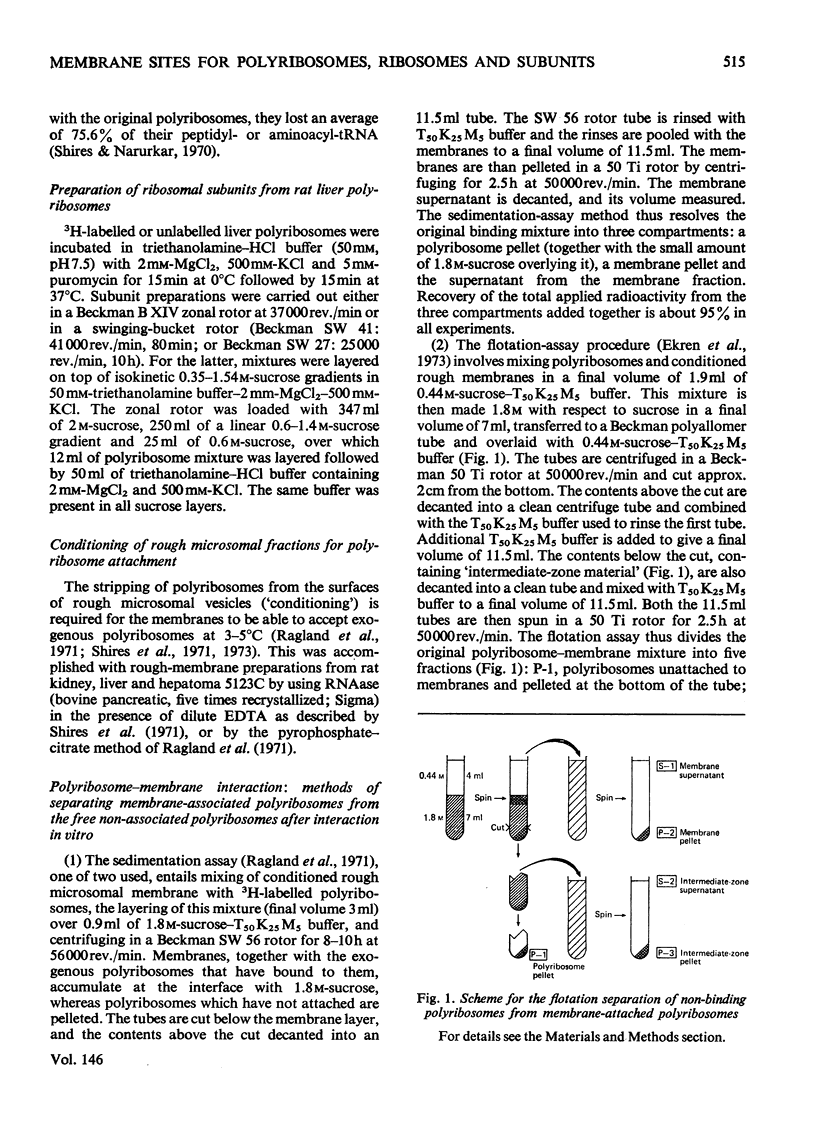
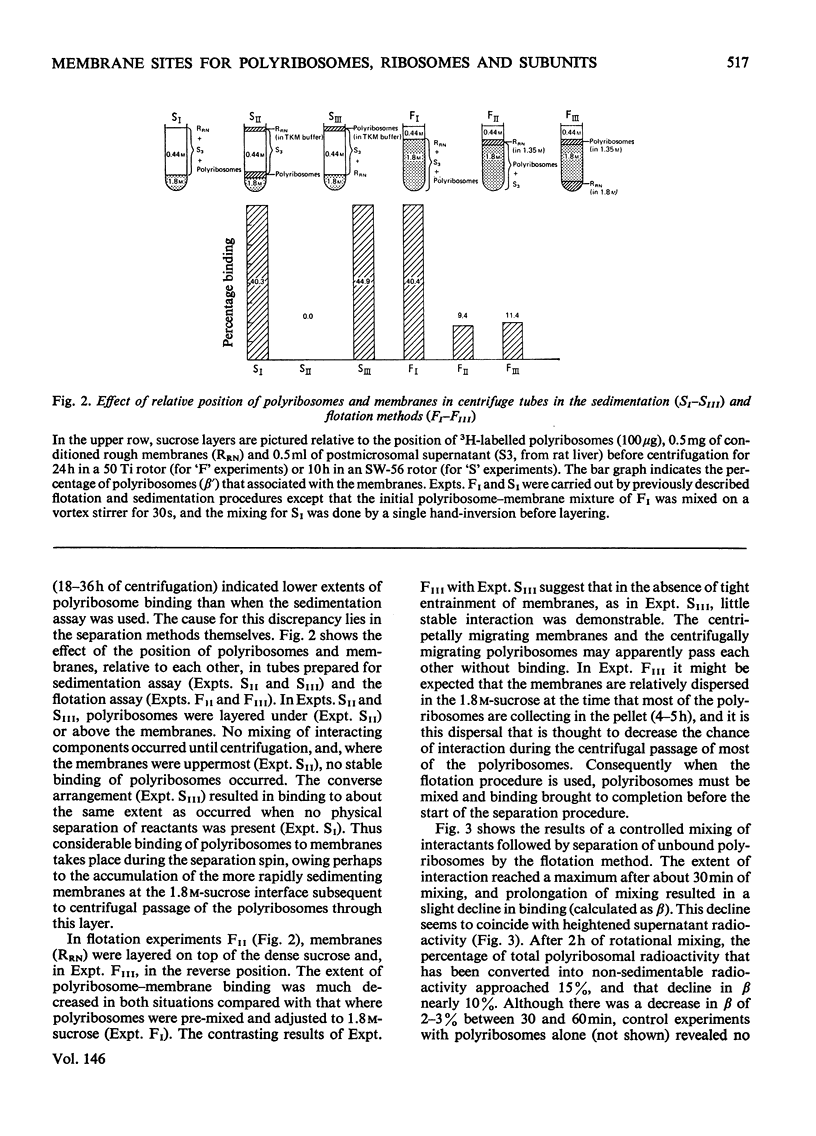
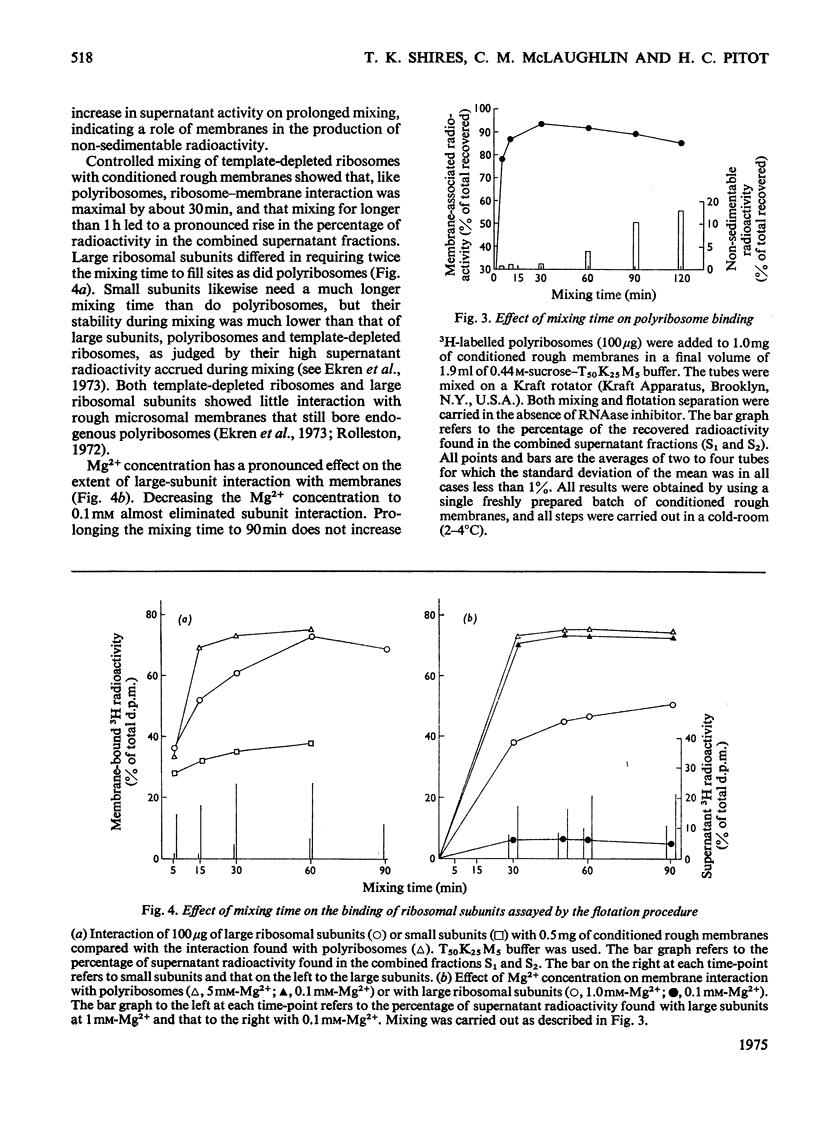
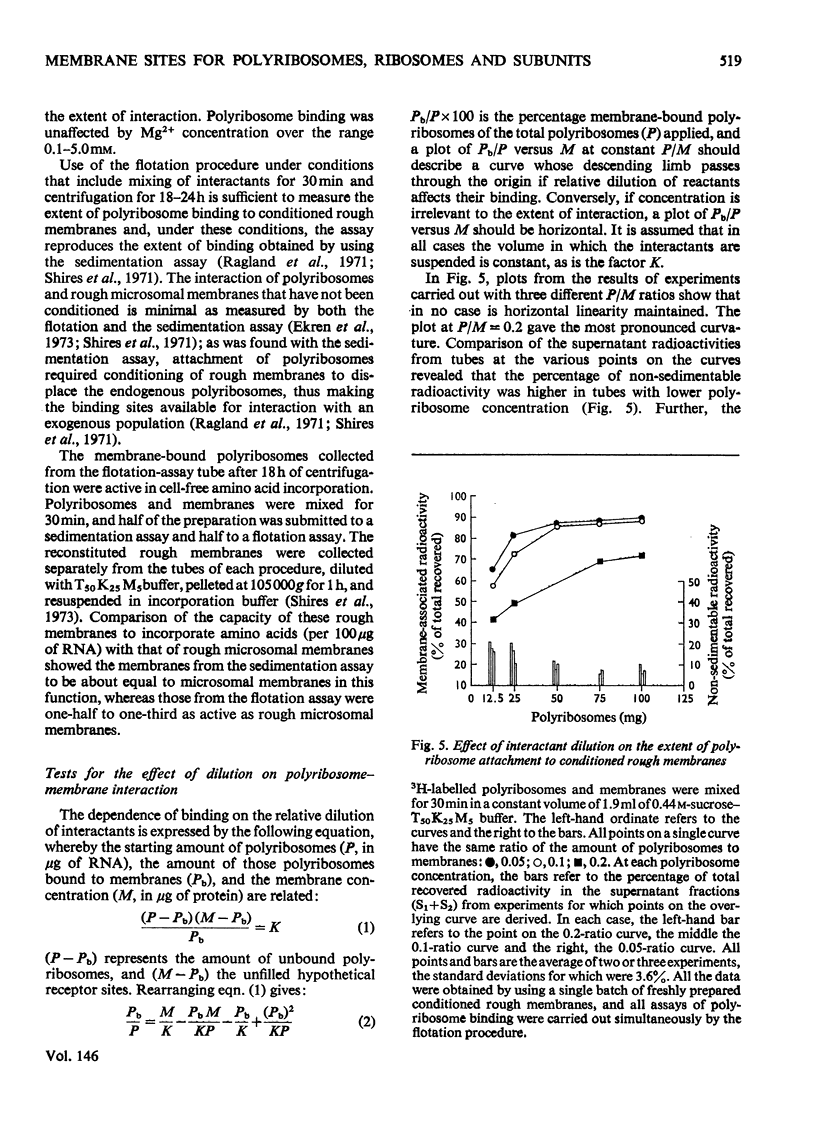
Abstract
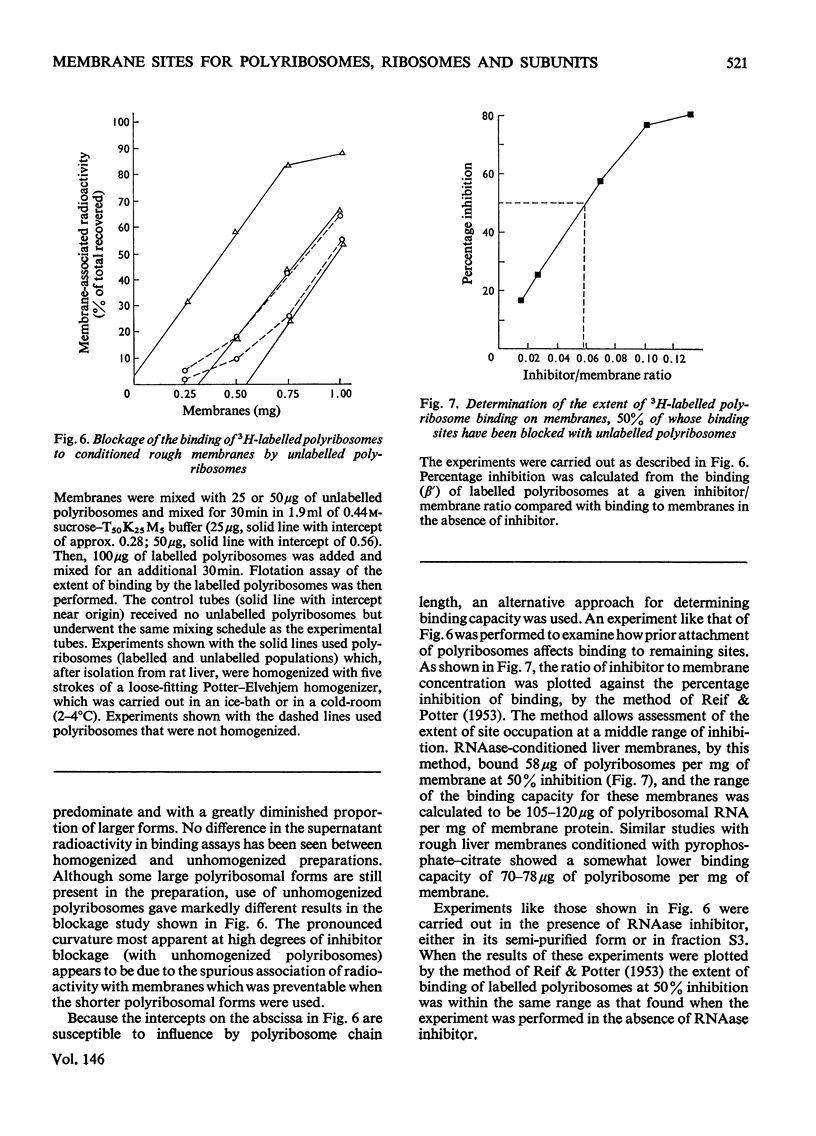
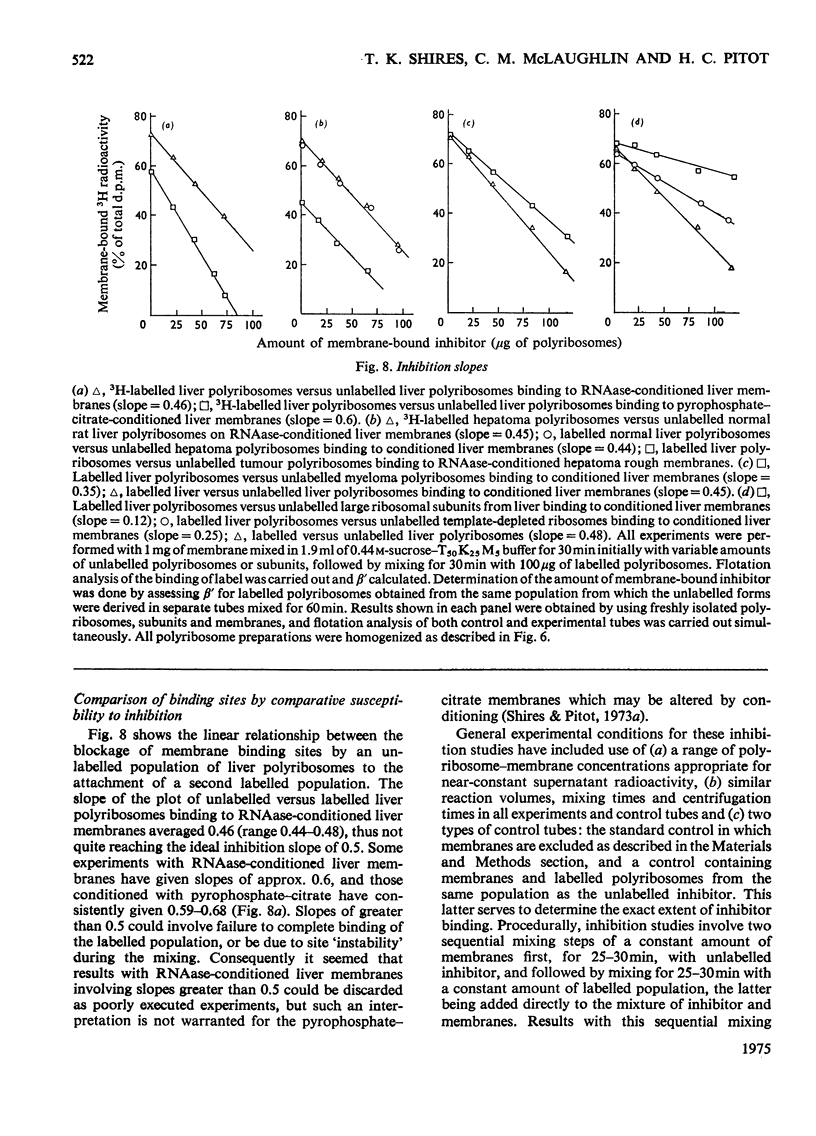
Differences in the binding sites for polyribosomes, template-depleted ribosomes and large ribosomal subunits were found in microsomal derivatives of the rough endoplasmic reticulum. 1. The stoicheiometry of polyribosome and ribosome interaction in vitro with membranes was shown to be influenced by the relative concentration of interactants and the duration of their mixing. Large ribosomal subunits required a more prolonged mixing schedule to achieve saturation of membranes than did polyribosomes. 2. By using a procedure which minimized the effects on binidng by the stoicheiometric variables, competition between populations of polyribosomes, ribosomes and subunits for membrane sites showed that subunits, and to a lesser extent ribosomes, failed to block polyribosome attachment. 3. Polyribosomes isolated from liver, kidney and hepatoma 5123C entirely bound to a common membrane site, but some polyribosomes from myeloma MOPC-21 bound to other sites, perhaps influenced by their unique nascent proteins. 4. Subunit-binding sites appear on rough membranes only after endogenous polyribosomes have been removed, but no evidence that resulting changes in surface constituents are responsible was found. Large-subunit binding was largely abolished by lowering MgC12 concentration of 0.1 mM, whereas under the same conditions polyribosome binding was undiminished. 5. The large-subunit site appears to be distinct from the polyribosome site not only in the restriction of its affinity for particles but also spatially, to the extent that bound subunits do not hinder access of polyribosomes to their sites.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ACKERMANN W. W., POTTER V. R. Enzyme inhibition in relation to chemotherapy. Proc Soc Exp Biol Med. 1949 Oct;72(1):1–9. doi: 10.3181/00379727-72-17313. [DOI] [PubMed] [Google Scholar]
- Adelman M. R., Sabatini D. D., Blobel G. Ribosome-membrane interaction. Nondestructive disassembly of rat liver rough microsomes into ribosomal and membranous components. J Cell Biol. 1973 Jan;56(1):206–229. doi: 10.1083/jcb.56.1.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agsteribbe C. A., Borst P., Aaij C. A study of minor RNA components of rat-liver microsomes. Biochim Biophys Acta. 1971 Aug 26;246(2):233–238. doi: 10.1016/0005-2787(71)90132-8. [DOI] [PubMed] [Google Scholar]
- Berdinskikh N. K., Kozak V. V., Khomenko A. K., Shliakhovenko V. A. Vliianie rezhima gomogenizatsii na sokhrannost' poliribosom pecheni krys. Vopr Med Khim. 1971 May-Jun;17(3):298–301. [PubMed] [Google Scholar]
- Ekren T., Shires T., Pitot H. C. Determining the affinity in vitro of hepatic ribosomal subunits for derivatives of the rough endoplasmic reticulum. Biochem Biophys Res Commun. 1973 Sep 5;54(1):283–289. doi: 10.1016/0006-291x(73)90920-0. [DOI] [PubMed] [Google Scholar]
- Gribnau A. A., Schoenmakers J. G., Bloemendal H. Purification of rat liver RNase inhibitor and its effect on polyribosome integrity. Arch Biochem Biophys. 1969 Mar;130(1):48–52. doi: 10.1016/0003-9861(69)90007-1. [DOI] [PubMed] [Google Scholar]
- Grove B. K., Johnson T. C. The effect of ribonuclease on ribosomal RNA and subsequent polypeptide synthesis. Biochem Biophys Res Commun. 1973 Nov 1;55(1):45–51. doi: 10.1016/s0006-291x(73)80057-9. [DOI] [PubMed] [Google Scholar]
- Hüvös P., Vereczkey L., Gaál O. Incorporating activity of ribosomes and integrity of ribosomal RNA. Biochem Biophys Res Commun. 1970 Nov 25;41(4):1020–1026. doi: 10.1016/0006-291x(70)90187-7. [DOI] [PubMed] [Google Scholar]
- Ikehara Y., Pitot H. C. Localization of polysome-bound albumin and serine dehydratase in rat liver cell fractions. J Cell Biol. 1973 Oct;59(1):28–44. doi: 10.1083/jcb.59.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jothy S., Tay S., Simpkins H. The role of membrane phospholipids in the interaction of ribosomes with endoplasmic-reticulum membrane. Biochem J. 1973 Mar;132(3):637–640. doi: 10.1042/bj1320637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan S. W., Webb T. E., Morris H. P. Diversity and nature of ribosomal pools in hepatoma 7800 and host liver. Biochem J. 1968 Oct;109(4):617–623. doi: 10.1042/bj1090617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lawford G. R. The effect of incubation with puromycin on the dissociation of rat liver ribosomes into active subunits. Biochem Biophys Res Commun. 1969 Sep 24;37(1):143–150. doi: 10.1016/0006-291x(69)90892-4. [DOI] [PubMed] [Google Scholar]
- Leslie R. A., Mansbridge J. N. Effect of sedimentation through sucrose solutions on the protein-synthesizing ability of rat liver microsomes. Biochem J. 1970 May;117(5):893–898. doi: 10.1042/bj1170893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisowska-Bernstein B., Lamm M. E., Vassalli P. Synthesis of immunoglobulin heavy and light chains by the free ribosomes of a mouse plasma cell tumor. Proc Natl Acad Sci U S A. 1970 Jun;66(2):425–432. doi: 10.1073/pnas.66.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milstein C., Brownlee G. G., Harrison T. M., Mathews M. B. A possible precursor of immunoglobulin light chains. Nat New Biol. 1972 Sep 27;239(91):117–120. doi: 10.1038/newbio239117a0. [DOI] [PubMed] [Google Scholar]
- Moyer G. H., Murray R. K., Khairallah L. H., Suss R., Pitot H. C. Ultrastructural and biochemical characteristics of endoplasmic reticulum fractions of the Morris 7800 and Reuber H-35 hepatomas. Lab Invest. 1970 Jul;23(1):108–118. [PubMed] [Google Scholar]
- Munro H. N., Fleck A. Recent developments in the measurement of nucleic acids in biological materials. A supplementary review. Analyst. 1966 Feb;91(79):78–88. doi: 10.1039/an9669100078. [DOI] [PubMed] [Google Scholar]
- Pitot H. C., Shires T. K. Introductory remarks: membrane-polysome interactions. Fed Proc. 1973 Jan;32(1):76–79. [PubMed] [Google Scholar]
- REIF A. E., POTTER V. R. In vivo inhibition of succinoxidase activity in normal and tumor tissues by antimycin A. Cancer Res. 1953 Jan;13(1):49–57. [PubMed] [Google Scholar]
- Ragland W. L., Shires T. K., Pitot H. C. Polyribosomal attachment to rat liver and hepatoma endoplasmic reticulum in vitro. A method for its study. Biochem J. 1971 Jan;121(2):271–278. doi: 10.1042/bj1210271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolleston F. S., Mak D. The binding of polyribosomes to smooth and rough endoplasmic-reticulum membranes. Biochem J. 1973 Apr;131(4):851–853. doi: 10.1042/bj1310851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolleston F. S. The binding of ribosomal subunits to endoplasmic reticulum membranes. Biochem J. 1972 Sep;129(3):721–731. doi: 10.1042/bj1290721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott-Burden T., Hawtrey A. O. Further in vitro studies on the reattachment of ribosomes to ribosome-free membranes. Hoppe Seylers Z Physiol Chem. 1972 Nov;353(11):1727–1734. doi: 10.1515/bchm2.1972.353.2.1727. [DOI] [PubMed] [Google Scholar]
- Scott-Burden T., Hawtrey A. O. Preparation of ribosome-free membranes from rat liver microsomes by means of lithium chloride. Biochem J. 1969 Dec;115(5):1063–1069. doi: 10.1042/bj1151063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott-Burden T., Hawtrey A. O. Studies on the reattachment of ribosomes and ribosomal subunits to ribosome-free membranes prepared from rat liver microsomes by means of lithium chloride. Hoppe Seylers Z Physiol Chem. 1971 Apr;352(4):575–582. doi: 10.1515/bchm2.1971.352.1.575. [DOI] [PubMed] [Google Scholar]
- Shires T. K., Ekren T., Narurkar L. M., Pitot H. C. Protein synthesis on rat liver polysome-membrane complexes formed in vitro and disposition of the discharges chains. Nat New Biol. 1973 Apr 18;242(120):198–201. doi: 10.1038/newbio242198a0. [DOI] [PubMed] [Google Scholar]
- Shires T. K., Narurkar L., Pitot H. C. The association in vitro of polyribosomes with ribonuclease-treated derivatives of hepatic rough endoplasmic reticulum. Characteristics of the membrane binding sites and factors influencing association. Biochem J. 1971 Nov;125(1):67–79. doi: 10.1042/bj1250067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shires T. K., Pitot H. C. Correlation of rat liver membrane binding of polysomes in vitro with function of the complexes formed. Biochem Biophys Res Commun. 1973 Jan 23;50(2):344–351. doi: 10.1016/0006-291x(73)90846-2. [DOI] [PubMed] [Google Scholar]
- Shires T. K., Pitot H. C. Functional studies of polysome-membrane interactions in vitro. Adv Enzyme Regul. 1973;11:255–272. doi: 10.1016/0065-2571(73)90019-8. [DOI] [PubMed] [Google Scholar]
- Vassalli P., Lisowska-Bernstein B., Lamm M. E. Cell-free synthesis of rat immunoglobulin. 3. Analysis of the cell-free made chains and of their mode of assembly. J Mol Biol. 1971 Feb 28;56(1):1–19. doi: 10.1016/0022-2836(71)90080-5. [DOI] [PubMed] [Google Scholar]
- Webb T. E., Morris H. P. Properties of the inactive ribosomal components in rat liver and hepatoma. Biochem J. 1969 Nov;115(3):575–582. doi: 10.1042/bj1150575. [DOI] [PMC free article] [PubMed] [Google Scholar]


