Abstract
Prompt diagnosis of equine septic arthritis is crucial for successful treatment. Serum amyloid A (SAA) has been suggested as a reliable biomarker. However, we previously found that synovial fluid SAA increases in nonaffected joints of horses with septic arthritis. We hypothesized that systemic SAA may leak into the nonaffected joints. If this is the case, we also hypothesized that locally produced joint SAA isoforms may be better candidates for septic arthritis biomarkers. Thus, our objectives were 1) to evaluate the temporal kinetics of systemic and synovial fluid SAA in horses with septic arthritis (n = 5), non-septic synovitis (n = 5), and systemic inflammation (n = 5), examining both affected and contralateral joints; and 2) investigate putative locally produced joint SAA isoforms and detect amino-acid differences between them. We confirmed that SAA increases significantly in synovial fluid in nonaffected joints of horses with systemic inflammation (≤352 mg/L), as well as in contralateral nonaffected joins in horses with septic arthritis (≤1,830 mg/L) compared to baseline at time 0 (<0.2 mg/L). We also identified a putative locally produced joint SAA peptide in synovial fluid (FGDSGHGAADSR) that differed in 1 amino acid from 2 systemic peptides found both in plasma and synovial fluid. The putative joint SAA isoform was present in joints of horses with both septic arthritis and systemic inflammation (ion intensities 104–106). Thus, the increase of synovial fluid SAA may be both due to the leakage of SAA from serum into joints and local production of joint SAA isoforms.
Keywords: horses, isoforms, septic arthritis, serum amyloid A, synovitis, systemic inflammation
Septic arthritis is a common disease that affects horses of all ages and can be life-threatening if not treated immediately. Therefore, a prompt and accurate diagnosis is crucial to initiate aggressive treatment in order to reduce risk and ensure a good prognosis.
Serum amyloid A (SAA) is an acute-phase protein that is widely used for the detection of inflammation in human 24 and veterinary medicine.6,12 In horses, it is suggested as a useful marker of joint inflammation and infection. 22 SAA in synovial fluid in healthy horses is usually undetectable (<0.2–0.7 mg/L) but it increases significantly with septic arthritis (up to 100–1,500 mg/L). 35 Following infection or inflammation, SAA concentration increases rapidly in serum and synovial fluid, and was also reported to decrease rapidly once the noxious stimulus was removed due to its short half-life.13,21,22 The concentration of SAA in synovial fluid is not affected by repeated arthrocentesis, antibiotic treatment, or arthroscopic lavage.10,33–35 For these reasons, it has been considered a reliable marker in the diagnosis and monitoring of equine septic arthritis cases. However, we found previously that SAA increases significantly in the healthy joints contralateral to the joints with experimental septic arthritis. 45 Based on this finding, we hypothesized that SAA leaks from the systemic circulation into synovial fluid of healthy nonaffected joints. Thus, our first objective was to test this hypothesis by comparing SAA concentrations in serum and synovial fluid obtained from affected and nonaffected joints of horses with experimentally induced septic arthritis, non-septic synovitis, and systemic inflammation.
Given that we hypothesized that total SAA in synovial fluid represents both systemic SAA leaked into synovial fluid and locally produced joint SAA, our second objective was to investigate the potential ability of locally produced joint SAA isoforms to allow differentiation of septic arthritis, non-septic synovitis, and systemic inflammation. SAA is a family of closely related proteins, and several SAA isoforms have been identified in humans and animals, including in several species of domestic animals.1,5,37 SAA is mainly produced in the liver, but it is also synthetized by other tissues, including synovial tissue in humans18,29 and horses.3,8,10,25 In vitro production of SAA by synovial fibroblasts and chondrocytes has been demonstrated for humans and horses.11,30 In equine synovial fluid, 2 highly alkaline (isoelectric point: 10.0, 10.2) local isoforms of SAA have been detected using isoelectric focusing electrophoresis. 8 However, the sequence difference between systemic and joint SAA isoforms in horses remains unknown. Thus, identification of locally produced joint SAA isoforms may be useful for the development of tests used in the diagnosis and monitoring of septic arthritis in horses. Hence, we studied the temporal kinetics of SAA in serum and synovial fluid of horses with different experimentally induced inflammatory conditions (non-septic synovitis, septic arthritis, and systemic inflammation) and evaluated potential differences between peptide sequences of SAA in synovial fluid and plasma obtained from horses with these different experimental inflammatory conditions.
Materials and methods
Experimental design
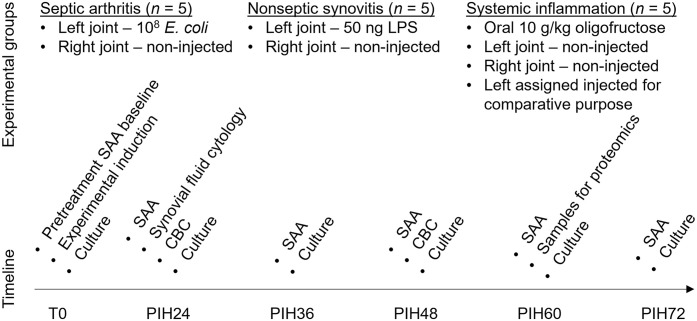
Our study was conducted in compliance with the guidelines of the Canadian Council on Animal Care after review and approval by the University of Saskatchewan Animal Care and Use Committee and Animal Research Ethics Board (protocol 20160095). We used 15 adult horses, with a median age of 12 y (range: 6–16 y) and median weight of 470 kg (range: 305–572 kg) in our study. Horses were considered to be healthy and free of musculoskeletal disease based on thorough physical examination, lameness examination, and blood work (CBC; biochemistry profile including measurement of systemic blood SAA). Horses were randomly assigned to one of the following groups: septic arthritis (n = 5); non-septic synovitis (n = 5); or systemic inflammation (n = 5). For the non-septic synovitis and septic arthritis groups, the left middle carpal joint of horses was injected with either lipopolysaccharide (LPS) or Escherichia coli, respectively; systemic inflammation was induced by an overdose of oligofructose as described below. For the systemic inflammation group, the left middle carpal joint was assigned as the treated joint (injected joint); however, no intra-articular treatment was administered. In addition, the right contralateral middle carpal joints in all 3 groups were not treated (hereafter, non-injected joints) and were used as internal controls. Throughout the period of the study, horses were offered hay and water ad libitum. Samples of blood and synovial fluid from both joints were collected from all 15 horses at time zero (T0) immediately before the induction of experimental conditions, and at regular intervals after that as described below (Fig. 1).
Figure 1.
Roadmap for the experimental design and outcomes of serum amyloid A (SAA) concentration and identification of SAA isoforms in blood and synovial fluid in horses with experimentally induced septic arthritis, non-septic synovitis, and systemic inflammation. Synovial fluid cytology and CBC were used for confirmation of successful induction of experimental models. E. coli = Escherichia coli; LPS = lipopolysaccharide; PIH24 = post-induction hour 24, etc.; T0 = pretreatment baseline.
Experimental induction of non-septic synovitis and septic arthritis
Acute synovitis was induced using a published LPS model. 16 Briefly, 50 ng of LPS (E. coli O55:B5 strain; Sigma-Aldrich) was diluted in 1 mL of sterile PBS solution, aliquoted, and then stored at −80°C until used. Aliquots of LPS (50 ng/mL) were thawed prior to inducing non-septic synovitis and stored on ice until the assigned middle carpal joint was injected.
Septic arthritis was induced using a model for septic arthritis in horses with an inoculation dose of 1.0 × 108 cfu of E. coli using a published bacterial/septic arthritis model. 16 Briefly, the strain that we used was isolated from a clinical case that had been presented to the Veterinary Medical Center, University of Saskatchewan (Saskatoon, SK, Canada), and had been diagnosed and treated for septic arthritis. The stock culture was divided into 50-µL aliquots of 1.0 × 1010 cfu/mL and thereafter stored at −80°C. Bacteria were cultured in blood culture medium at 37°C in aerobic conditions to the mid-exponential phase of growth. Bacterial cells were harvested by centrifugation, washed in sterile PBS, and resuspended in fresh sterile PBS to 1.0 × 108 cfu/mL, as described previously. 16
Horses in the septic arthritis and non-septic synovitis groups at T0 were sedated with detomidine hydrochloride (0.015 mg/kg, IV), and the selected joint was prepared aseptically. The assigned carpus was held in flexion by an assistant, a 20-ga needle attached to a 6-mL syringe was then placed into the dorsolateral pouch of the middle carpal joint and used to collect 4–6 mL of synovial fluid, which provided the baseline value for each horse and was labeled as T0. Following the first collection of synovial fluid sample at T0, the syringe was then changed for a 3-mL Luer-Lok syringe containing either 1 mL of 50 ng/mL LPS (for non-septic synovitis group) or 1.0 × 108 cfu of E. coli (for septic arthritis group), and the solution was injected into the joint.
Experimental induction of systemic inflammation
Systemic inflammation was induced experimentally using an oligofructose model, which has been shown to reproduce consistent signs of systemic inflammation response syndrome in horses, such as severe diarrhea, increased heart rate, and blood leukocyte count. 2 Briefly, horses were administered an overdose of oligofructose (Orafti P95 oligofructose; Beneo) at a dose of 10 g/kg dissolved in 4 L of warm water and administrated via nasogastric tube.
Experimental procedures and sampling
Blood and synovial fluid samples from the injected and non-injected middle carpal joint were obtained before intra-articular injection of LPS and E. coli or before administration of oligofructose, and these samples served as baseline values for each horse (T0, baseline value). Thereafter, blood and synovial fluid samples from injected and non-injected joints were collected at post-induction hours (PIH) 24, 36, 48, 60, and 72. Note that in the systemic inflammation group, no intra-articular treatment was administered either to injected or to the non-injected contralateral joint; hence, both could be considered as “healthy not treated controls” even though, for the comparative purpose of our study, they were assigned to the injected or non-injected category. Bandages were placed on both carpi, maintained throughout the study, and replaced after each arthrocentesis. Following collection of samples at PIH72, all horses were euthanized with an overdose of pentobarbital sodium (85 mg/kg, IV), and samples of liver, synovial membrane, and articular cartilage from the joints of interest were collected. Samples were snap frozen in liquid nitrogen and then stored at −80°C for another research study.
Horses underwent physical examination with assessment of general appearance, rectal temperature, gastrointestinal motility, heart rate, and respiratory rate before inducing experimental models and then every 6 h until the end of our study. Lameness was evaluated using the American Association of Equine Practitioners (AAEP) system (scale of 0–5; 0 = lameness not perceptible under any circumstance; 5 = a lameness producing minimal weight bearing in motion and/or at rest, or a complete inability to move). 31 In addition, pain was assessed using a composite measure pain scale (CMPS) modified for orthopedic pain. 20 The CMPS that we used evaluated the following 6 behavior categories: gross pain behavior, weight bearing, head position, location of horse in stall, response to open door, and response to approach from observer, in combination with an overall subjective pain score. For each behavioral category, a pain score of 0–4 was given.
Rescue analgesia
Nonselective nonsteroidal anti-inflammatory drugs were not used for rescue analgesia, because the effects of NSAIDs have been reported to lower concentrations of several acute-phase proteins, 40 and phenylbutazone has been shown to significantly decrease the WBC count following induction of an equine experimental model of synovitis. 28 Although intra-articular injection of opioids was shown to have mild anti-inflammatory effects in horses with LPS-induced synovitis,19,36,43 the effects of systemic morphine administration on joint inflammation or SAA expression have not been demonstrated.8,19 For these reasons, we elected to use opioids as the administered rescue analgesia to minimize interference with SAA expression in our study. Lameness and pain scores were used to determine if horses needed rescue analgesia. Rescue analgesia was administrated if 1) a horse had a score of 4 in any of the CMPS categories; 2) if the total CMPS was ≥15, or 3) lameness was ≥grade 4. The administered rescue analgesia consisted of morphine (0.1 mg/kg, IM). One horse in the non-septic synovitis group required rescue analgesia (0.1 mg/kg, IM) at PIH24 and did not require additional rescue analgesia thereafter to complete the study. After induction of the experimental model of septic arthritis, all horses required rescue analgesia (0.1 mg/kg, IM) at PIH24 and, subsequently, additional doses were administered (q8h, IM) to complete the study. Two horses in the systemic inflammation group required rescue analgesia at PIH48 and until completing the study (0.1 mg/kg, q8h, IM).
Sample collection and processing
Following collection of synovial fluid, samples were divided into 3 aliquots: aliquot 1 in a 0.5-mL EDTA tube (Microtainer; Becton Dickinson) for SAA quantification, aliquot 2 in 0.5-mL EDTA tube (Microtainer) for cytologic evaluation (if sufficient amount was obtained), and aliquot 3 in a sterile 1.5-mL microcentrifuge tube (Fisherbrand; Fisher) for bacterial culture. First aliquots of synovial fluid collected at times T0, PIH24, PIH36, PIH48, PIH60, and PIH72 were centrifuged at 3,500 × g for 10 min at ambient temperature. Synovial fluid supernatant was then transferred into low-retention microcentrifuge tubes (Fisherbrand; Fisher) and stored at −80°C for SAA quantification.
At times T0 and PIH24, the second aliquot of synovial fluid was submitted for cytology without centrifugation (PDS; Prairie Diagnostic Services, Saskatoon, SK, Canada) for subjective assessment of color, turbidity, and viscosity as well as quantification of total protein (TP), total nucleated cell count (NCC), and percentage of neutrophils (PN) to confirm inflammation. In normal synovial fluid, TP is usually <31.1 g/L, NCC <5 × 109 cells/L, and PN <10%. 23 Measurements above these values were considered to indicate inflammation. We used criteria of TP >40 g/L, NCC >30 × 109 cells/L, and >80% PN27,39 to confirm the septic arthritis model, with subsequent positive bacterial culture confirming septic arthritis and negative culture confirming non-septic synovitis.
For bacterial culture, the third aliquots of synovial fluid collected from all horses at times PIH24, PIH48, and PIH72 were placed into sterile 1.5-mL microcentrifuge tubes. Blood culture medium (Signal; Oxoid) was inoculated with 200 µL of synovial fluid and was incubated in aerobic conditions on a shaking platform (180 rpm) at 37°C for 72 h or until bacterial growth was identified. If bacterial growth was observed, the medium was then streaked on Columbia agar with 5% sheep blood culture plate to obtain isolated colonies. Bacteria were identified to species level using matrix-assisted laser desorption/ionization–time-of-flight mass spectrometry at PDS.
Blood was collected from the jugular vein at times T0, PIH24, PIH36, PIH48, PIH60, and PIH72 using blood collection system tubes (Vacutainer Serum; Becton Dickinson) and allowed to clot for 30 min. Thereafter, tubes were centrifuged at 3,500 × g for 10 min at ambient temperature, and serum was collected and transferred into low-retention microcentrifuge tubes and stored at −80°C for later SAA quantification. In addition, blood was collected in EDTA tubes for determination of CBC at times T0 (baseline), PIH24, and PIH48 to confirm systemic inflammation. We used an increase in WBC count with left shift to confirm systemic inflammation. To obtain plasma for proteomics analysis, we collected blood in blood collection tubes (P100; Becton Dickinson), which are coated with K2EDTA and a protease inhibitor cocktail.
SAA analysis
When the collection of all samples was completed, serum and synovial fluid samples were thawed at room temperature. To reduce viscosity, 10 µL of hyaluronidase was added to 490 µL of synovial fluid as reported previously. 45 Quantification of serum and synovial fluid SAA was then performed on an automated chemistry analyzer (Hoffmann-La Roche) using a human SAA turbidimetric immunoassay (LZ test SAA; Eiken) that has been validated in serum for equine assays 9 and used in previous equine studies. The assay’s limit of quantification (LOQ) reported by the manufacturer was 0.2 mg/L. The tests were performed at the Animal Health Laboratory at the University of Guelph (Guelph, ON, Canada).
Proteomics analysis of SAA isoforms
To identify potential locally produced joint SAA isoforms, the samples of synovial fluid and plasma collected at PIH60 were used for comparative proteomics analysis using liquid chromatography–tandem mass spectrometry (LC-MS/MS). The work was done in Dr. Katselis’s Mass Spectrometry Laboratory for Omics Research (Health Sciences, College of Medicine, University of Saskatchewan) with a quadrupole time-of-flight instrument (6550 iFunnel, Chip Cube LC-MS interface; Agilent). Synovial fluid samples included samples from the injected joint in septic arthritis (n = 5), non-septic synovitis (n = 5), and systemic inflammation (n = 5) groups, and the assigned non-injected joint from the systemic inflammation group (n = 5). Both injected (n = 5) and non-injected (n = 5) joints could be considered as “healthy not-treated controls” in the systemic inflammation group. Plasma samples from all 15 horses from the 3 experimental groups (5 samples/group) were analyzed. The proteomics analysis was performed as described previously. 17 Before and after sample analysis, HeLa standard digests were run in triplicate to ensure peak analytical instrument performance. In addition, solvent blanks were run between samples to ensure that there was no carryover. Raw mass spectra data were searched against all mammalian SAA sequences in UniProtKB combined SwissProt and TrEMBL databases (2018 release; www.uniprot.org).
Statistical analysis
The dependent variables included TP, NCC, PN, and systemic and synovial fluid SAA concentrations. Groups were independent variables. Values of SAA below the LOQ (0.2 mg/L) of the assay were interpreted as “0” for statistical analysis. Distribution and central tendency of the dependent variables were assessed based on descriptive statistics (x̄, SD, medians, and histograms). One-way ANOVA with Tukey multiple comparisons was carried out to compare clinical parameters PIH24 between groups. Two-way repeated measures ANOVA with Tukey multiple comparison was performed to compare serum and synovial fluid SAA concentrations between groups at each time. One-way repeated measures ANOVA with Sidak multiple comparison was performed to compare SAA concentration between injected and non-injected joints within each group. Normality of data was assessed using residual and QQ plots. Significance level p ≤ 0.05 was considered significant. All group comparison analyses were performed and graphed with Prism v.8.2.1 for Windows (GraphPad).
To determine the sensitivity (Se) and specificity (Sp) of synovial fluid SAA for the differentiation of septic arthritis from non-septic synovitis and systemic inflammation, we carried out receiver operating characteristic (ROC) curve analysis (SPSS Statistics for Windows, v.28.0; IBM). All samples in injected joint in septic arthritis group starting at PIH24 were considered as true positive; all other samples were considered as true negatives. To evaluate how sensitivity and specificity changed depending on the duration of inflammation, we analyzed whole datasets, including synovial fluid SAA values at PIH24–72, as well as subsets for PIH24–36 and PIH48–72.
Results
Experimental model induction
Successful induction of synovitis and septic arthritis was confirmed at PIH24 by cytologic examination of synovial fluid for all experimental horses with septic arthritis and non-septic synovitis (Fig. 2; Suppl. Table 1), together with positive bacterial cultures of E. coli for septic arthritis and negative bacterial culture for non-septic synovitis and systemic inflammation groups at PIH24 and thereafter at all times. Systemic inflammation was confirmed based on clinical signs and CBC.
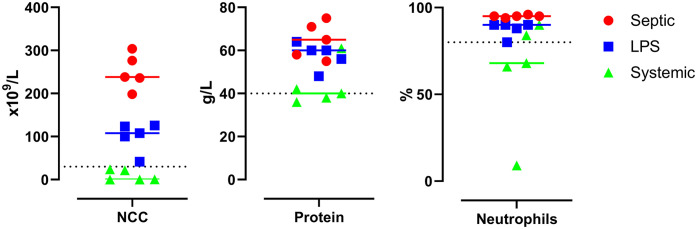
Figure 2.
Effects of experimentally induced septic arthritis, non-septic synovitis, and systemic inflammation in horses on synovial fluid of injected joints: nucleated cell count (NCC), total protein, and percent neutrophils at PIH24 in septic arthritis (Septic), lipopolysaccharide-induced non-septic synovitis (LPS), and systemic inflammation (Systemic) groups. Dotted line in each graph is the threshold commonly used in the diagnosis of septic arthritis (synovial fluid protein >40 g/L, NCC >30 × 109 cells/L, and >80% neutrophils).
Mean NCC in the septic arthritis, non-septic synovitis, and systemic inflammation groups were, in units of × 109 cells/L: 251 ± 41 (range: 198–304), 100 ± 34 (range: 42–125), and 9.3 ± 12.4 (range: 0.2–24), respectively. NCC increased above the reported criterion for septic arthritis in both the septic arthritis and non-septic synovitis groups. In the systemic inflammation group, the NCC remained consistently below this value. There was a significant difference between groups (F2,12 = 75; p < 0.001), and all groups were different from each other (p < 0.05).
Mean TP in the septic arthritis, non-septic synovitis, and systemic inflammation groups were, in units of g/L: 65 ± 8 (range: 55–75), 58 ± 6 (range: 48–64), and 43 ± 10 (range: 36–61), respectively. There was a significant difference between groups (F2,12 = 8.5; p < 0.001). TP in septic arthritis and non-septic synovitis was significantly higher compared to the systemic inflammation group (p < 0.05) but was not significantly different between each other. In the systemic inflammation group, TP was above the criterion reported for septic arthritis in 2 of 5 horses.
Mean PN in the septic arthritis, non-septic synovitis, and systemic inflammation groups were, in %: 95 ± 1 (range: 94–96), 88 ± 4 (range: 80–90), and 63 ± 32 (range: 9–90), respectively. There was a significant difference between groups (F2,12 = 3.9; p < 0.05), with the septic arthritis group significantly higher compared to the systemic inflammation group (p < 0.05). In the septic arthritis and non-septic synovitis groups, PN was above the criterion reported for septic arthritis in all horses, whereas in the systemic inflammation group only in 2 of 5 horses (the same 2 horses in which TP was increased).
In the systemic inflammation group, 2 of 5 horses had increased percentage of TP and neutrophils above the criteria for septic arthritis in the joints assigned as treated, suggesting transient inflammation. However, lack of reported sensitivity and specificity for these cytologic parameters for the diagnosis of septic joint inflammation is a limitation.
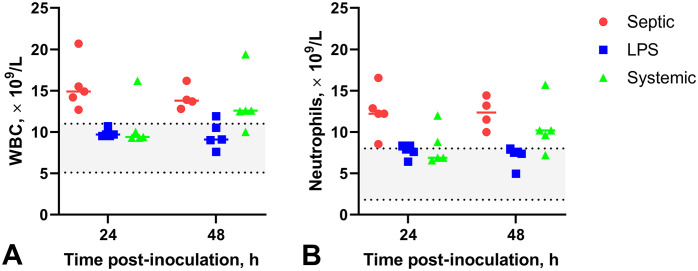
There was a significant difference between groups in both WBC and neutrophil counts at PIH24 (F2,12 = 7.7, p < 0.01, and F2,12 = 7.4, p < 0.01, respectively) and PIH48 (F2,11 = 4.6, p < 0.05, and F2,11 = 6.3, p < 0.05, respectively; Fig. 3; Suppl. Table 2). At PIH24, WBCs and segmented neutrophils were significantly increased in the septic arthritis group compared to the non-septic synovitis and systemic inflammation groups; at PIH48, WBCs and segmented neutrophils were significantly increased in the septic arthritis group compared to the non-septic synovitis group, but there was no significant difference between septic arthritis and systemic inflammation groups. Hence, a systemic inflammatory response occurred in the septic arthritis and systemic inflammation groups, and an absent-to-borderline systemic response occurred in the non-septic synovitis group.
Figure 3.
Effects of experimentally induced septic arthritis, non-septic synovitis, and systemic inflammation in horses in blood on WBC count (A) and neutrophil count (B). A. WBC count at PIH24 and 48. WBCs are consistently elevated in the septic arthritis (Septic) horses. Most samples in systemic inflammation horses (Systemic) had increased WBC by PIH48; only one horse in the non-septic synovitis group (LPS) had mildly increased WBC at PIH48. B. Neutrophilia in all horses with septic arthritis and most horses with systemic inflammation at PIH48. Some horses with non-septic synovitis had borderline neutrophilia. Left shift and slight toxic change were also noted. Gray area between the dotted lines represents the normal RI. LPS = lipopolysaccharide.
Post-induction period: clinical parameters
In the septic arthritis group, all horses developed fever, tachycardia, and tachypnea at PIH24. Thereafter, vital parameters were within normal limits except heart rate, which remained increased (median: 56 bpm; range: 48–64 bpm). All horses were grade 4 lame at PIH24, became grade 5 at PIH36, and remained with a grade 5 lameness up to PIH72. At PIH24, the injected carpus had heat, mild swelling, and was painful on palpation. By PIH72, all horses had developed marked swelling; flexion of the injected carpus was painful.
In the non-septic synovitis group, horses developed various degrees of lameness at PIH24: grade 3 lameness (4 horses) to grade 4 lameness (1 horse). Lameness was no longer present in 4 of the horses at PIH36, and the remaining horse’s lameness had resolved by PIH72. Vital parameters of 4 horses in the non-septic synovitis group were within normal limits at all times (heart rate <44 bpm; respiratory rate <28 bpm). The heart rate of 1 horse increased at PIH24 (52 bpm) coinciding with the time of highest lameness score observed (grade 4). Thereafter all vital parameters in this horse returned to normal limits until the completion of our study. Throughout our study, all horses in the non-septic synovitis group remained bright, alert, and responsive to stimuli, eating and passing normal feces.
In the systemic inflammation group, all 5 horses developed diarrhea within 24 h of oligofructose administration and were tachycardic at PIH24. Two of 5 horses developed laminitis by PIH48. The heart rates of these 2 horses remained increased (median: 60; range: 52–68 bpm), and diarrhea persisted up to 60 h; treatment consisted of lactated Ringer solution at a maintenance rate of 2.5 mL/kg/h until diarrhea resolved. Diarrhea had resolved by PIH36 in the other 3 horses, and vital parameters remained within normal limits in these 3 horses until the completion of our study.
Post-induction period: serum SAA concentration
Serum SAA concentrations at T0 were below the LOQ (<0.2 mg/L) of the immunoassay in all groups (Fig. 4A, 4B). For the septic arthritis group, the serum SAA concentration increased following induction of synovial joint infection at PIH24 (median: 1,130 mg/L; range: 445–1,480 mg/L) and reached peak concentrations at PIH72 (median: 3,790 mg/L; range: 3,390–3,830 mg/L). In the non-septic synovitis group, the serum SAA concentration increased moderately at PIH24 (median: 89 mg/L; range: 42–145 mg/L), and reached its peak at PIH36 (median: 127 mg/L; range: 56–222 mg/L). In the systemic inflammation group, the serum SAA concentration began to increase at PIH24 (median: 76 mg/L; range: 57–140 mg/L), reaching peak concentrations at PIH72 (median: 2,910 mg/L; range: 2,640–3,380 mg/L).
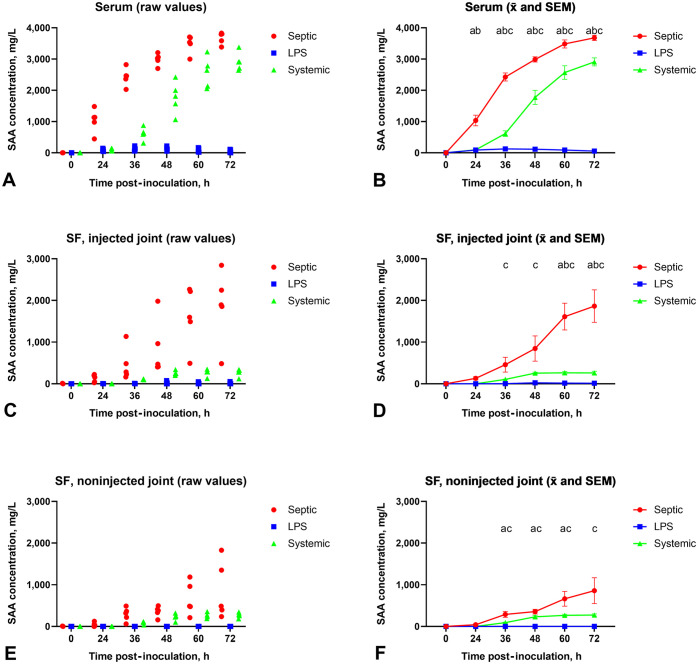
Figure 4.
Effects of experimentally induced septic arthritis (Septic), non-septic synovitis (LPS), and systemic inflammation (Systemic) in horses on the serum amyloid A (SAA) concentration in serum (A, B), synovial fluid (SF) of injected joints (C, D), and SF of non-injected joints (E, F). Individual SAA measurements are plotted in A, C, and E. x̄ and SEM are plotted in B, D, and F, with statistical significance (p ≤ 0.05) between groups at each time marked as follows: a = septic arthritis vs. non-septic synovitis; b = septic arthritis vs. systemic inflammation; c = systemic inflammation vs. non-septic synovitis. LPS = lipopolysaccharide.
Comparing serum SAA concentration between groups, there was significant interaction between group and time (F10,60 = 107; p < 0.001). Comparisons with Tukey correction revealed that serum SAA concentration was significantly higher in the septic arthritis group than in the non-septic synovitis and systemic inflammation groups at PIH24, PIH36, PIH48, PIH60, and PIH72 (p < 0.05). The serum SAA concentration was significantly higher in the systemic inflammation group compared to the non-septic synovitis group at PIH36, PIH48, PIH60, and PIH72 (p < 0.05).
Post-induction period: synovial fluid SAA concentration
Synovial fluid SAA concentrations at T0 (baseline value) were below the LOQ (<0.2 mg/L) of the immunoassay in all 3 groups for both injected and non-injected joints (Fig. 4C–F). In the septic arthritis group, the synovial fluid SAA concentration in the injected joint began to increase following induction of bacterial joint infection at PIH24 (median: 155 mg/L; range: 26–224 mg/L) and reached its peak concentration at PIH72 (median: 1,890 mg/L; range: 484–2,840 mg/L). The synovial fluid SAA concentration in the non-injected joint began to increase concurrently at PIH24 (median: 29 mg/L; range: 2.5–123 mg/L) and peaked at PIH72 (median: 485 mg/L; range 237–1,830 mg/L). There was a significant interaction between time and joint in the septic arthritis group (F5,40 = 3.5; p = 0.01). Synovial fluid SAA concentration was significantly higher in the injected joint compared to the non-injected joint at PIH60 and PIH72 (p < 0.05 with Sidak correction). There were no significant differences between the injected and non-injected joints at PIH24, PIH36, and PIH48.
For the non-septic synovitis group, there were no significant differences between any times and between the left and right joints.
In the systemic inflammation group, both carpal joints were treated the same and SAA concentrations were comparable. The synovial fluid SAA concentration of the joint assigned as the injected joint was <0.5 mg/L at PIH24, started to increase by PIH36 (median: 106 mg/L; range: 96–118 mg/L), and reached peak concentrations at PIH60 (median: 287 mg/L; range: 130–352 mg/L), whereas in the joint assigned as the non-injected joint, the synovial fluid SAA concentration began to increase also by PIH36 (median: 101 mg/L; range: 35–111 mg/L) and peaked at PIH72 (median: 284 mg/L; range: 35–303 mg/L). No significant differences were found in synovial fluid SAA concentrations between assigned injected and non-injected joints at any of the measured post-induction times in horses with systemic inflammation.
When synovial fluid SAA concentrations were compared between the injected joints of the 3 groups, there was a significant interaction between time and group (F10,60 = 14.2; p < 0.001). The septic arthritis group had significantly higher SAA concentrations than the non-septic synovitis and systemic inflammation groups at PIH60 and PIH72 (p < 0.05 with Tukey correction). Synovial fluid SAA concentrations in the systemic inflammation group were significantly higher compared to the non-septic synovitis group at PIH36–72.
Comparing the synovial fluid SAA concentration in the non-injected joint among the 3 groups, there was a significant interaction between time and group (F10,60 = 4.7; p < 0.001). There were no significant differences in SAA concentration between septic arthritis and systemic inflammation groups. Compared to the non-septic synovitis group, the synovial fluid SAA concentration was significantly higher in the septic group at PIH36–60, and in the systemic inflammation group at PIH36–72 (p < 0.05 with Tukey correction).
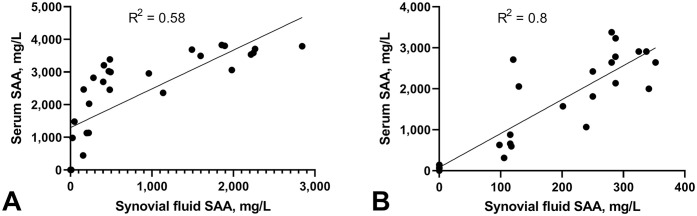
The SAA concentration in synovial fluid and serum in the septic arthritis and systemic inflammation groups was significantly and positively correlated (Fig. 5). In the systemic inflammation group, the correlation was linear, despite high variability (R 2 = 0.80); linear fit was poor in the septic arthritis group (R 2 = 0.58). Correlation for non-septic synovitis was not performed because of very low SAA values.
Figure 5.
Correlation of serum amyloid A (SAA) concentration to synovial fluid SAA concentration (injected joint) A. in septic arthritis group and B. systemic inflammation group. Lines are best-fit regression lines and demonstrate higher correlation between the serum and synovial fluid SAA concentration at different times in the systemic inflammation group (R 2 = 0.8) compared to the septic arthritis group (R 2 = 0.58).
ROC curve analysis of synovial fluid SAA for detection of septic arthritis
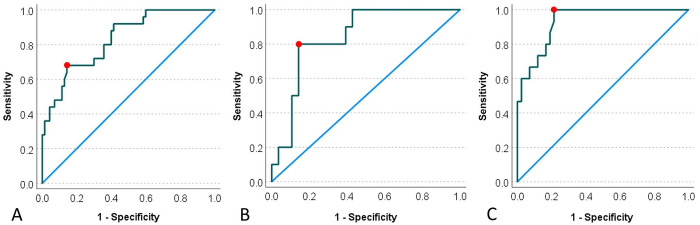
By ROC curve analysis of the whole dataset of PIH24–72 in our experimental model, synovial fluid SAA of 400 mg/L provided optimal classification with Se of 68% and Sp of 85.7% (Fig. 6A). At 24.9 mg/L, Se was 100%, but Sp was only 40%; at 1,840 mg/L, Se was only 28%, but Sp was 100%. When the dataset was restricted to PIH24–36, synovial fluid SAA at 139 mg/L provided optimal classification with Se of 80% and Sp of 85.7% (Fig. 6B). At 18.8 mg/L, Se was 100%, and Sp was 57%; at 813 mg/L, Se was only 10%, but Sp was 100%. When the dataset was restricted to PIH48–72, synovial fluid SAA at 400 mg/L provided optimal classification with Se of 100% and Sp of 79% (Fig. 6C). At 1,842 mg/L, Se was 53%, and Sp was 100%. Hence, the sensitivity of synovial fluid SAA to differentiate septic arthritis from non-septic synovitis or systemic inflammation increases with time and, at 400 mg/L, the sensitivity reaches 100% with inflammation of >48 h.
Figure 6.
Comparison of serum amyloid A (SAA) threshold concentration for optimal sensitivity and specificity to differentiate septic arthritis from non-septic arthritis and systemic inflammation at different stages of experimentally induced disease using receiver operating characteristic (ROC) curve analysis. A. ROC analysis for the entire experimental period (PIH24–72) determined that a SAA threshold at 400 mg/L (red dot) will differentiate septic arthritis from non-septic synovitis and systemic inflammation with Se of 68% and Sp of 85.7%. B. ROC analysis for the early experimental period (PIH24–36) determined that a SAA threshold at 139 mg/L (red dot) will differentiate septic arthritis from non-septic synovitis and systemic inflammation with Se of 80% and Sp of 85.7%. C. ROC analysis for the late experimental period (PIH48–72) determined that a SAA threshold at 400 mg/L (red dot) will differentiate septic arthritis from non-septic synovitis and systemic inflammation with Se of 100% and Sp of 79%. PIH = post-induction hour.
Proteomics results
We identified 3 different isoforms of an SAA peptide, 2 were present in both plasma and synovial fluid, and 1 was found in synovial fluid but not plasma. Peptide FGDSGHGAADSR, which mapped to an equine SAA isoform (UniProtKB TrEMBL accession F6ZL17), was consistently found in 4 of 5 samples of synovial fluid in horses with septic arthritis (injected joint) and 3 of 4 synovial fluid samples in the systemic inflammation group (both joints); this peptide was not present in synovial fluid of the injected joint of the non-septic synovitis group and was absent from plasma samples (n = 15) in all groups. Two other peptides mapping to different isoforms of equine SAA—FGDSGHGAEDSR (UniProtKB TrEMBL F6ZPQ6) and FGDSGHGEADSR (UniProtKB TrEMBL A0A9L0INR1)—were found both in synovial fluid and plasma samples (Suppl. Tables 3, 4) in all groups (Fig. 7). These peptides differed from the putative synovial fluid peptide by one substitution of glutamic acid (E) by alanine (A). Because these 2 peptides were found in plasma, we suspect they are produced in the liver as part of the systemic acute-phase response, and are referred to as systemic peptide 1 (SP1) and systemic peptide 2 (SP2); the peptide found only in synovial fluid is referred to as joint peptide (JP; Table 1). Ion intensities in synovial fluid for SP1 and SP2 were on the order of magnitude 105–107; ion intensities for JP were ~10 times lower, on the order of magnitude 104–106 (Suppl. Tables 3, 4).
Figure 7.
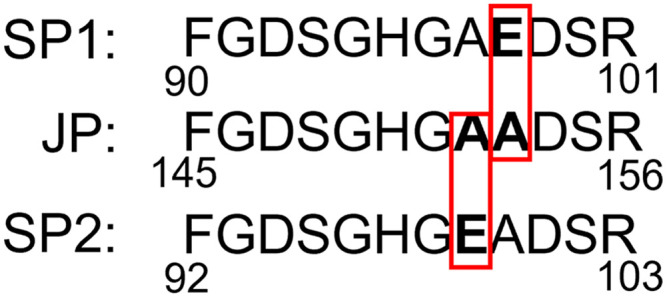
Amino-acid sequence comparison between a peptide of a putative SAA isoform found only in synovial fluid (JP = joint peptide) with the corresponding peptide of an SAA isoform found in both synovial fluid and plasma (systemic peptide 1, SP1; systemic peptide 2, SP2). Amino-acid sequences of all peptides were identified by liquid chromatography–tandem mass spectrometry (LC-MS/MS), demonstrating substitution of glutamic acid (E) by alanine (A) in the SAA peptide found only in synovial fluid. Position of these peptides within the reported sequences (F6ZL17 for JP, F6ZPQ6 for SP1, and A0A9L0INR1 for SP2) published in UniProtKB unreviewed (TrEMBL) database is indicated by the numbers below the sequence.
Table 1.
Identification of the putative serum amyloid A (SAA) peptides in plasma (systemic peptide 1, SP1; systemic peptide 2, SP2) and synovial fluid (joint peptide, JP) of experimental horses.
| Plasma | Injected joint | Non-injected joint | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Samples | SP1 | SP2 | JP | SP1 | SP2 | JP | SP1 | SP2 | JP |
| S1 | Y | N | N | Y | Y | Y | NP | NP | NP |
| S2 | Y | Y | N | Y | Y | N | NP | NP | NP |
| S3 | Y | N | N | Y | Y | Y | NP | NP | NP |
| S4 | Y | Y | N | Y | N | Y | NP | NP | NP |
| S5 | Y | Y | N | Y | Y | Y | NP | NP | NP |
| L1 | N | N | N | Y | N | N | NP | NP | NP |
| L2 | N | N | N | N | N | N | NP | NP | NP |
| L3 | Y | N | N | Y | N | N | NP | NP | NP |
| L4 | Y | N | N | N | N | N | NP | NP | NP |
| L5 | Y | N | N | N | N | N | NP | NP | NP |
| X1 | Y | N | N | N | N | Y | N | N | N |
| X2 | N | Y | N | Y | N | Y | N | N | Y |
| X3 | Y | Y | N | Y | Y | N | Y | N | Y |
| X4 | Y | N | N | Y | Y | Y | Y | Y | Y |
| X5 | Y | N | N | NP | NP | NP | N | N | N |
L1–L5 = horses in lipopolysaccharide-induced non-septic synovitis group; N = no; NP = analysis not performed; S1–S5 = horses in septic arthritis group; X1–X5 = horses in systemic inflammation group; Y = yes.
Discussion
Using 3 experimental inflammatory models in horses (i.e., non-septic synovitis, septic arthritis, and systemic inflammation), we demonstrated leakage of systemic SAA into nonaffected healthy joints, which potentially complicates diagnostic interpretation of SAA in equine arthritis cases, and we identified an SAA peptide found in synovial fluid of horses with septic arthritis and systemic inflammation, but absent from plasma of all horses; this peptide may represent a putative locally produced joint SAA isoform, but its diagnostic potential for septic arthritis is questionable.
SAA increased rapidly in serum and synovial fluid after experimental induction of septic arthritis. A marked increase was observed at 24 h after induction and continued to increase at 72 h at the end of our study. Serum SAA in the septic arthritis group was not different from the systemic inflammation group but was significantly increased compared to the non-septic synovitis and systemic inflammation groups by PIH60. The lack of statistical significance before PIH60 is likely due to high variation in the septic arthritis group; in particular, one horse had lower SAA values than all other horses.
A study reported the cutoff value for SAA concentration in synovial fluid for the diagnosis of septic arthritis determined by ELISA to be 24.0 mg/L, 38 with Se of 0.93 and Sp of 0.77. Another study suggested cutoff values for the diagnosis of septic arthritis for serum and synovial fluid SAA at 60.7 (Se 82.4; Sp 88.9) and 1.14 mg/L (Se 80; Sp 73), 32 respectively, as determined by turbidimetric assay (Eiken LZ SAA test). Both studies used clinical cases of joint disease, and the wide discrepancy between the synovial fluid SAA cutoff values proposed in these studies may be due to different tests used or different Se and Sp selected. Even based on the more conservative reported cutoff value of 24.0 mg/L, all contralateral control joints in the septic arthritis group and all joints in the systemic inflammation group in our study could be potentially misdiagnosed as septic. ROC analysis in our experimental model showed that synovial fluid SAA measurement at 400 mg/L optimized sensitivity and specificity for diagnosis of septic arthritis, and when measurements PIH48 and later were considered, this cutoff yielded Se of 100% and Sp of 79%. On the other hand, if all SAA measurements PIH24 and later were included, Se and Sp for diagnosis of septic arthritis were 68% and 86%, respectively. Compared to the reported cutoff value of 24.0 mg/L, 38 in our experimental model, a synovial fluid SAA concentration of 22.8 mg/L achieved Se of 100%; however, the Sp with this value was only 32%. Our data are based on an experimental model and should be interpreted cautiously, but they suggest that synovial fluid SAA sensitivity to differentiate septic arthritis from non-septic synovitis and systemic inflammation increases with time, and the previously reported cutoffs may need to be revised higher.
We found that LPS-induced non-septic synovitis did not cause significant synovial fluid or systemic SAA increase, which is compatible with studies using the same concentration of LPS.22,45 However, in studies using 20–60 times higher doses of LPS (1–3 µg), there was significant increase in both blood and synovial fluid SAA, as well as synovial fluid nucleated cells. 8
Our study also confirmed our previous results, which found a significant increase of synovial fluid SAA in contralateral non-injected control joints of the septic arthritis group. 45 In addition, we found a synovial fluid SAA increase in the systemic inflammation model up to ~350 mg/L. Between PIH24 and PIH48, there was no significant difference between synovial fluid SAA in injected joint in the septic arthritis and systemic inflammation groups, and between injected and non-injected joints in the septic arthritis group, suggesting leakage of systemic SAA into joints. Significant positive correlation with good linear fit between SAA concentration in serum and synovial fluid in horses with systemic inflammation provides further support to our suspicion of significant leakage of SAA from serum into synovial fluid. We also found that, in the septic arthritis group, synovial fluid SAA continues to increase even when serum SAA plateaus. This potentially indicates an additional source of synovial fluid SAA other than the leakage of serum SAA into the joint, namely local production of SAA in the joint. This led us to the second objective of our study, which was to identify potential locally produced joint SAA isoforms using LC-MS/MS.
SAA exists in several isoforms, 44 which may be hepatic or extrahepatic. Identification of joint-specific SAA isoform could potentially enhance the diagnostic accuracy of this biomarker for equine septic arthritis. Tissue-specific SAA expression has been described in a range of domestic animals, as well as humans. 41 Low-level production of extrahepatic SAA in cattle and horses was reported from a wide variety of tissues, including lung, mammary gland, pancreas, synovial membrane, thymus, thyroid gland, and uterus. 3 Mouse SAA3 isoform is produced in macrophages.41,42 In cattle, the milk SAA3 isoform was identified in cows with mastitis. 15 In mares, endometritis leads to endometrial SAA mRNA expression. 4 Expression of SAA isoforms from synovial cells was demonstrated in vitro in rabbit, human, and equine cell cultures.11,26,30 Studies in horses and dogs with joint disease also reported the intraarticularly produced SAA isoforms with highly alkaline isoelectric points.8,14
The amino-acid sequence of the equine extrahepatic SAA isoforms is still poorly understood. The peptide that we found in synovial fluid but not in plasma may belong to the locally produce joint isoform of SAA. Three SAA isoforms in equine serum have been identified, with amino-acid sequence variability in positions 16, 44, and 59 of the SAA protein (UniProt accession P19857). 7 Although we found corresponding peptides in our data as well, they were found in both plasma and synovial fluid in our experimental models. However, we found a consistent substitution of glutamic acid by alanine in one of the peptides found only in synovial fluid but not plasma. This change may, at least in part, explain the alkaline isoelectric point observed in intraarticular equine SAA isoforms in a previous study. 8 Interestingly, the putative locally produced joint peptide was also found in the synovial fluid of horses with systemic inflammation, in both left and right joints. This suggests that severe systemic inflammation may induce SAA production locally within the joint. Therefore, based on our data, locally produced joint SAA may not be more useful than total SAA measurement to differentiate joint infection from non-infectious joint inflammation or systemic inflammation. Further, based on our study, we cannot conclude if the putative isoform we identified is specific to the joint or whether it may be produced in other tissues. However, we suspect it is locally produced because it was consistently absent from the systemic circulation. In addition, although several SAA isoforms have been identified in humans and domestic animals, isoforms of SAA are not yet used in clinical practice as biomarkers in any other species. Finally, although we found that the ion intensities for the putative locally produced joint peptide is ~10 times lower than the systemic isoforms, we cannot yet measure concentrations of various SAA isoforms. Therefore, further study is needed to develop assays able to measure concentrations of various SAA isoforms and to determine the relevance of SAA isoforms for the diagnosis of septic arthritis.
Our study has several limitations. Low numbers of experimental animals led to high variation in measured values. Contralateral joints in septic arthritis and non-septic synovitis were not analyzed by LC-MS/MS to evaluate the presence of SAA isoforms. Also, it is not known which isoforms are detected by the turbidimetric SAA assay that we used. Because we found the difference of one amino acid, we suspect that it did not affect the antigenic properties of these peptides that are detected in a similar way by the assay. Finally, we did not investigate the origin of the putative locally produced SAA isoform.
Overall, our study provides further evidence that, when synovial fluid SAA increases with systemic inflammation or local inflammation in a distant site, SAA leaks from serum into the synovial fluid of nonaffected joints. This can potentially cause misdiagnosis if synovial fluid SAA is interpreted without other clinical pathology data; synovial fluid SAA should be interpreted with caution. In horses with experimental septic arthritis, the serum and synovial fluid SAA concentrations continued to increase at 72 h after the start of our experiment, suggesting that the SAA increase may last longer than previously thought. 22 Finally, we identified a joint SAA peptide, which may correspond to the locally produced SAA isoform. The increase of synovial fluid SAA may be both due to the leakage of SAA from serum into joints and local production of joint SAA isoforms. Further elucidating the sequence and structure of synovial fluid SAA isoforms is needed to evaluate its potential utility for the diagnosis of septic arthritis in horses.
Supplemental Material
Supplemental material, sj-pdf-1-vdi-10.1177_10406387241299873 for Temporal kinetics of serum amyloid A (SAA) concentration and identification of SAA isoforms in blood and synovial fluid of horses with experimentally induced septic arthritis, non-septic synovitis, and systemic inflammation by Roman V. Koziy, George S. Katselis, Seiji Yoshimura, Elemir Simko and José L. Bracamonte in Journal of Veterinary Diagnostic Investigation
Supplemental material, sj-pdf-2-vdi-10.1177_10406387241299873 for Temporal kinetics of serum amyloid A (SAA) concentration and identification of SAA isoforms in blood and synovial fluid of horses with experimentally induced septic arthritis, non-septic synovitis, and systemic inflammation by Roman V. Koziy, George S. Katselis, Seiji Yoshimura, Elemir Simko and José L. Bracamonte in Journal of Veterinary Diagnostic Investigation
Supplemental material, sj-pdf-3-vdi-10.1177_10406387241299873 for Temporal kinetics of serum amyloid A (SAA) concentration and identification of SAA isoforms in blood and synovial fluid of horses with experimentally induced septic arthritis, non-septic synovitis, and systemic inflammation by Roman V. Koziy, George S. Katselis, Seiji Yoshimura, Elemir Simko and José L. Bracamonte in Journal of Veterinary Diagnostic Investigation
Acknowledgments
We thank Dr. Paulos Chumala for his assistance with mass spectrometry.
Footnotes
The authors declared no conflicts of interest related to this report.
Funding: Funding for our work was provided by the Mark and Pat DuMont Equine Orthopedics Research Fund and the Townsend Equine Health Research Fund.
ORCID iDs: Roman V. Koziy  https://orcid.org/0000-0003-1009-7814
https://orcid.org/0000-0003-1009-7814
George S. Katselis  https://orcid.org/0000-0003-0869-7300
https://orcid.org/0000-0003-0869-7300
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Roman V. Koziy, Departments of Veterinary Pathology, University of Saskatchewan, Saskatoon, SK, Canada.
George S. Katselis, Western College of Veterinary Medicine, and Canadian Centre for Rural and Agricultural Health, Department of Medicine, College of Medicine, University of Saskatchewan, Saskatoon, SK, Canada
Seiji Yoshimura, Large Animal Clinical Sciences, University of Saskatchewan, Saskatoon, SK, Canada.
Elemir Simko, Departments of Veterinary Pathology, University of Saskatchewan, Saskatoon, SK, Canada.
José L. Bracamonte, Large Animal Clinical Sciences, University of Saskatchewan, Saskatoon, SK, Canada
References
- 1. Alsemgeest SP, et al. First evidence for the existence of multiple isoforms of bovine serum amyloid-A (apoSAA). Scand J Immunol 1995;41:407–413. [DOI] [PubMed] [Google Scholar]
- 2. Anderson SL, Singh B. Neutrophil apoptosis is delayed in an equine model of colitis: implications for the development of systemic inflammatory response syndrome. Equine Vet J 2017;49:383–388. [DOI] [PubMed] [Google Scholar]
- 3. Berg LC, et al. Serum amyloid A is expressed in histologically normal tissues from horses and cattle. Vet Immunol Immunopathol 2011;144:155–159. [DOI] [PubMed] [Google Scholar]
- 4. Christoffersen M, et al. Evaluation of the systemic acute phase response and endometrial gene expression of serum amyloid A and pro- and anti-inflammatory cytokines in mares with experimentally induced endometritis. Vet Immunol Immunopathol 2010;138:95–105. [DOI] [PubMed] [Google Scholar]
- 5. De Buck M, et al. Structure and expression of different serum amyloid A (SAA) variants and their concentration-dependent functions during host insults. Curr Med Chem 2016;23:1725–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eckersall PD, Bell R. Acute phase proteins: biomarkers of infection and inflammation in veterinary medicine. Vet J 2010;185:23–27. [DOI] [PubMed] [Google Scholar]
- 7. Hultén C, et al. The acute phase serum amyloid A protein (SAA) in the horse: isolation and characterization of three isoforms. Vet Immunol Immunopathol 1997;57:215–227. [DOI] [PubMed] [Google Scholar]
- 8. Jacobsen S. Use of serum amyloid A in equine medicine and surgery. Vet Clin Pathol 2023;52(Suppl 1):8–18. [DOI] [PubMed] [Google Scholar]
- 9. Jacobsen S, Andersen PH. The acute phase protein serum amyloid A (SAA) as a marker of inflammation in horses. Equine Vet Educ 2007;19:38–46. [Google Scholar]
- 10. Jacobsen S, et al. Serum amyloid A isoforms in serum and synovial fluid in horses with lipopolysaccharide-induced arthritis. Vet Immunol Immunopathol 2006;110:325–330. [DOI] [PubMed] [Google Scholar]
- 11. Jacobsen S, et al. Evaluation of a commercially available human serum amyloid A (SAA) turbidometric immunoassay for determination of equine SAA concentrations. Vet J 2006;172:315–319. [DOI] [PubMed] [Google Scholar]
- 12. Jacobsen S, et al. Concentrations of serum amyloid A in serum and synovial fluid from healthy horses and horses with joint disease. Am J Vet Res 2006;67:1738–1742. [DOI] [PubMed] [Google Scholar]
- 13. Jacobsen S, et al. Production of serum amyloid A in equine articular chondrocytes and fibroblast-like synoviocytes treated with proinflammatory cytokines and its effects on the two cell types in culture. Am J Vet Res 2016;77:50–58. [DOI] [PubMed] [Google Scholar]
- 14. Kjelgaard-Hansen M, et al. Serum amyloid A isoforms in serum and synovial fluid from spontaneously diseased dogs with joint diseases or other conditions. Vet Immunol Immunopathol 2007;117:296–301. [DOI] [PubMed] [Google Scholar]
- 15. Kovačević-Filipović M, et al. Serum amyloid A isoforms in serum and milk from cows with Staphylococcus aureus subclinical mastitis. Vet Immunol Immunopathol 2012;145:120–128. [DOI] [PubMed] [Google Scholar]
- 16. Koziy RV, et al. Use of standard diagnostic techniques to determine eradication of infection in experimental equine septic arthritis. Can J Vet Res 2019;83:24–33. [PMC free article] [PubMed] [Google Scholar]
- 17. Koziy RV, et al. Discovery proteomics for the detection of putative markers for eradication of infection in an experimental model of equine septic arthritis using LC-MS/MS. J Proteomics 2022;261:104571. [DOI] [PubMed] [Google Scholar]
- 18. Kumon Y, et al. Local expression of acute phase serum amyloid A mRNA in rheumatoid arthritis synovial tissue and cells. J Rheumatol 1999;26:785–790. [PubMed] [Google Scholar]
- 19. Lindegaard C, et al. Anti-inflammatory effects of intra-articular administration of morphine in horses with experimentally induced synovitis. Am J Vet Res 2010;71:69–75. [DOI] [PubMed] [Google Scholar]
- 20. Lindegaard C, et al. Analgesic efficacy of intra-articular morphine in experimentally induced radiocarpal synovitis in horses. Vet Anaesth Analg 2010;37:171–185. [DOI] [PubMed] [Google Scholar]
- 21. Long A, Nolen-Walston R. Equine inflammatory markers in the twenty-first century: a focus on serum amyloid A. Vet Clin North Am Equine Pract 2020;36:147–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ludwig EK, et al. Serum and synovial fluid serum amyloid A response in equine models of synovitis and septic arthritis. Vet Surg 2016;45:859–867. [DOI] [PubMed] [Google Scholar]
- 23. Mahaffey EA. Synovial fluid. In: Cowell RL, Tyler RD, eds. Diagnostic Cytology and Hematology of the Horse. 2nd ed. Mosby, 2002:163–170. [Google Scholar]
- 24. Malle E, De Beer FC. Human serum amyloid A (SAA) protein: a prominent acute-phase reactant for clinical practice. Eur J Clin Invest 1996;26:427–435. [DOI] [PubMed] [Google Scholar]
- 25. McDonald TL, et al. Elevated extrahepatic expression and secretion of mammary-associated serum amyloid A 3 (M-SAA3) into colostrum. Vet Immunol Immunopathol 2001;83:203–211. [DOI] [PubMed] [Google Scholar]
- 26. Mitchell TI, et al. Serum amyloid A (SAA3) produced by rabbit synovial fibroblasts treated with phorbol esters or interleukin 1 induces synthesis of collagenase and is neutralized with specific antiserum. J Clin Invest 1991;87:1177–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Morton AJ. Diagnosis and treatment of septic arthritis. Vet Clin North Am Equine Pract 2005;21:627–649. [DOI] [PubMed] [Google Scholar]
- 28. Morton AJ, et al. Preferential and non-selective cyclooxygenase inhibitors reduce inflammation during lipopolysaccharide-induced synovitis. Res Vet Sci 2005;78:189–192. [DOI] [PubMed] [Google Scholar]
- 29. O’Hara R, et al. Acute-phase serum amyloid A production by rheumatoid arthritis synovial tissue. Arthritis Res 2000;2:142–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. O’Hara R, et al. Local expression of the serum amyloid A and formyl peptide receptor-like 1 genes in synovial tissue is associated with matrix metalloproteinase production in patients with inflammatory arthritis. Arthritis Rheum 2004;50:1788–1799. [DOI] [PubMed] [Google Scholar]
- 31. Pluim M, et al. Short- and long-term follow-up of 150 sports horses diagnosed with tendinopathy or desmopathy by ultrasonographic examination and treated with high-power laser therapy. Res Vet Sci 2018;119:232–238. [DOI] [PubMed] [Google Scholar]
- 32. Robinson CS, et al. Are serum amyloid A or D-lactate useful to diagnose synovial contamination or sepsis in horses? Vet Rec 2017;181:425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sanchez Teran AF, et al. Effects of repeated intra-articular administration of amikacin on serum amyloid A, total protein and nucleated cell count in synovial fluid from healthy horses. Equine Vet J Suppl 2012;(43):12–16. [DOI] [PubMed] [Google Scholar]
- 34. Sanchez-Teran AF, et al. Effect of arthroscopic lavage on systemic and synovial fluid serum amyloid A in healthy horses. Vet Surg 2016;45:223–230. [DOI] [PubMed] [Google Scholar]
- 35. Sanchez-Teran AF, et al. Effect of repeated through-and-through joint lavage on serum amyloid A in synovial fluid from healthy horses. Vet J 2016;210:30–33. [DOI] [PubMed] [Google Scholar]
- 36. Santos LCP, et al. Effects of intraarticular ropivacaine and morphine on lipopolysaccharide-induced synovitis in horses. Vet Anaesth Analg 2009;36:280–286. [DOI] [PubMed] [Google Scholar]
- 37. Soler L, et al. Serum amyloid A3 (SAA3), not SAA1 appears to be the major acute phase SAA isoform in the pig. Vet Immunol Immunopathol 2011;141:109–115. [DOI] [PubMed] [Google Scholar]
- 38. Stack JD, et al. Comparison of serum amyloid A measurements in equine synovial fluid with routine diagnostic methods to detect synovial infection in a clinical environment. Front Vet Sci 2019;6:325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Steel CM. Equine synovial fluid analysis. Vet Clin North Am Equine Pract 2008;24:437–454. [DOI] [PubMed] [Google Scholar]
- 40. Tarp S, et al. Effect of nonsteroidal antiinflammatory drugs on the C-reactive protein level in rheumatoid arthritis: a meta-analysis of randomized controlled trials. Arthritis Rheum 2012;64:3511–3521. [DOI] [PubMed] [Google Scholar]
- 41. Uhlar CM, Whitehead AS. Serum amyloid A, the major vertebrate acute-phase reactant. Eur J Biochem 1999;265:501–523. [DOI] [PubMed] [Google Scholar]
- 42. Upragarin N, et al. Extrahepatic production of acute phase serum amyloid A. Histol Histopathol 2005;20:1295–1307. [DOI] [PubMed] [Google Scholar]
- 43. Van Loon JPAM, et al. Intra-articular opioid analgesia is effective in reducing pain and inflammation in an equine LPS induced synovitis model. Equine Vet J 2010;42:412–419. [DOI] [PubMed] [Google Scholar]
- 44. Witkowska-Piłaszewicz OD, et al. Serum amyloid A in equine health and disease. Equine Vet J 2019;51:293–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yoshimura S, et al. Use of serum amyloid A in serum and synovial fluid to detect eradication of infection in experimental septic arthritis in horses. Can J Vet Res 2020;84:198–204. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-vdi-10.1177_10406387241299873 for Temporal kinetics of serum amyloid A (SAA) concentration and identification of SAA isoforms in blood and synovial fluid of horses with experimentally induced septic arthritis, non-septic synovitis, and systemic inflammation by Roman V. Koziy, George S. Katselis, Seiji Yoshimura, Elemir Simko and José L. Bracamonte in Journal of Veterinary Diagnostic Investigation
Supplemental material, sj-pdf-2-vdi-10.1177_10406387241299873 for Temporal kinetics of serum amyloid A (SAA) concentration and identification of SAA isoforms in blood and synovial fluid of horses with experimentally induced septic arthritis, non-septic synovitis, and systemic inflammation by Roman V. Koziy, George S. Katselis, Seiji Yoshimura, Elemir Simko and José L. Bracamonte in Journal of Veterinary Diagnostic Investigation
Supplemental material, sj-pdf-3-vdi-10.1177_10406387241299873 for Temporal kinetics of serum amyloid A (SAA) concentration and identification of SAA isoforms in blood and synovial fluid of horses with experimentally induced septic arthritis, non-septic synovitis, and systemic inflammation by Roman V. Koziy, George S. Katselis, Seiji Yoshimura, Elemir Simko and José L. Bracamonte in Journal of Veterinary Diagnostic Investigation