from 2015 to 2030. Diabetes Care 41, 963–970 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Dall TM et al. The economic burden of elevated blood glucose levels in 2017: diagnosed and undiagnosed diabetes, gestational diabetes mellitus, and prediabetes. Diabetes Care 42, 1661–1668 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang P et al. Global healthcare expenditure on diabetes for 2010 and 2030. Diabetes Res. Clin. Pract 87, 293–301 (2010). [DOI] [PubMed] [Google Scholar]
- 12.The DCCT Research Group. The diabetes control and complications trial (DCCT). Design and methodologic considerations for the feasibility phase. Diabetes 35, 530–545 (1986). [PubMed] [Google Scholar]
- 13.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med 329, 977–986 (1993). [DOI] [PubMed] [Google Scholar]; This study was the first large-scale clinical trial to investigate intensive glycaemic control for the management of T1DM and provides the foundation for modern T1DM treatment.
- 14.Lachin JM, Genuth S, Cleary P, Davis MD & Nathan DM Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N. Engl. J. Med 342, 381–389 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nathan DM et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N. Engl. J. Med 353, 2643–2653 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group. Epidemiology of diabetes interventions and complications (EDIC). Design, implementation, and preliminary results of a long-term follow-up of the diabetes control and complications trial cohort. Diabetes Care 22, 99–111 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Writing Group for the DCCT/EDIC Research Group. Coprogression of cardiovascular risk factors in type 1 diabetes during 30 years of follow-up in the DCCT/EDIC study. Diabetes Care 39, 1621–1630 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diabetes Control Complications Trial (DCCT)/Epidemiology of Diabetes Intervention and Complications (EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular outcomes in type 1 diabetes: the DCCT/EDIC study 30-year follow-up. Diabetes Care 39, 686–693 (2016).26861924 [Google Scholar]
- 19.Cleary PA et al. The effect of intensive glycemic treatment on coronary artery calcification in type 1 diabetic participants of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study. Diabetes 55, 3556–3565 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nathan DM et al. Intensive diabetes therapy and carotid intima-media thickness in type 1 diabetes mellitus. N. Engl. J. Med 348, 2294–2303 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.UK Prospective Diabetes Study Group. UK Prospective Diabetes Study (UKPDS). VIII. Study design, progress and performance. Diabetologia 34, 877–890 (1991). [PubMed] [Google Scholar]
- 22.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 352, 837–853 (1998). [PubMed] [Google Scholar]; This study was the first to demonstrate microvascular benefits with intensive glycaemic control in patients with T2DM.
- 23.UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 352, 854–865 (1998). [PubMed] [Google Scholar]; Integral stratification in UKPDS demonstrated that treatment of T2DM with metformin results in a statistically significant reduction in all-cause mortality, myocardial infarction and composite macrovascular disease, leading to metformin as the first-line agent for the management of T2DM.
- 24.Holman RR, Paul SK, Bethel MA, Matthews DR & Neil HA 10-year follow-up of intensive glucose control in type 2 diabetes. N. Engl. J. Med 359, 1577–1589 (2008). [DOI] [PubMed] [Google Scholar]
- 25.Gerstein HC et al. Effects of intensive glucose lowering in type 2 diabetes. N. Engl. J. Med 358, 2545–2559 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]; ACCORD, a study that was terminated early, was the first study to identify that intensive treatment could lead to death in patients with high-risk T2DM — data that has impacted guidelines considerably.
- 26.Accord Study Group. Nine-year effects of 3.7 years of intensive glycemic control on cardiovascular outcomes. Diabetes Care 39, 701–708 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel A et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N. Engl. J. Med 358, 2560–2572 (2008). [DOI] [PubMed] [Google Scholar]; ADVANCE demonstrated small benefits with intensive control in patients with T2DM, primarily driven by decreased nephropathy.
- 28.Zoungas S et al. Follow-up of blood-pressure lowering and glucose control in type 2 diabetes. N. Engl. J. Med 371, 1392–1406 (2014). [DOI] [PubMed] [Google Scholar]
- 29.Abraira C et al. Cardiovascular events and correlates in the Veterans Affairs Diabetes Feasibility Trial. Veterans Affairs Cooperative Study on Glycemic Control and Complications in Type II Diabetes. Arch. Intern. Med 157, 181–188 (1997). [PubMed] [Google Scholar]
- 30.Abraira C et al. Design of the cooperative study on glycemic control and complications in diabetes mellitus type 2: Veterans Affairs Diabetes Trial. J. Diabetes Complicat 17, 314–322 (2003). [DOI] [PubMed] [Google Scholar]
- 31.Duckworth W et al. Glucose control and vascular complications in veterans with type 2 diabetes. N. Engl. J. Med 360, 129–139 (2009). [DOI] [PubMed] [Google Scholar]; VADT demonstrated no macrovascular benefit of intensive control in older patients with longstanding diabetes and complications.
- 32.Hayward RA et al. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med 372, 2197–2206 (2015). [DOI] [PubMed] [Google Scholar]
- 33.Reaven PD et al. Intensive glucose control in patients with type 2 diabetes – 15-year follow-up. N. Engl. J. Med 380, 2215–2224 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marso SP, Kennedy KF, House JA & McGuire DK The effect of intensive glucose control on all-cause and cardiovascular mortality, myocardial infarction and stroke in persons with type 2 diabetes mellitus: a systematic review and meta-analysis. Diab Vasc. Dis. Res 7, 119–130 (2010). [DOI] [PubMed] [Google Scholar]
- 35.Riddle MC et al. Epidemiologic relationships between A1C and all-cause mortality during a median 3.4-year follow-up of glycemic treatment in the ACCORD trial. Diabetes Care 33, 983–990 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.American Diabetes Association. 6. Glycemic targets: standards of medical care in diabetes–2020. Diabetes Care 43, S66–S76 (2020). [DOI] [PubMed] [Google Scholar]; The ADA provides the guidelines utilized by many diabetes specialists and argues for a HbA1c target of <7%.
- 37.Garber AJ et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm – 2017 executive summary. Endocr. Pract 23, 207–238 (2017). [DOI] [PubMed] [Google Scholar]; The AACE–ACE guidelines suggest the tightest glycaemic control of the available guidelines (HbA1c <6.5%), based primarily on long-term microvascular benefit from tighter control.
- 38.Qaseem A et al. Hemoglobin A1c targets for glycemic control with pharmacologic therapy for nonpregnant adults with type 2 diabetes mellitus: a guidance statement update from the American College of Physicians. Ann. Intern. Med 168, 569–576 (2018). [DOI] [PubMed] [Google Scholar]; The ACP suggests less stringent HbA1c targets (HbA1c 7–8%), citing harms of intensive control including hypoglycaemia, death, cost and patient burden.
- 39.National Institute for Health and Care Excellence. Type 2 diabetes in adults: management (NICE, 2015). [PubMed] [Google Scholar]
- 40.US Department of Veterans Affairs & US Department of Defense. VA/DoD clinical practice guideline for the management of type 2 diabetes mellitus in primary care (VA/DoD, 2017). [Google Scholar]
- 41.Redmon B, et al. Diagnosis and management of type 2 diabetes mellitus in adults (ICSI, 2014). [Google Scholar]
- 42.Scottish Intercollegiate Guidelines Network. Management of diabetes: a national clinical guideline (SIGN, 2017). [Google Scholar]
- 43.Buse JB et al. 2019 Update to: Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 43, 487–493 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; The consensus report provides clinicians with strong recommendations for pharmacotherapy based on emerging evidence that the drug employed might matter as much as or more than the glycaemic target.
- 44.Schoenborn NL et al. Patient perceptions of diabetes guideline frameworks for individualizing glycemic targets. JAMA Intern. Med 179, 1642–1649 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ray KK et al. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta-analysis of randomised controlled trials. Lancet 373, 1765–1772 (2009). [DOI] [PubMed] [Google Scholar]
- 46.Turnbull FM et al. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia 52, 2288–2298 (2009). [DOI] [PubMed] [Google Scholar]
- 47.Rawshani A et al. Risk factors, mortality, and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med 379, 633–644 (2018). [DOI] [PubMed] [Google Scholar]
- 48.Currie CJ et al. Survival as a function of HbA(1c) in people with type 2 diabetes using differing glucose-lowering regimens. Diabetologia 59, S157–S157 (2016). [Google Scholar]
- 49.Currie CJ & Poole CD Survival as a function of HbA(1c) in people with type 2 diabetes reply. Lancet 375, 1434–1435 (2010). [DOI] [PubMed] [Google Scholar]
- 50.Raghavan S et al. Diabetes mellitus-related all-cause and cardiovascular mortality in a national cohort of adults. J. Am. Heart Assoc 8, e011295 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Laiteerapong N et al. The legacy effect in type 2 diabetes: impact of early glycemic control on future complications (the Diabetes & Aging Study). Diabetes Care 42, 416–426 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gaede P et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N. Engl. J. Med 348, 383–393 (2003). [DOI] [PubMed] [Google Scholar]
- 53.Gaede P, Lund-Andersen H, Parving HH & Pedersen O Effect of a multifactorial intervention on mortality in type 2 diabetes. N. Engl. J. Med 358, 580–591 (2008). [DOI] [PubMed] [Google Scholar]
- 54.Ueki K et al. Effect of an intensified multifactorial intervention on cardiovascular outcomes and mortality in type 2 diabetes (J-DOIT3): an open-label, randomised controlled trial. Lancet Diabetes Endocrinol. 5, 951–964 (2017). [DOI] [PubMed] [Google Scholar]
- 55.Rawshani A et al. Relative prognostic importance and optimal levels of risk factors for mortality and cardiovascular outcomes in type 1 diabetes mellitus. Circulation 139, 1900–1912 (2019). [DOI] [PubMed] [Google Scholar]
- 56.Nordwall M et al. Impact of HbA1c, followed from onset of type 1 diabetes, on the development of severe retinopathy and nephropathy: the VISS study (vascular diabetic complications in Southeast Sweden). Diabetes Care 38, 308–315 (2015). [DOI] [PubMed] [Google Scholar]
- 57.Groop PH et al. The presence and severity of chronic kidney disease predicts all-cause mortality in type 1 diabetes. Diabetes 58, 1651–1658 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stark Casagrande S, Fradkin JE, Saydah SH, Rust KF & Cowie CC The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988–2010. Diabetes Care 36, 2271–2279 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Edelman SV & Polonsky WH Type 2 diabetes in the real world: the elusive nature of glycemic control. Diabetes Care 40, 1425–1432 (2017). [DOI] [PubMed] [Google Scholar]
- 60.Sorli C et al. Efficacy and safety of once-weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a double-blind, randomised, placebo-controlled, parallel-group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol. 5, 251–260 (2017). [DOI] [PubMed] [Google Scholar]
- 61.Rodbard HW et al. Semaglutide added to basal insulin in type 2 diabetes (SUSTAIN 5): a randomized, controlled trial. J. Clin. Endocrinol. Metab 103, 2291–2301 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sacks DB Measurement of hemoglobin A(1c): a new twist on the path to harmony. Diabetes Care 35, 2674–2680 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Little RR, Rohlfing C & Sacks DB The national glycohemoglobin standardization program: over 20 years of improving hemoglobin A1c measurement. Clin. Chem 65, 839–848 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Welsh KJ, Kirkman MS & Sacks DB Role of glycated proteins in the diagnosis and management of diabetes: research gaps and future directions. Diabetes Care 39, 1299–1306 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kilpatrick ES, Rigby AS & Atkin SL Variability in the relationship between mean plasma glucose and HbA1c: implications for the assessment of glycemic control. Clin. Chem 53, 897–901 (2007). [DOI] [PubMed] [Google Scholar]
- 66.Yudkin JS et al. Unexplained variability of glycated haemoglobin in non-diabetic subjects not related to glycaemia. Diabetologia 33, 208–215 (1990). [DOI] [PubMed] [Google Scholar]
- 67.Beck RW, Connor CG, Mullen DM, Wesley DM & Bergenstal RM The fallacy of average: how using HbA1c alone to assess glycemic control can be misleading. Diabetes Care 40, 994–999 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Diabetes Research in Children Network (DIRECNET) Study Group. Relationship of A1C to glucose concentrations in children with type 1 diabetes: assessments by high-frequency glucose determinations by sensors. Diabetes Care 31, 381–385 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dagogo-Jack S Pitfalls in the use of HbA1c as a diagnostic test: the ethnic conundrum. Nat. Rev. Endocrinol 6, 589–593 (2010). [DOI] [PubMed] [Google Scholar]
- 70.Snieder H et al. HbA(1c) levels are genetically determined even in type 1 diabetes: evidence from healthy and diabetic twins. Diabetes 50, 2858–2863 (2001). [DOI] [PubMed] [Google Scholar]
- 71.Khera PK et al. Evidence for interindividual heterogeneity in the glucose gradient across the human red blood cell membrane and its relationship to hemoglobin glycation. Diabetes 57, 2445–2452 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cohen RM et al. Red cell life span heterogeneity in hematologically normal people is sufficient to alter HbA1c. Blood 112, 4284–4291 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tuttle KR et al. Diabetic kidney disease: a report from an ADA consensus conference. Diabetes Care 37, 2864–2883 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Campbell L, Pepper T & Shipman K HbA1c: a review of non-glycaemic variables. J. Clin. Pathol 72, 12–19 (2019). [DOI] [PubMed] [Google Scholar]
- 75.Nielsen LR et al. HbA1c levels are significantly lower in early and late pregnancy. Diabetes Care 27, 1200–1201 (2004). [DOI] [PubMed] [Google Scholar]
- 76.Bry L, Chen PC & Sacks DB Effects of hemoglobin variants and chemically modified derivatives on assays for glycohemoglobin. Clin. Chem 47, 153–163 (2001). [PubMed] [Google Scholar]
- 77.Boudreau V et al. Screening for cystic fibrosis-related diabetes: matching pathophysiology and addressing current challenges. Can. J. Diabetes 40, 466–470 (2016). [DOI] [PubMed] [Google Scholar]
- 78.Pani LN et al. Effect of aging on A1C levels in individuals without diabetes: evidence from the Framingham Offspring Study and the National Health and Nutrition Examination Survey 2001–2004. Diabetes Care 31, 1991–1996 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wu L et al. Effect of age on the diagnostic efficiency of HbA1c for diabetes in a Chinese middle-aged and elderly population: the Shanghai Changfeng Study. PLoS ONE 12, e0184607 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bergenstal RM et al. Racial differences in the relationship of glucose concentrations and hemoglobin A1c levels. Ann. Intern. Med 167, 95–102 (2017). [DOI] [PubMed] [Google Scholar]
- 81.Herman WH et al. Differences in A1C by race and ethnicity among patients with impaired glucose tolerance in the diabetes prevention program. Diabetes Care 30, 2453–2457 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhong GC, Ye MX, Cheng JH, Zhao Y & Gong JP HbA1c and risks of all-cause and cause-specific death in subjects without known diabetes: a dose-response meta-analysis of prospective cohort studies. Sci. Rep 6, 24071 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Herman WH Are there clinical implications of racial differences in HbA1c? Yes, to not consider can do great harm! Diabetes Care 39, 1458–1461 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bower JK, Brancati FL & Selvin E No ethnic differences in the association of glycated hemoglobin with retinopathy: the National Health and Nutrition Examination Survey 2005–2008. Diabetes Care 36, 569–573 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tsugawa Y, Mukamal KJ, Davis RB, Taylor WC & Wee CC Should the hemoglobin A1c diagnostic cutoff differ between blacks and whites? A cross-sectional study. Ann. Intern. Med 157, 153–159 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Selvin E Are there clinical implications of racial differences in HbA1c? A difference, to be a difference, must make a difference. Diabetes Care 39, 1462–1467 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hudson PR, Child DF, Jones H & Williams CP Differences in rates of glycation (glycation index) may significantly affect individual HbA1c results in type 1 diabetes. Ann. Clin. Biochem 36, 451–459 (1999). [DOI] [PubMed] [Google Scholar]
- 88.Cohen RM, Holmes YR, Chenier TC & Joiner CH Discordance between HbA1c and fructosamine. Evidence for a glycosylation gap and its relation to Diabetic nephropathy. Diabetes Care 26, 163–167 (2003). [DOI] [PubMed] [Google Scholar]
- 89.Hempe JM, Gomez R, McCarter RJ Jr. & Chalew SA High and low hemoglobin glycation phenotypes in type 1 diabetes: a challenge for interpretation of glycemic control. J. Diabetes Complicat 16, 313–320 (2002). [DOI] [PubMed] [Google Scholar]
- 90.Nayak AU, Nevill AM, Bassett P & Singh BM Association of glycation gap with mortality and vascular complications in diabetes. Diabetes Care 36, 3247–3253 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hempe JM et al. The hemoglobin glycation index identifies subpopulations with harms or benefits from intensive treatment in the ACCORD trial. Diabetes Care 38, 1067–1074 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Basu S, Raghavan S, Wexler DJ & Berkowitz SA Characteristics associated with decreased or increased mortality risk from glycemic therapy among patients with type 2 diabetes and high cardiovascular risk: machine learning analysis of the ACCORD trial. Diabetes Care 41, 604–612 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.van Steen SC et al. Haemoglobin glycation index and risk for diabetes-related complications in the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) trial. Diabetologia 61, 780–789 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gorst C et al. Long-term glycemic variability and risk of adverse outcomes: a systematic review and meta-analysis. Diabetes Care 38, 2354–2369 (2015). [DOI] [PubMed] [Google Scholar]
- 95.Sacks DB A1C versus glucose testing: a comparison. Diabetes Care 34, 518–523 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Parrinello CM & Selvin E Beyond HbA1c and glucose: the role of nontraditional glycemic markers in diabetes diagnosis, prognosis, and management. Curr. Diab. Rep 14, 548 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kim WJ & Park CY 1,5-Anhydroglucitol in diabetes mellitus. Endocrine 43, 33–40 (2013). [DOI] [PubMed] [Google Scholar]
- 98.Buse JB, Freeman JL, Edelman SV, Jovanovic L & McGill JB Serum 1,5-anhydroglucitol (GlycoMark): a short-term glycemic marker. Diabetes Technol. Ther 5, 355–363 (2003). [DOI] [PubMed] [Google Scholar]
- 99.Kishimoto M et al. 1,5-Anhydro-D-glucitol evaluates daily glycemic excursions in well-controlled NIDDM. Diabetes Care 18, 1156–1159 (1995). [DOI] [PubMed] [Google Scholar]
- 100.Dungan KM et al. 1,5-anhydroglucitol and postprandial hyperglycemia as measured by continuous glucose monitoring system in moderately controlled patients with diabetes. Diabetes Care 29, 1214–1219 (2006). [DOI] [PubMed] [Google Scholar]
- 101.Battelino T et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care 42, 1593–1603 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Beck RW et al. Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care 42, 400–405 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lu J et al. Association of time in range, as assessed by continuous glucose monitoring, with diabetic retinopathy in type 2 diabetes. Diabetes Care 41, 2370–2376 (2018). [DOI] [PubMed] [Google Scholar]
- 104.Bergenstal RM et al. Glucose management indicator (GMI): a new term for estimating A1C from continuous glucose monitoring. Diabetes Care 41, 2275–2280 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Runge AS et al. Does time-in-range matter? Perspectives from people with diabetes on the success of current therapies and the drivers of improved outcomes. Clin. Diabetes 36, 112–119 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Polonsky WH, Hessler D, Ruedy KJ, Beck RW & Group DS The impact of continuous glucose monitoring on markers of quality of life in adults with type 1 diabetes: further findings from the DIAMOND randomized clinical trial. Diabetes Care 40, 736–741 (2017). [DOI] [PubMed] [Google Scholar]
- 107.Rodbard D Continuous glucose monitoring: a review of successes, challenges, and opportunities. Diabetes Technol. Ther 18, S3–S13 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Onisie O, Crocket H & de Bock M The CGM grey market: a reflection of global access inequity. Lancet Diabetes Endocrinol. 7, 823–825 (2019). [DOI] [PubMed] [Google Scholar]
- 109.Graham C Continuous glucose monitoring and global reimbursement: an update. Diabetes Technol. Ther 19, S60–S66 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Khunti K, Wolden ML, Thorsted BL, Andersen M & Davies MJ Clinical inertia in people with type 2 diabetes: a retrospective cohort study of more than 80,000 people. Diabetes Care 36, 3411–3417 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Brown SA et al. Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N. Engl. J. Med 381, 1707–1717 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lind M et al. HbA1c level as a risk factor for retinopathy and nephropathy in children and adults with type 1 diabetes: Swedish population based cohort study. BMJ 366, l4894 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rodriguez-Gutierrez R & McCoy RG Measuring what matters in diabetes. JAMA 321, 1865–1866 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pedersen-Bjergaard U et al. Comparison of the HAT study, the largest global hypoglycaemia study to date, with similar large real-world studies. Diabetes Obes. Metab 21, 844–853 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Banting FG, Best CH, Collip JB, Campbell WR & Fletcher AA Pancreatic extracts in the treatment of diabetes mellitus. Can. Med. Assoc. J 12, 141–146 (1922). [PMC free article] [PubMed] [Google Scholar]
- 116.Nathan DM & DCCT/EDIC Research Group. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study at 30 years: overview. Diabetes Care 37, 9–16 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rahbar S, Blumenfeld O & Ranney HM Studies of an unusual hemoglobin in patients with diabetes mellitus. Biochem. Biophys. Res. Commun 36, 838–843 (1969). [DOI] [PubMed] [Google Scholar]
- 118.Gebel E The start of something good: the discovery of HbA(1c) and the American Diabetes Association Samuel Rahbar Outstanding Discovery Award. Diabetes Care 35, 2429–2431 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Koenig RJ, Araujo DC & Cerami A Increased hemoglobin AIc in diabetic mice. Diabetes 25, 1–5 (1976). [DOI] [PubMed] [Google Scholar]
- 120.Koenig RJ et al. Correlation of glucose regulation and hemoglobin AIc in diabetes mellitus. N. Engl. J. Med 295, 417–420 (1976). [DOI] [PubMed] [Google Scholar]
- 121.Gabbay KH Editorial: glycosylated hemoglobin and diabetic control. N. Engl. J. Med 295, 443–444 (1976). [DOI] [PubMed] [Google Scholar]
- 122.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 33, S62–S69 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lenters-Westra E, Schindhelm RK, Bilo HJ & Slingerland RJ Haemoglobin A1c: historical overview and current concepts. Diabetes Res. Clin. Pract 99, 75–84 (2013). [DOI] [PubMed] [Google Scholar]
- 124.World Health Organization. Use of glycated haemoglobin (HbA1c) in the diagnosis of diabetes mellitus: abbreviated report of a WHO consultation (WHO, 2011). [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


